Abstract
Ethionamide (ETH) is a second-line drug for the treatment of tuberculosis. As a prodrug, ETH has to be activated by EthA. ethA is controlled by its repressor EthR. 2-Phenylethyl-butyrate (2-PEB) inhibits EthR binding, enhances expression of EthA, and thereby enhances the growth-inhibitory effects of ethionamide, isoxyl, and thiacetazone in Mycobacterium tuberculosis strains with resistance to ETH due to inhA promoter mutations but not ethA mutations.
TEXT
With more than 9.3 million new cases and more than 1.8 million deaths every year (15), tuberculosis (TB) still remains a global health problem. The rise of drug-resistant strains of Mycobacterium tuberculosis emphasizes the need for new treatment protocols. Ethionamide (ETH) is a second-line prodrug, which needs to be activated by the mycobacterial Baeyer-Villiger monooxygenase EthA to exert its antimicrobial activity by inhibiting the enoyl-acyl carrier protein (ACP) reductase InhA. ETH does not bind directly to InhA but forms a covalent adduct with NAD. This ETH-NAD adduct inhibits InhA (13). InhA is part of a fatty acid synthase type II system (FASII) which synthesizes mycolic acids, essential components of the unique mycobacterial cell wall (12, 17).
The expression of ethA is under the control of its natural repressor EthR (2), which contributes to the limited natural drug susceptibility of M. tuberculosis. As a result, ETH-based tuberculosis therapy may be unsuccessful, even when prescribed at high hepatotoxic doses (7). Acquired ETH resistance often is found associated with two types of genetic alterations: (i) mutations in the promoter region of the mabA-inhA operon (e.g., T−8C [mutation at position −8 to the start codon], T−8A, C−15T), resulting in InhA overexpression (5, 9, 10), and (ii) mutations in ethA, leading to a nonfunctional monooxygenase EthA (6, 9). Like ETH, thioamide drugs such as thiacetazone (TAC) and isoxyl (ISO) are activated by EthA (6), but these have different targets.
It has recently been shown that chemical compounds like benzylacetate, 2-phenylethyl-butyrate (2-PEB), and BDM31343 (16) inhibit EthR binding and thus enhance the expression of EthA. In turn, increased expression of EthA enhances the antibacterial effect of ETH on M. tuberculosis H37Rv (14). Here we show that 2-PEB enhances the growth-inhibitory effect of the three EthA-activated antibiotics ETH, ISO, and TAC on M. tuberculosis H37Rv and on drug-susceptible and drug-resistant clinical isolates of M. tuberculosis.
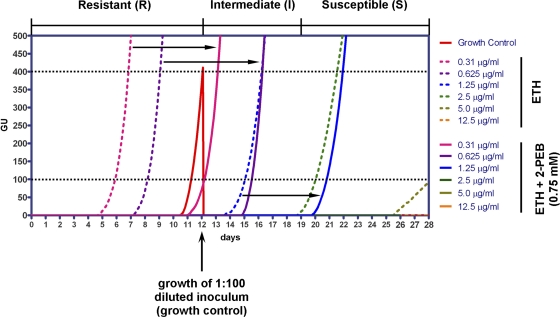
Initially, the laboratory strain H37Rv and 5 pansusceptible clinical isolates of M. tuberculosis were challenged with different concentrations of ETH (ranging from 12.5 μg/ml to 0.16 μg/ml) with and without the addition of 0.75 mM 2-PEB. Drug susceptibility was assessed using the MGIT 960 instrumentation (Becton Dickinson) and the TBeXIST software as described recently (11). Briefly, 0.8 ml of MGIT 960 SIRE supplement (Becton Dickinson) and 0.2 ml of the drug solution were added to the MGIT tubes. The tubes were inoculated with 0.5 ml of test strain suspension. As a control, a drug-free MGIT tube was inoculated with 0.5 ml of a 1:100-diluted (sterile H2O) suspension of the test strain. Growth of the bacteria was monitored by EpiCenter software (version 5.6.6), equipped with the TBeXiST module (Becton Dickinson), and was expressed as growth units (GU). A strain was considered to be resistant (R) to a drug when the test tube reached ≥100 GU earlier than the drug-free control tube reached a GU value of 400. Susceptibility (S) of a strain was defined when the control tube reached 400 GU and the test tube remained ≤100 GU for more than 7 days after the control tube had reached 400 GU. A strain was considered to be intermediate (I) when the test tube reached ≥100 GU within 7 days after the control tube reached 400 GU. Figure 1 depicts the growth curves for strain H37Rv as a representative. The addition of 2-PEB enhanced the growth-inhibitory activity of ETH, i.e., the strains changed their resistance profile by shifting from resistant to intermediate or from intermediate to susceptible at a given ETH concentration upon addition of 2-PEB. As summarized in Table 1, 2-PEB enhanced the growth-inhibitory effect of ETH in drug-susceptible clinical isolates (5/5) and in M. tuberculosis H37Rv.
Fig 1.
Growth of M. tuberculosis H37Rv at different concentrations of ETH in the presence or absence of 2-PEB. Inoculation of a 1:100-diluted H37Rv suspension serves as a growth control (growth curve shown in red). Dotted lines at GU values of 100 and 400 indicate the thresholds for defining growth of strains under test conditions and growth of the control, respectively. See text for definition of resistant (R), intermediate (I), and susceptible (S). Black arrows indicate the shift of the growth curves with the addition of 2-PEB.
Table 1.
Potentiation of the growth-inhibitory effect of ETH, ISO, and TAC on clinical isolates of M. tuberculosis
| Strain | ETH |
ISO |
TAC |
Characteristic(s)c of genetic locus: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concn (μg/ml) | Resistance profile: |
Potentiation | Concn (μg/ml) | Resistance profile: |
Potentiation | Concn (μg/ml) | Resistance profile: |
Potentiation | |||||||
| − 2-PEB | + 2-PEB | − 2-PEB | + 2-PEB | − 2-PEB | + 2-PEB | katG | inhA | ethA | |||||||
| Drug-susceptible strainsa | |||||||||||||||
| H37Rv | 0.16 | R | R | 0.1 | R | R | 0.03 | R | R | wt | wt | wt | |||
| 0.31 | R | R | 0.3 | R | R | 0.08 | I | I | |||||||
| 0.625 | R | I | 0.9 | I | S | Yes | 0.22 | I | I | Yes | |||||
| 1.25 | I | S | Yes | 2.7 | S | S | 0.66 | I | S | ||||||
| 2.5 | S | S | |||||||||||||
| 5 | S | S | |||||||||||||
| 12.5 | S | S | |||||||||||||
| 176914 | 0.16 | R | R | 0.1 | R | I | 0.03 | R | I | wt | wt | wt | |||
| 0.31 | R | R | 0.3 | I | I | 0.08 | I | S | |||||||
| 0.625 | R | I | 0.9 | S | S | Yes | 0.22 | S | S | Yes | |||||
| 1.25 | R | I | Yes | 2.7 | S | S | 0.66 | S | S | ||||||
| 2.5 | R | I | |||||||||||||
| 5 | I | S | |||||||||||||
| 12.5 | I | S | |||||||||||||
| 176861 | 0.16 | R | R | wt | wt | wt | |||||||||
| 0.31 | R | R | |||||||||||||
| 0.625 | R | I | |||||||||||||
| 1.25 | R | I | Yes | NDd | ND | ||||||||||
| 2.5 | I | I | |||||||||||||
| 5 | I | I | |||||||||||||
| 12.5 | I | I | |||||||||||||
| 176747 | 0.16 | R | R | 0.1 | R | R | 0.03 | R | R | wt | wt | wt | |||
| 0.31 | R | R | 0.3 | R | R | 0.08 | R | R | |||||||
| 0.625 | R | R | 0.9 | I | S | Yes | 0.22 | R | R | Yes | |||||
| 1.25 | R | I | Yes | 2.7 | S | S | 0.66 | R | I | ||||||
| 2.5 | R | I | |||||||||||||
| 5 | I | S | |||||||||||||
| 12.5 | S | S | |||||||||||||
| 176587 | 0.16 | R | R | wt | wt | wt | |||||||||
| 0.31 | R | R | |||||||||||||
| 0.625 | R | I | |||||||||||||
| 1.25 | I | I | Yes | ND | ND | ||||||||||
| 2.5 | I | I | |||||||||||||
| 5 | I | I | |||||||||||||
| 12.5 | I | S | |||||||||||||
| 176389 | 0.16 | R | R | wt | wt | wt | |||||||||
| 0.31 | R | R | |||||||||||||
| 0.625 | R | I | |||||||||||||
| 1.25 | I | I | Yes | ND | ND | ||||||||||
| 2.5 | I | I | |||||||||||||
| 5 | I | I | |||||||||||||
| 12.5 | I | S | |||||||||||||
| Drug-resistant strainsb | |||||||||||||||
| 2694 | 1.25 | R | R | 0.1 | R | R | 0.03 | R | R | wt | C−15T | wt | |||
| 2.5 | R | R | 0.3 | R | I | 0.08 | R | R | |||||||
| 5 | R | S | Yes | 0.9 | S | S | Yes | 0.22 | I | S | Yes | ||||
| 12.5 | S | S | 2.7 | S | S | 0.66 | I | S | |||||||
| 117 | 1.25 | R | R | 0.1 | R | R | 0.03 | R | R | wt | C−15T | wt | |||
| 2.5 | R | R | 0.3 | R | R | 0.08 | R | R | |||||||
| 5 | R | I | Yes | 0.9 | I | S | Yes | 0.22 | R | S | Yes | ||||
| 12.5 | S | S | 2.7 | S | S | 0.66 | R | S | |||||||
| 4269 | 1.25 | R | R | 0.1 | R | R | 0.03 | R | R | wt | C−15T | N345K | |||
| 2.5 | R | R | 0.3 | R | R | 0.08 | R | R | |||||||
| 5 | R | R | No | 0.9 | R | R | No | 0.22 | R | R | No | ||||
| 12.5 | R | R | 2.7 | I | I | 0.66 | R | R | |||||||
| 130 | 1.25 | R | R | S315T1 | wt | bp 1054 del, stop at pos. 372 | |||||||||
| 2.5 | R | R | |||||||||||||
| 5 | R | R | No | ND | ND | ||||||||||
| 12.5 | R | R | |||||||||||||
| 186137 | 1.25 | R | R | S315T1 | wt | Frameshift at pos. 7 | |||||||||
| 2.5 | R | R | |||||||||||||
| 5 | R | R | No | ND | ND | ||||||||||
| 12.5 | R | R | |||||||||||||
| 186038 | 1.25 | R | R | S315T1 | wt | W256 stop | |||||||||
| 2.5 | R | R | |||||||||||||
| 5 | R | R | No | ND | ND | ||||||||||
| 12.5 | R | R | |||||||||||||
| 177836 | 1.25 | R | R | S315T1 | wt | S266R, bp 1054 del, stop at pos. 372 | |||||||||
| 2.5 | R | R | |||||||||||||
| 5 | R | R | No | ND | ND | ||||||||||
| 12.5 | R | R | |||||||||||||
Strains 176914, 176861, 176747, 176587, and 176389 are susceptible to all first-line drugs.
Strains 2694, 117, and 4269 are resistant to INH (0.1 μg/ml) due to mutations in the promoter region of the mabA-inhA operon (C−15T). Strains 130, 186137, 186038, and 177836 are multidrug resistant (INH, RIF), with INH resistance due to mutations in the katG gene.
wt, wild type; del, deletion; stop, stop codon; pos., position.
ND, not done.
We next studied 7 strains with resistance to ETH and isoniazid (INH). These strains can be divided into two groups, those with a wild-type inhA-mabA promoter (4/7) and those with a C−15T mutation (3/7). The strains were challenged with different concentrations of ETH, ranging from 12.5 μg/ml to 1.25 μg/ml, with and without addition of 0.75 mM 2-PEB. When 2-PEB was added, an increased growth-inhibitory effect of ETH was found in 2/3 of the drug-resistant strains with a C−15T mutation (2694 and 117) (Table 1).
ISO and TAC share the same activator as ETH (6). Therefore, we investigated a putative potentiation of these two antibiotics by coincubation with 2-PEB. A subset of the above-described tested strains (176914, 176747, 2694, 4269, 117, and H37Rv) were chosen for study. Five of six strains (176914, 176747, 2694, 117, and H37Rv) showed increased susceptibility to ISO when 2-PEB was added (Table 1), including the two strains (2694 and 117) with resistance to INH associated with C−15T inhA-mabA promoter mutations. In the same 5 strains, a potentiation of TAC in combination with 2-PEB was observed (Table 1).
To address the molecular mechanism of ETH resistance, particularly in those strains without an inhA-mabA promoter mutation, we amplified and sequenced the ethR ethA gene region. Primers 5′-GATGCAGAGGCGGTGTTC-3′ and 5′-GTGTTCGGCGTCCACCCA-3′ were used to amplify a 3.2-kbp fragment comprised of ethA (Rv3845c) and its upstream and downstream sequences. Amplified gene fragments were sequenced using a BigDye Terminator cycle sequencing ready reaction kit (Applied Biosystems, Inc.) and an ABI 3130 DNA genetic analyzer (Applied Biosystems, Inc.). All INH-susceptible strains showed a wild-type ethR ethA sequence (Table 1). Of the strains exhibiting ETH resistance and INH low-level resistance, i.e., those with a C−15T inhA-mabA promoter mutation, one strain (4269) in addition exhibited an altered ethR ethA sequence, due to two nucleotide exchanges. One of these mutations is silent (TCG1326TCT, S442S), and the second leads to an Asn345Lys conversion. The ETH-resistant strains which exhibit high-level resistance to INH because of mutation S315T1 in KatG all carry mutations in ethA. Strains 186137, 130, and 186036 do not express fully mature EthA, either because of a frameshift mutation at position 7 (186137) or position 307 (130) or because of a conversion of a Trp256 stop codon (186038). Strain 177836 has a point mutation which leads to a Ser266Arg conversion in addition to a single base pair deletion at position 1054, which leads to a stop codon at amino acid position 372.
The expression of EthA is under the control of its natural repressor EthR (2), and thus the effect of ETH, ISO, and TAC can be enhanced by compounds that prevent EthR from binding to the ethA promoter, e.g., 2-PEB (14) or BDM31381 (16). Upon coadministration, 2-PEB enhanced the potency of ETH toward all (6/6) drug-susceptible strains and 2/3 INH-resistant strains with a C−15T mutation. Analysis of the ethA ethR region provides a rationale as to why 2-PEB does not enhance the growth-inhibitory effect of ETH in strain 4269. This strain encodes a mutated EthA, which is supposed to be enzymatically inactive in converting the prodrugs into their active forms. Of note, 2-PEB does not enhance the growth-inhibitory effect of ETH on multidrug-resistant (MDR) strains with mutations in katG but wild type in the inhA promoter (130, 186137, 186038, and 177836), since these MDR strains were found to carry mutations in ethA as well.
We subsequently characterized the ethA ethR genotype of additional ETH-resistant clinical isolates collected at the National Center for Mycobacteria. In addition to critical concentration testing of INH, RIF, and ETH, we characterized resistance-associated loci by molecular techniques. In total, for 6/25 strains, ETH resistance was associated with mutations in the inhA promoter region, while ethA was wild type. All other strains (19/25) were resistant to ETH because of mutations in ethA either alone (13/19) or in combination with mutations in the inhA promoter region (6/19). A large number of the ethA mutations resulted in a truncated EthA protein or affected EthA expression by altering the ribosomal binding site or the initiation codon (Table 2; also see Table S1 in the supplemental material). We hypothesize that the presence of the different resistance mutations in clinical strains reflects the history of drug treatment. The simultaneous presence of mutations in inhA and ethA most likely indicates that inhA promoter mutations alone may not suffice to confer clinical ETH resistance. According to this hypothesis, the presence of inhA promoter mutations reflects previous treatment with INH, while a mutation in ethA indicates resistance development following treatment with ETH.
Table 2.
Genotypes of ethionamide-resistant clinical isolatesa
| Genotype | No. of ethionamide-resistant clinical strains (n = 25) |
||||
|---|---|---|---|---|---|
| INH-resistant strains (n = 4) |
Multidrug-resistant strains (n = 21) |
||||
| inhA promoter mutation (n = 3) | katG mutation (n = 1) | katG mutation (n =12) | inhA promoter mutation (n = 5) | inhA promoter + katG mutation (n = 4) | |
| ethA mutb | 1 | 12 | 3 | 3 | |
| ethA wt | 3 | 2 | 1 | ||
For detailed genotypic characterization, see Table S1 in the supplemental material.
mut, mutated.
The preclinical development of chemical analogues of ISO and TAC with a higher antitubercular activity has great potential (1, 3, 4, 8). Future studies will have to investigate whether those compounds are boosted by 2-PEB, as this would possibly promote them as valuable alternatives for existing second-line drugs. A further possibility to improve the efficacy of 2-PEB (Joint Food and Agriculture Organization [FAO]/WHO Expert Committee on Food Additives, JEFCA no. 991) is through application of rational drug design and testing in synthetic mammalian gene circuits (14).
In conclusion, our data show that the growth-inhibitory effect of the three thioamide drugs ethionamide, isoxyl, and thiacetazone can be enhanced by adding 2-PEB. The addition of 2-PEB increased the growth-inhibitory effect of these drugs against laboratory strain M. tuberculosis H37Rv and drug-susceptible and drug-resistant clinical isolates of M. tuberculosis. However, the ability of 2-PEB to enhance the potency of these drugs apparently is limited to strains with a wild-type ethA locus.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Swiss National Science Foundation (31003A_135705).
We thank Claudia Ritter for preparing genomic DNA and support with the MGIT 960 system.
Footnotes
Published ahead of print 21 November 2011
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Alahari A, et al. 2007. Thiacetazone, an antitubercular drug that inhibits cyclopropanation of cell wall mycolic acids in mycobacteria. PLoS One 2: e1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baulard AR, et al. 2000. Activation of the pro-drug ethionamide is regulated in mycobacteria. J. Biol. Chem. 275: 28326–28331 [DOI] [PubMed] [Google Scholar]
- 3. Bermudez LE, et al. 2003. Thiosemicarbazole (thiacetazone-like) compound with activity against Mycobacterium avium in mice. Antimicrob. Agents Chemother. 47: 2685–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhowruth V, et al. 2006. Symmetrical and unsymmetrical analogues of isoxyl; active agents against Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 16: 4743–4747 [DOI] [PubMed] [Google Scholar]
- 5. Brossier F, Veziris N, Truffot-Pernot C, Jarlier V, Sougakoff W. 2011. Molecular investigation of resistance to the antituberculous drug ethionamide in multidrug-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 55: 355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeBarber AE, Mdluli K, Bosman M, Bekker LG, Barry CE., III 2000. Ethionamide activation and sensitivity in multidrug-resistant Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 97: 9677–9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hollinrake K. 1968. Acute hepatic necrosis associated with ethionamide. Br. J. Dis. Chest 62: 151–154 [DOI] [PubMed] [Google Scholar]
- 8. Liav A, Angala SK, Brennan PJ, Jackson M. 2008. N-D-aldopentofuranosyl-N′-[p-(isoamyloxy)phenyl]-thiourea derivatives: potential anti-TB therapeutic agents. Bioorg. Med. Chem. Lett. 18: 2649–2651 [DOI] [PubMed] [Google Scholar]
- 9. Morlock GP, Metchock B, Sikes D, Crawford JT, Cooksey RC. 2003. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 47: 3799–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muller B, et al. inhA promoter mutations: a gateway to extensively drug-resistant tuberculosis in South Africa? Int. J. Tuberc. Lung Dis. 15: 344–351 [PubMed] [Google Scholar]
- 11. Springer B, Lucke K, Calligaris-Maibach R, Ritter C, Bottger EC. 2009. Quantitative drug susceptibility testing of Mycobacterium tuberculosis by use of MGIT 960 and EpiCenter instrumentation. J. Clin. Microbiol. 47: 1773–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vilcheze C, Jacobs WR., Jr 2007. The mechanism of isoniazid killing: clarity through the scope of genetics. Annu. Rev. Microbiol. 61: 35–50 [DOI] [PubMed] [Google Scholar]
- 13. Wang F, et al. 2007. Mechanism of thioamide drug action against tuberculosis and leprosy. J. Exp. Med. 204: 73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weber W, et al. 2008. A synthetic mammalian gene circuit reveals antituberculosis compounds. Proc. Natl. Acad. Sci. U. S. A. 105: 9994–9998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. WHO 2009. WHO TB factsheet. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/2009/tbfactsheet_2009_one_page.pdf [Google Scholar]
- 16. Willand N, et al. 2009. Synthetic EthR inhibitors boost antituberculous activity of ethionamide. Nat. Med. 15: 537–544 [DOI] [PubMed] [Google Scholar]
- 17. Winder FG, Collins PB. 1970. Inhibition by isoniazid of synthesis of mycolic acids in Mycobacterium tuberculosis. J. Gen. Microbiol. 63: 41–48 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.