Abstract
A multidrug-resistant Acinetobacter baumannii isolate recovered from a patient hospitalized in Switzerland after a transfer from Serbia produced the NDM-1 carbapenemase. The blaNDM-1 gene was part of a chromosomally located Tn125 composite transposon bracketed by two copies of the same insertion sequence, ISAba125. This transposon was also associated with the acquisition and expression of the blaNDM-2 gene in an A. baumannii isolate in Germany. Tn125 appears to be the main vehicle for dissemination of blaNDM genes in that species.
TEXT
The carbapenemase NDM-1, initially identified in Escherichia coli and Klebsiella pneumoniae, has been found mostly in enterobacterial species (12, 16, 17). However, recent reports have described the occurrence of blaNDM genes in Acinetobacter baumannii. Several NDM-1-positive A. baumannii isolates have been identified in India (10), and two NDM-positive A. baumannii isolates have been recovered in Germany, one being from a patient transferred from a Serbian hospital and producing NDM-1 (6), whereas the other produced NDM-2 (one amino acid substitution with respect to NDM-1) and had been recovered from a patient transferred from an Egyptian hospital (isolate ML) (9). Although the Indian subcontinent is considered a reservoir of NDM-1 producers (17), recent reports indicate that at least the Balkan states and the Middle East regions could also be potential reservoirs (13, 21).
In a recent work, Pfeifer et al. (18) reported that the blaNDM-1 gene identified in A. baumannii isolate 161/07 from Germany (from a patient transferred from Serbia) (6) was located inside a composite transposon bracketed by two copies of insertion sequence ISAba125.
A retrospective survey focusing on multidrug-resistant Gram-negative isolates identified several non-clonally related NDM-1-producing isolates from three patients who had been hospitalized in Geneva University Hospitals, Switzerland, from March 2009 to October 2010. One E. coli and one K. pneumoniae isolate were recovered from the same patient, who had been transferred from Serbia (22). In both isolates, the blaNDM-1 gene was identified on the same 150-kb IncA/C-type plasmid (20). Further investigations showed that this patient also carried a multidrug-resistant A. baumannii isolate that had been recovered from rectal swabs.
A. baumannii isolate JH was resistant to all β-lactams, including carbapenems (MICs of imipenem, ertapenem, doripenem, and meropenem measured by Etest [AB bioMérieux; Solna, Sweden] were all >32 μg/ml) according to the CLSI guidelines (3). It was also resistant to gentamicin, amikacin, chloramphenicol, tetracycline, and fluoroquinolones and remained susceptible to tobramycin and netilmicin, with MICs of colistin, rifampin, and tigecycline being at 0.5, 1, and 1 μg/ml, respectively.
PCR and sequencing revealed that A. baumannii JH harbored the blaNDM-1 gene. Screening for additional β-lactamase genes and for 16S RNA methylase genes as reported previously (1, 23) showed that A. baumannii JH was coharboring another carbapenemase gene, namely, blaOXA-23, whereas no 16S RNA methylase gene was identified, in accordance with the susceptibility observed for some aminoglycosides. Multilocus sequence typing was performed, following the Institut Pasteur scheme, as described previously (4), and showed that isolate JH belonged to the ST1 type, whereas an NDM-2-positive A. baumannii ML isolate recently identified from Egypt belonged to the ST103 type (9), both types differing significantly. Interestingly, ST1-type A. baumannii isolates have recently been identified in Greece (carbapenem resistant by production of OXA-58) (14) and Italy (carbapenem susceptible or resistant by production of OXA-58) (5).
In order to investigate the genetic structures at the origin of the blaNDM-1 gene in A. baumannii JH and of the blaNDM-2 gene in A. baumannii ML, cloning experiments were performed as described previously (24). Expression in E. coli TOP10 gave E. coli TOP10(pAbNDM1) and E. coli TOP10(pAbNDM-2), expressing NDM-1 and NDM-2, respectively. They exhibited the exact same resistance pattern, with identical MICs of all β-lactams, in accordance with our previous observations (9).
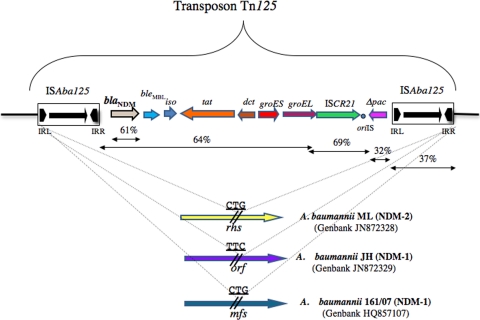
Sequencing of the whole insert of both recombinant plasmids (an XbaI 15-kb fragment for pAbNDM1 and an HindIII 17-kb fragment for pAbNDM2) allowed characterization of the structures surrounding the blaNDM-1 and blaNDM-2 genes. Both genes were located inside the exact same composite transposon, named Tn125, made of two copies of insertion sequence ISAba125 bracketing a 7,925-bp fragment, thus forming a 10,099-bp transposon. ISAba125 is a 1,087-bp element belonging to the IS30 family, containing a single open reading frame corresponding to the 322-amino-acid-long transposase. The two copies of ISAba125 were located in the same direct orientation, and they differed by 6 bp, leading only to three amino acid changes. However, their respective inverted repeats were identical.
In both isolates, transposon Tn125 was bracketed by a 3-bp target site duplication (CTG in isolate ML and TTC in isolate JH) corresponding to the signature of the transposition process. Further sequence analysis showed that transposon Tn125 was located on the chromosome in both isolates. In A. baumannii JH, it was inserted into a gene similar to that identified in the chromosome of several A. baumannii strains (in silico analysis) and encoding a putative protein of unknown function. In A. baumannii ML, it was inserted into the rhs gene, encoding an Rhs protein of unknown function, though Rhs elements are known to be accessory elements often present as multiple copies in genomes (27). These data suggest that the same transposition targeted independently the chromosomes of two distinct A. baumannii strains.
Detailed analysis of the Tn125 content revealed that the distance between ISAba125 and the blaNDM-1 or blaNDM-2 gene was 93 bp, as previously observed in blaNDM-1-positive enterobacterial isolates in which either a truncated or complete copy of ISAba125 had been identified (Fig. 1) (20). The expression of the blaNDM-1/blaNDM-2 genes was therefore under the control of a hybrid promoter whose −35 sequence was located in the left inverted repeat of ISAba125, as demonstrated in E. coli (19). Downstream of blaNDM-1/blaNDM-2, eight open reading frames (ORFs) were identified (Fig. 1). The first corresponded to the bleMBL gene, encoding a 121-amino-acid-long protein conferring resistance to bleomycin, as previously found in enterobacterial isolates (19, 20). Then, a gene encoding a 212-amino-acid-long putative phosphoribosylanthranilate isomerase was identified, whose product shares 98% amino acid identity with the protein encoded by a gene identified downstream of blaNDM-1 on plasmid pNDM-HK from E. coli (Fig. 1). It was followed by a gene encoding a 343-amino-acid-long putative twin-arginine translocation pathway signal sequence domain protein sharing similarities with that of Brevundimonas diminuta (GenBank accession no. EEGF96543.1) and then by a gene encoding a 135-amino-acid long periplasmidic divalent cation tolerance protein 64% identical with that of Xanthomonas albilineans (GenBank accession no. CBA14859.1). Downstream, genes encoding the GroES and GroEL chaperonin proteins, respectively, were identified. The GroES protein (97 amino acids long) shared 85% identity with those encoded by genes located on IncA/C-type and blaCMY-2-positive plasmids in E. coli (2) and Salmonella enterica serovar Typhimurium (28). The GroEL protein (547 amino acids long) shared 92% identity with that encoded by an IncHI2 plasmid from E. coli APEC01 (8). Finally, the mobilized fragment bracketed by the two ISAba125 elements ended with a gene encoding the putative transposase of an ISCR-like element, termed ISCR21, sharing 96% identity with that identified in E. coli APEC01 and 93% with that of ISCR19 involved in the acquisition of the blaOXA-18 gene in Pseudomonas aeruginosa (15). ISCR elements are peculiar insertion sequences belonging to the IS91 family, likely mobilizing genes located at their left-hand extremity by rolling-circle transposition (25). According to the sequence of its transposase, ISCR21 belongs to the ISCR3/ISCR5 group (26). The putative oriIS sequence defining the origin of replication of ISCR21 was identified, sharing a high degree of identity (16 out of 19 bp) with those of ISCR3. At the right-hand extremity of ISCR21 and before the ISAba125 right copy of Tn125, a truncated gene encoding a putative phospholipid acetyltransferase was identified, with the corresponding protein sequence sharing 91% amino acid identity with that of Acinetobacter junii (GenBank accession no. EEY94339.1) (Fig. 1).
Fig 1.
Features of transposon Tn125, carrying the blaNDM-1 and blaNDM-2 genes. Genes and their transcription orientations are indicated by arrows. The lengths of the target genes and the exact location of the target site are not to scale. oriIS of ISCR21 is indicated by a circle. The 3-bp Tn125 target sites identified in each isolate are underlined and uppercase. GC content is indicated in percentages. Gene names are abbreviated according to their corresponding proteins: iso for phosphoribosylanthranilate isomerase; tat for the twin-arginine translocation pathway signal sequence protein; dvt for the divalent cation tolerance protein; Δpac for truncated phospholipid acetyltransferase; rhs for the Rhs protein; orf for an unknown open reading frame; and mfs for major facilitator superfamily metabolite/H+ symporter. IRL and IRR indicate inverted repeat left and right, respectively.
Analyzing both the location of the diverse mobile elements and the GC content of the genes identified in transposon Tn125 (Fig. 1), our hypothesis here is that the original mobilization of the blaNDM-1 gene occurred through a rolling-circle transposition process involving ISCR21. This IS could have mobilized a DNA fragment encompassing all the genes from groEL and groES to blaNDM-1 (actually exhibiting similar GC percentages) from its still-unknown bacterial progenitor. Then, this transposed fragment may have targeted the chromosome of an A. junii-related strain (corresponding to sequences with a much lower GC content) (Fig. 1). Then, two copies of ISAba125 (known to be present in different Acinetobacter species, since it is has been found in A. baumannii and Acinetobacter genomospecies 3/Acinetobacter pittii according to the GenBank databases) could have targeted the blaNDM-1-surrounding sequences, thus forming Tn125, which could transpose to other Acinetobacter sp. isolates.
It is noteworthy that the NDM-1-producing A. baumannii isolate 161/07 from Germany belonged to ST25, distinct from our two isolates; however, the blaNDM-1 gene was also identified as part of a composite transposon (18). In silico analysis revealed that this isolate harbored an identical transposon Tn125. Its target site was also chromosomal but corresponded to another gene, namely, mfs, encoding a putative major facilitator superfamily metabolite/H+ symporter (18). A detailed analysis using sequences available in the GenBank databases (accession no. HQ857107) showed that the ISAba125 extremities were wrongly annotated in that study, the target site duplication being consequently 3 and not 8 bp long.
Transposon Tn125 was bracketed by two copies of insertion sequence ISAba125 that are identical to those reported to be multiple copies in the chromosome of A. baumannii ACICU (7) (Fig. 1). It is noteworthy that the target site duplication bracketing Tn125 in isolate 161/07 corresponded to CTG, exactly identical to that identified in isolate ML, despite the fact that the two target genes have no genetic link. This would suggest that this CTG sequence may represent a hot spot of transposition for ISAba125 and consequently for transposition of Tn125.
The current dissemination of blaNDM-1 and blaNDM-2 in A. baumannii is likely linked to a Tn125 and not plasmid related, in contrast to what is observed in the Enterobacteriaceae, in which it is supported mainly by plasmids in which blaNDM-1-surrounding sequences contain a single copy of ISAba125 that is most often truncated.
Nucleotide sequence accession number.
The nucleotide and protein sequences of the Tn125 transposons and their close genetic environments have been registered in GenBank under accession no. JN872328 (isolate ML) and JN872329 (isolate JH).
ACKNOWLEDGMENTS
This work was mostly funded by the INSERM (U914), France, and by grants from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539), Université Paris XI, and from the European Community (TEMPOtest-QC; HEALTH-2009-241742).
We thank Y. Pfeifer for strain 161/07.
Footnotes
Published ahead of print 5 December 2011
REFERENCES
- 1. Berçot B, Poirel L, Nordmann P. 2011. Updated multiplex PCR for detection of 16S rRNA methylases; high prevalence among NDM-1 producers. Diagn. Microbiol. Infect. Dis. 71:442–445 [DOI] [PubMed] [Google Scholar]
- 2. Call DR, et al. 2010. blaCMY-2-positive IncA/C plasmids from Escherichia coli and Salmonella enterica are a distinct component of a larger lineage of plasmids. Antimicrob. Agents Chemother. 54:590–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clinical Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. CLSI M100-S21 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S. 2010. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One 5:e10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Popolo A, Giannouli M, Triassi M, Brisse S, Zarrilli R. 2011. Molecular epidemiological investigation of multidrug-resistant Acinetobacter baumannii strains in four Mediterranean countries with a multilocus sequence typing scheme. Clin. Microbiol. Infect. 17:197–201 [DOI] [PubMed] [Google Scholar]
- 6. Göttig S, et al. 2010. Global spread of New Delhi metallo-β-lactamase 1. Lancet Infect. Dis. 10:828–829 [DOI] [PubMed] [Google Scholar]
- 7. Iacono M, et al. 2008. Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob. Agents Chemother. 52:2616–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson TJ, Wannemeuhler YM, Scaccianoce JA, Johnson SJ, Nolan LK. 2006. Complete DNA sequence, comparative genomics, and prevalence of an IncHI2 plasmid occurring among extraintestinal pathogenic Escherichia coli isolates. Antimicrob. Agents Chemother. 50:3929–3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaase M, et al. 2011. NDM-2 carbapenemase in Acinetobacter baumannii from Egypt. J. Antimicrob. Chemother. 66:1260–1262 [DOI] [PubMed] [Google Scholar]
- 10. Karthikeyan K, Thirunarayan MA, Krishnan P. 2010. Coexistence of blaOXA-23 with blaNDM-1 and armA in clinical isolates of Acinetobacter baumannii from India. J. Antimicrob. Chemother. 65:2253–2254 [DOI] [PubMed] [Google Scholar]
- 11. Reference deleted.
- 12. Kumarasamy KK, et al. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Livermore DM, Walsh TR, Toleman M, Woodford N. 2011. Balkan NDM-1: escape or transplant? Lancet Infect. Dis. 11:164.. [DOI] [PubMed] [Google Scholar]
- 14. Mezzatesta ML, et al. Epidemiological characterization and distribution of carbapenem-resistant Acinetobacter baumannii clinical isolates in Italy. Clin. Microbiol. Infect., in press [DOI] [PubMed] [Google Scholar]
- 15. Naas T, et al. 2008. Genetic structure associated with blaOXA-18, encoding a clavulanic acid-inhibited extended-spectrum oxacillinase. Antimicrob. Agents Chemother. 52:3898–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17:1791–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nordmann P, Poirel L, Toleman MA, Walsh TR. 2011. Does broad spectrum ß-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria? J. Antimicrob. Chemother. 66:689–692 [DOI] [PubMed] [Google Scholar]
- 18. Pfeifer Y, et al. 2011. Molecular characterization of blaNDM-1 in an Acinetobacter baumannii strain isolated in Germany in 2007. J. Antimicrob. Chemother. 66:1998–2001 [DOI] [PubMed] [Google Scholar]
- 19. Poirel L, Bonnin RA, Nordmann P. 2011. Analysis of the resistome of a multidrug-resistant NDM-1-producing Escherichia coli strain by high-throughput genome sequencing. Antimicrob. Agents Chemother. 55:4224–4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poirel L, Dortet L, Bernabeu S, Nordmann P. 2011. Genetic features of blaNDM-1-positive Enterobacteriaceae. Antimicrob. Agents Chemother. 55:5403–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poirel L, Fortineau N, Nordmann P. 2011. International transfer of NDM-1-producing Klebsiella pneumoniae from Iraq to France. Antimicrob. Agents Chemother. 55:1821–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poirel L, et al. 2011. Molecular analysis of NDM-1-producing enterobacterial isolates from Geneva, Switzerland. J. Antimicrob. Chemother. 66:1730–1733 [DOI] [PubMed] [Google Scholar]
- 23. Poirel L, Walsh TR, Cuvillier V, Nordmann P. 2011. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 70:119–123 [DOI] [PubMed] [Google Scholar]
- 24. Rodriguez-Martinez J-M, Nordmann P, Fortineau N, Poirel L. 2010. VIM-19, a metallo-ß-lactamase with increased carbapenemase activity from Escherichia coli and Klebsiella pneumoniae. Antimicrob. Agents Chemother. 54:471–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toleman MA, Bennett PM, Walsh TR. 2006. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 70:296–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Toleman MA, Walsh TR. 2008. Evolution of the ISCR3 group of ISCR elements. Antimicrob. Agents Chemother. 52:3789–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y-D, Zhao S, Hill CW. 1998. Rhs elements comprise three subfamilies which diverged prior to acquisition by Escherichia coli. J. Bacteriol. 180:4102–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Welch TJ, et al. 2007. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One 2:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]