Abstract
The 19-kDa carboxyl-terminal fragment of the merozoite surface protein-1 (MSP-119) has been shown to regulate antibody (Ab)-mediated protective immunity to blood-stage malaria infection. But the serological memory to this antigen tends to be short-lived, and little is known of the mechanisms that regulate the formation of B cell memory to MSP-119 antigen. We studied the formation of B cell memory response after immunization with the recombinant 19-kDa Plasmodium falciparum merozoite surface protein 1 (PfMSP-119). Immunization with PfMSP-119 resulted in delayed increase in germinal center (GC) B cell numbers. This poor GC reaction correlated with short-lived PfMSP-119-specific antibodies in serum and the short life of PfMSP-119-specific plasma cells and memory B cells (MBCs) in spleen and bone marrow. PfMSP-119-specific MBCs were capable of producing antigen (Ag)-specific Ab-secreting cell (ASC) responses that were short-lived following challenge immunization of the immune mice with antigen or transgenic Plasmodium berghei parasite expressing PfMSP-119 in place of native P. berghei MSP-119 at 8 weeks after the last immunization or following adoptive transfer into naive hosts. However, no protection was achieved in PfMSP-119 immune mice or recipient mice with PfMSP-119-specific MBCs following challenge with transgenic P. berghei. Our findings suggest that PfMSP-119-specific IgG production by short-lived plasma cells combined with the poor ability of the PfMSP-119-induced MBCs to maintain the anamnestic IgG responses failed to contribute to protection against infection.
INTRODUCTION
Despite major efforts at control, Plasmodium falciparum infections continue to be a major health burden for millions of people in tropical countries (13, 48). In areas of endemicity, immunity to malaria is gradually acquired by repeated exposures. But this immunity is rapidly lost after exposure to the parasite ceases, indicating that the immunity is short-lived and needs constant boosting for maintenance (reviewed in reference 26). Though a number of studies support the hypothesis that antibody (Ab)-producing B cells are important mediators of protective immunity (15), antibody levels have been reported to decline rapidly, indicating poor memory B cells (MBCs) and long-lived plasma cell (LLPC) development (1, 24, 25). However, some studies have shown development and maintenance of memory B cells after infection (10, 32, 44, 45). Therefore, the role and maintenance of B cell responses to blood-stage malaria antigens in the situation of infection, and vaccination has remained controversial. A better understanding of B cell responses during P. falciparum infections is essential toward designing a preventive malaria vaccine.
P. falciparum merozoite surface protein 1 (PfMSP-1) is the most abundant protein on the surface of invasive blood-stage merozoites, and its 19-kDa C-terminal, cysteine-rich region of PfMSP-1 (PfMSP-119) is highly conserved and effective in induction of a protective immune response against malaria parasite infection (9, 23, 24). The protection has been correlated with high levels of induction of growth-inhibitory antibodies and not with effector CD4+ T cells (20, 21). But there is a poor understanding of the B cell responses to PfMSP-119 that protect against blood-stage infection in vivo in humans.
In this study we investigated the development of memory B cells and their differentiation into antibody-secreting cells (ASCs) and the protective capacity upon PfMSP-119 administration. Our findings indicate that memory responses to PfMSP-119 both in the bone marrow (BM) and spleen were impaired, and adoptive transfer of MBCs resulted in the formation of short-lived antibody-secreting plasma cells and a short-lived antibody response. Furthermore, these newly formed ASCs and antibodies failed to protect recipient mice against challenge infection. Taken together, our data demonstrated that immunization with PfMSP-119 generated short-lived Ab titers, which corresponded with the short life span of plasma cells, produced memory B cells that were unable to mount a robust anamnestic Ab response upon reimmunization or parasite challenge, and failed to contribute to protection against infection.
MATERIALS AND METHODS
Expression and purification of P. falciparum MSP-119 recombinant protein.
A synthetic gene encoding P. falciparum MSP-119 (PfMSP-119) was designed to modify the native gene sequence for optimal expression in Escherichia coli. The amino acid sequence of PfMSP-119 corresponding to residues 1526 to 1619 of the P. falciparum Welcome strain (GenBank accession number P04933) was back-translated to nucleotide sequence based on an E. coli codon frequency table (available at http://www.kazusa.or.jp/codon). Except for methionine and glycine that came from cloning the restriction enzyme site at the N terminus and a hexahistidine tag following these two amino aids, no additional amino acid was introduced in the coded recombinant PfMSP-119 (rPfMSP-119). After modification, the AT content of the synthetic gene sequence was 51% versus 64% for the native gene. The designed synthetic gene was synthesized commercially and cloned in a pET28(+) plasmid expression vector for expression in E. coli. The cloned synthetic gene was sequenced from both ends and was found to be free from any error. Expression of the recombinant PfMSP-119 was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Sigma), and the recombinant protein present in the soluble fraction was purified by metal affinity chromatography using nickel-nitrilotriacetic acid (NTA) matrix (Qiagen) as described in a previous study (28). Purified recombinant PfMSP-119 was analyzed for purity, homogeneity, and conformation by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) under reducing and nonreducing conditions, immunoblot analysis, and reverse-phase (RP) chromatography. The conformational integrity of the PfMSP-119 was assessed by its reactivity with disulfide-dependent, conformation-specific PfMSP-119 monoclonal antibodies (MAbs 5.2 and 12.10). Based on relative migration on SDS-polyacrylamide gels and the presence of a single, dominant reactive band on immunoblots, the recombinant PfMSP-119 antigen appeared to be properly folded (see reference 29). Mass spectrum analysis and N-terminal sequencing further confirmed the molecular identity of the purified PfMSP-119. The final preparations of PfMSP-119, contained less than 0.25 endotoxin units (EU) per 25 μg of protein.
Mice and Plasmodium parasites.
Six- to 8-week-old BALB/c female mice were obtained from the Jackson Laboratory and were housed under specific-pathogen-free conditions in the animal facilities at the International Centre of Genetic Engineering and Biotechnology (ICGEB). All experiments were performed in compliance with the ICGEB Institutional Animal Care and Use Committee regulations. A transgenic Plasmodium berghei parasite, PbPfM19 that expressed P. falciparum MSP-119 in place of native P. berghei MSP-119 (PbMSP-119) (a kind gift from B. S. Crabb (The Walter and Eliza Hall Institute of Medical Research, Parkville, Australia) was used to infect mice (11).
Immunization, adoptive transfer, and challenge.
To activate B cells in vivo, groups of 15 to 20 mice were immunized subcutaneously with 20 μg of rPfMSP-119 or phosphate-buffered saline (PBS) emulsified in complete Freund's adjuvant (CFA). On days 30 and 90, mice were boosted with the same amount of antigen or PBS emulsified in incomplete Freund's adjuvant (IFA).
After the last immunization, mice were rested for 8 weeks and were challenged with 1 × 104 live PbPfM19-parasitized red blood cells (PbPfM19-pRBCs). Parasitemia in infected mice was assessed from day 5 postchallenge by microscopic examination of Giemsa-stained blood films. Percent parasitemia was determined by counting the number of infected red blood cells per 1,000 erythrocytes.
For adoptive transfer experiments, we adopted the protocol of Ochesenbein et al. for our studies (33). RBC-cleared splenocytes and bone marrow cells from PfMSP-119 immune and age-matched adjuvant-treated control mice were depleted of CD138+ antibody-secreting cells (ASCs) by magnetic cell sorting (Miltenyi Biotec, Auburn, CA), according to the manufacturer's instructions. Purity of sorted cells was routinely >95%. For adoptive transfers, 3 × 107 pooled CD138− cells were injected intravenously (i.v.) into nonirradiated recipients. One day after transfer, recipients were challenged with either 20 μg of PfMSP-119 delivered in IFA or PBS or with 1 × 104 live PbPfM19-parasitized red blood cells. Parasitemia in infected mice was assessed from day 6 postchallenge by microscopic examination of Giemsa-stained blood films. Percent parasitemia was determined by counting the number of infected red blood cells per 1,000 erythrocytes.
Restimulation of B cell.
B cells isolated 4 weeks after the last immunization were stimulated using established protocols (19). Nondepleted and CD138+-depleted RBC-cleared splenocytes and bone marrow cells were cultured at 3 × 106 cells/ml in RPMI 1640 medium (Life Technologies) supplemented with 10% fetal bovine serum (Gibco), 2 mM l-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin (all from Life Technologies), and 50 μM β-mercaptoethanol (Gibco) at 37°C for 5 days in the presence or absence of 25 μg/ml of PfMSP-119. After 5 days of culture, ASCs were detected by enzyme-linked immunospot (ELISPOT) assays as described previously.
Antibody ELISA.
PfMSP-119-specific total IgG and IgG isotypes were measured by standard sandwich enzyme-linked immunosorbent assay (ELISA). ELISA plates were coated with 5 μg/ml recombinant PfMSP-119 overnight at 4°C. The plates were blocked with PBS containing 1% nonfat dry milk and incubated in serially diluted sera, and bound Ig was detected with biotinylated secondary Abs to anti-IgG, anti-IgG1, anti-IgG2a, anti-IgG2b, and anti-IgG3 followed by streptavidin peroxidase; plates were developed using H2O2 and O-phenylene diamine substrate (Sigma-Aldrich, St. Louis, MO). The absorbance was read at 490 nm. Endpoint titers were expressed as the reciprocal of the highest serum dilution for which the optical density (OD) was equal or greater than the OD (mean plus 2 standard deviations [SDs]) of nonimmune control serum.
ELISPOT assays.
PfMSP-119-specific bone marrow or spleen ASCs were determined by direct ex vivo ELISPOT assay or after 5 days of antigen stimulation. Ninety-six-well nitrocellulose plates (Multiscreen-HA; Millipore, Bedford, MA) were coated overnight at 4°C with recombinant PfMSP-119 at 10 μg/ml, followed by a 16-h culture with splenocytes, BM cells, or memory cell pool at 1 × 106 cells/ml in RPMI medium containing 50 μM 2-mercaptoethanol (ME) and 10% fetal calf serum (FCS). Plates were washed, and bound PfMSP-119-specific IgG-producing cells were revealed with goat anti-mouse IgG conjugated to horseradish peroxidase (Sigma), visualized by the addition of 3-aminoethyl carbazole. Spots were counted using a Bioreader 2000 ELISPOT plate reader system (Biosys).
Flow cytometry.
Spleen and bone marrow cells were stained with B220-peridinin chlorophyll protein (PerCP), IgG1-phycoerythrin (PE), CD138-PE, IgD-fluorescein isothiocyanate (FITC), IgM-FITC, IgG1-FITC, GL7-FITC, CD27-FITC (BD Biosciences), or peanut agglutinin (PNA)-FITC (Vector Laboratories), washed, and subsequently fixed and acquired on a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (Tree Star). Profiles are presented as 5% probability contours with outliers.
Statistical analysis.
Comparisons between experimental groups were done with the two-tailed Student t test for unpaired samples using the statistical analysis program of GraphPad Prism. For all tests, a value of P < 0.05 was considered significant.
RESULTS
Recombinant PfMSP-119 induces a short-lived humoral response.
The recombinant PfMSP-119 protein was produced in Escherichia coli and expressed as a soluble protein. PfMSP-119 was purified to homogeneity from soluble fractions by a combination of metal affinity and ion exchange chromatography and showed apparent mobility of ∼19 kDa on SDS-PAGE gels (see reference 29). Reverse-phase high-performance liquid chromatography (RP-HPLC) and gel permeation chromatography analyses suggested that the purified PfMSP-119 protein was >98.0% pure and was in a monomeric form. Analyses of conformational properties using PfMSP-119-specific monoclonal antibody showed that the conformational epitopes of PfMSP-119, which may be critical for generation of the antiparasitic immune response, remained intact in the recombinant protein. The final preparations of PfMSP-119 contained fewer than 0.25 endotoxin units (EU) per 25 μg of protein. Host cell proteins were not observed in any of the protein samples, as determined by an ELISA and Western blot analysis (29; also data not shown).
First, we evaluated kinetics of antibody production after PfMSP-119 immunization by ELISA. Following primary immunization, low or negligible anti-PfMSP-119 antibody titers were detected in the serum (Fig. 1A). However, after a second immunization at day 30, the level of anti-PfMSP-119 IgG response increased by 300-fold (P < 0.001) and peaked at day 56, and the antibody levels then declined to ∼90% of this peak by day 85 (Fig. 1A). A third immunization at day 90 further increased the PfMSP-119-specific IgG response by 900-fold compared to that seen after primary immunization (P < 0.0005) and peaked 10 days later (at day 100), but thereafter the antibody levels declined and were undetectable day 200. The relative titers of the four PfMSP-119 subclasses after the second or third immunization were as follows: IgG1 > IgG2a > IgG2b > IgG3 (Fig. 1B).
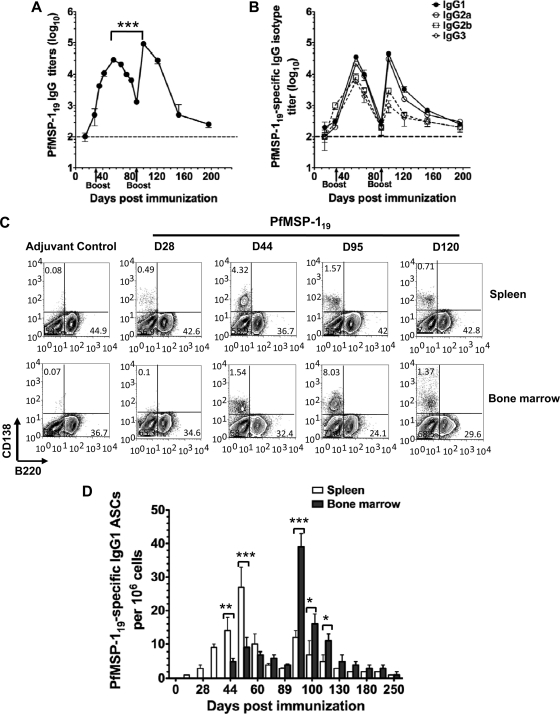
Fig 1.
PfMSP-119-specific B cell responses in mice is short-lived. Mice (n = 10) were immunized subcutaneously and boosted with 20 μg of PfMSP-119 emulsified in CFA/IFA at the base of the tail on days 0, 30, and 90. Age- and sex-matched control mice were immunized with adjuvant alone. (A and B) Anti-PfMSP-119 total IgG and IgG isotype Ab titers in mice were determined by ELISA. Results are shown as mean ± SD (n > 6). (C) Splenocytes and bone marrow cells were recovered from PfMSP-119-primed or adjuvant-treated control mice at different days postimmunization and were stained for B220 versus CD138 for fluorescence-activated cell sorter analysis. Contour plots represent live cells gated according to forward and side scatter profiles. Gates were set to show the percentages of plasma cells (B220− CD138+). (D) Cell suspensions of spleen and bone marrow were prepared from individual mice, and an ELISPOT assay was used to enumerate the number of PfMSP-119-specific IgG-producing ASCs. The values are geometric means ± SD values of three to four mice per time point. Experiments are representative of three repeats. **, P < 0.001; ***, P < 0.005 (statistically significant difference in ex vivo ASC response between spleen and bone marrow).
To further examine the short-lived nature of PfMSP-119-specific antibody response, we analyzed the frequencies of ASCs within the spleen and bone marrow by flow cytometry and ELISPOT assays (Fig. 1C and D). Splenocytes and bone marrow cells were stained with CD138 (syndecan-1), a marker for ASCs (38). Following the first immunization with PfMSP-119, the frequencies of CD138+ plasma cells (PCs) in the spleen and bone marrow expressing low levels of B220 (B220low) were comparable to those of adjuvant-treated control mice. The peak B220low CD138+ plasma cell population was seen in the spleen 14 days after a second immunization (at day 44), whereas it was seen in the bone marrow 5 days after the third immunization (at day 95) (Fig. 1C). At day 120, albeit lower, the bone marrow, but not the spleen, retained a small percentage of PCs (Fig. 1C). These results suggest that PfMSP-119 induced short-lived plasma cells.
Next, using ELISPOT assays, we determined the kinetics of PfMSP-119-specific ASCs. Consistent with our ELISA data shown in Fig. 1A, following primary immunization, there were no detectable PfMSP-119-specific IgG ASCs in the spleen and the bone marrow. Parallel to the increase in the overall frequency of total CD138+ plasma cells in spleen and bone marrow, an increase in the frequencies of PfMSP-119-specific, IgG-secreting ASCs was also observed after the second and third immunizations. A small number of PfMSP-119-specific IgG ASCs were initially detected in spleen at day 35, reached a maximum at day 56, and declined thereafter (Fig. 1D). In contrast, PfMSP-119-specific IgG and IgG1 ASCs were detected in the bone marrow after day 56 following the second immunization, but the peak response was seen at day 95 after the third immunization. Subsequent to this peak response, the number of PfMSP-119-specific IgG ASCs in bone marrow declined to almost background levels by day 150. Consistent with the flow cytometry data, our ELISPOT assay results indicate that initially PfMSP-119-specific IgG ASCs were detected in large numbers in the spleens (27 spots/106 cells in spleen versus 9 spots/106 cells in bone marrow), but during the late secondary response bone marrow became the major site of ASC homing (12 spots/106 cells in spleen versus 39 spots/106 in bone marrow; P < 0.02) (Fig. 1D). Consistent with the findings of PfMSP-119-specific antibody response, the ASC data suggest that the short-lived humoral response induced by PfMSP-119 immunization in mice was due to the failure to develop a sustainable population of long-lived plasma cells in the spleen or bone marrow.
B cells in response to PfMSP-119 immunization show delayed GC formation.
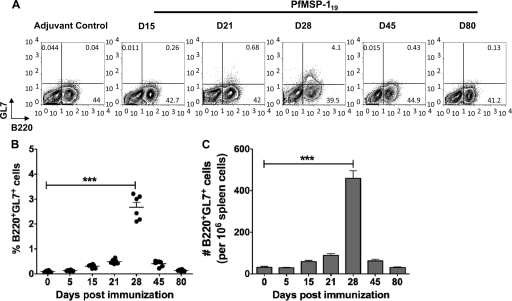
Next, we examined the presence of germinal center (GC)-derived B cells in the spleens of the PfMSP-119-immunized mice by staining for GL7 (39) on B220-positive (B220+) B cells. In response to T-dependent protein antigen immunization, GCs are usually apparent within 7 days of immunization and reach peak size before 2 weeks (27, 43). We observed that splenocytes showed low frequency and low absolute numbers of B220+ GL7+ GC B cells in the PfMSP-119-primed mice at day 7 after the primary immunization (Fig. 2). The response peaked only at day 28, indicating that a delayed GC reaction was initiated in the spleen of PfMSP-119-immunized mice after primary immunization (Fig. 2). Following the second and third immunizations with PfMSP-119, B220+ GL7+ GC B cell populations were no longer detectable in PfMSP-119-primed mice. These data suggest that the generation of GC B cells is delayed during primary immune response to PfMSP-119 and thereafter regresses rapidly after the second immunization and could possibly be the reasons for the delayed onset of the antibody response described above.
Fig 2.
A late germinal center B cell response is seen the PfMSP-119-primed mice. Splenocytes from PfMSP-119-primed or adjuvant-treated control mice were stained using germinal center markers, B220 and GL7. (A) Kinetics of B220+ GL7+ germinal center B cells in splenocytes is indicated. Numbers in the contour plots indicate the relative percentages of the B220+ GL7+ population. The cells were recovered from tissues of four mice per time point, and the results are representative of one experiment repeated three times. (B and C) Scatter plots of percentages and bar graph of total numbers of B220+ GL7+ cells in spleen of PfMSP-119-primed mice are depicted. The values are geometric means ± SD values of six mice per time point. Experiments are representative of three repeats. ***, P < 0.001.
PMSP-119 induces memory B cell responses.
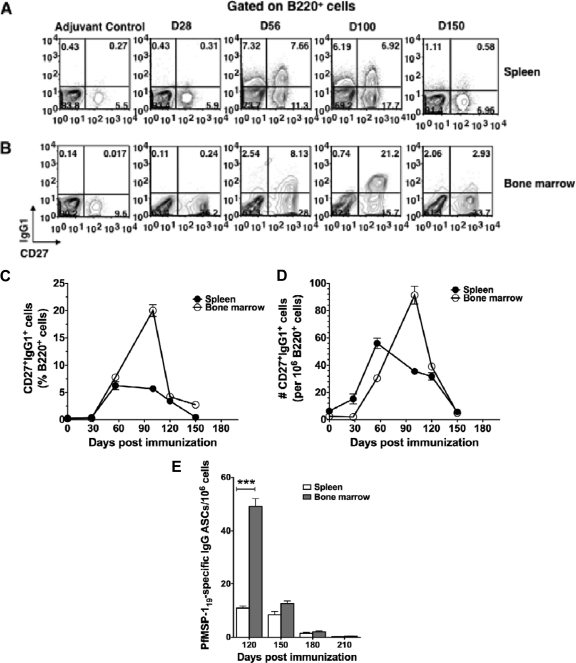
We then sought to examine whether PfMSP-119 immunization resulted in the formation of memory B cells. High expression of surface proteins IgG1 and CD27 typically defined in other bacterial and viral infections was used to gate on MBCs generated by PfMSP-119 immunization (cells expressing IgG1 and high levels of CD27 [IgG1+ CD27high]) (2, 4). Four weeks after the second immunization, PfMSP-119-primed mice showed 3- to 5-fold increased frequencies and total numbers of IgG1+ CD27high B cells in spleen (Fig. 3A, C, and D), whereas after the last immunization there was a 5- to 7-fold increase in the bone marrow (Fig. 3B, C, and D) compared with levels in adjuvant-treated control mice. However, irrespective of the tissue examined, over time we observed a reduction in both the frequencies and absolute numbers of B cells that expressed the IgG1+ CD27high phenotype.
Fig 3.
B cells with memory phenotype are generated in PfMSP-119-primed mice but these are short-lived. Splenocytes and bone marrow cells recovered from three mice per time point from PfMSP-119-primed or adjuvant-treated control mice at different days postimmunization were stained with B220, CD27, and IgG1. (A and B) The contour plot shows the expression of CD27 and IgG1 on gated B220+ cells. The average frequency (C) and total number (D) of CD27high IgG1+ cells in spleen and bone marrow cells were calculated, and results are depicted as line graphs. (E) Differentiation of memory B cells to Ag-specific ASCs in response to recall antigen in vitro. Spleen and bone marrow cells were isolated from PfMSP-119-primed mice at indicated days after the last immunization, depleted of CD138+ plasma cells, and cultured with PfMSP-119 for 5 days. The numbers of PfMSP-119-specific ASCs were determined by ELISPOT assay. The cells were recovered from tissues of four mice per time point, and the results are representative of three independent experiments. ***, P < 0.001 (statistically significant difference between spleen and bone marrow).
We then measured antigen-specific MBCs in spleen and bone marrow cells 4 weeks after the third immunizations. CD138− spleen and bone marrow cells (containing the memory B cell pool) were isolated at different times from PfMSP-119-primed mice and restimulated in vitro with recall antigen, and the differentiation of memory B cells into anti-PfMSP-119 ASCs was analyzed by ELISPOT assay. We found that the number of bone marrow-derived CD138− memory cells responded to restimulation and differentiated into a large number of PfMSP-119-specific IgG1 ASCs (49 ± 5.94 ASCs/106 cells) while relatively fewer CD138− memory cells isolated from spleen differentiated into IgG1 ASCs (10.8 ± 1.71 ASCs/106 cells) (Fig. 3E). Although the magnitude of the PfMSP-119-specific memory B cell response was highest in the bone marrow (4.5-fold; P < 0.001), it lasted for a short time, after which PfMSP-119-specific ASCs could no longer be detected (Fig. 3E). These results show that PfMSP-119 induced a much shorter B cell memory response in the spleen and bone marrow, suggesting perhaps that although the PfMSP-119 is capable of generating a memory response, it is unable to maintain the response long term.
PfMSP-119-induced memory B cells do not confer protection from blood-stage PbPfM19 infection.
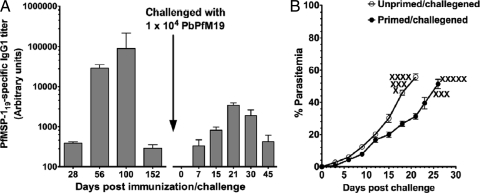
PfMSP-119-immunized were then challenged with PbPfM19 transgenic parasites to assess memory B cell function. First, we had assessed the ability of anti-MSP-119 antibodies from immunized mice to recognize the native PfMSP-119 in PbPfM19 transgenic parasites by immunofluorescence assay (IFA) before we proceeded with live-parasite challenge studies. Antibodies to PfMSP-119 had showed strong reactivity on the surface of merozoites of the PbPfM19 transgenic parasite with the characteristic circumferential staining (data not shown). We next tested whether PfMSP-119-immunized mice when directly challenged with PbPfM19 parasites were protected from lethal infection. To test this, a set of mice that were primed and boosted two times with PfMSP-119 in CFA/IFA were rested for 8 weeks after the last immunization and then challenged with 104 PbPfM19 transgenic parasites. Another set of unprimed adjuvant-treated control mice (age and sex matched) was also challenged with the same number of transgenic parasites. We then evaluated the contribution of infection in the maintenance of PfMSP-119-specific antibody titer and parasitemia during the course of infection. At the time of challenge, the immune mice possessed very low detectable PfMSP-119-specific antibody titers. A challenge infection with PbPfM19 led to a 10-fold increase in the PfMSP119-specific antibodies by day 21, indicating that there was a classical memory antibody response, but no such response was seen in the challenged adjuvant-treated control mice (Fig. 4A). As shown in Fig. 4B, PfMSP-119-immune mice were not protected and succumbed to the infection by day 28, with peak parasitemia of 51.4 ± 6.46, while the unprimed mice succumbed to the infection by day 21, with peak parasitemia of 55.7 ± 3.46. Although there was a week's difference in the survival of the mice in the two groups, there was no significant difference in the level of parasitemia between the two groups of mice (P > 0.10).
Fig 4.
PfMSP-119-induced memory B cells do not confer protection from blood-stage infection. Mice (n = 8) were immunized and boosted twice at days 30 and 90. Age- and sex-matched control mice were immunized with adjuvant alone. After the last immunization, mice were rested for 8 weeks and then challenged with 1 × 104 PbPfM19-parasitized RBCs. After parasite challenge, mice were bled at different days postchallenge for determining anti-PfMSP-119-specific IgG1 responses in serum (A) and parasitemia (B). The data are representative of two independent experiments. X, death of mice.
Adoptively transferred PfMSP-119-specific memory B cells are short-lived and do not offer protection against PbPfM19 parasites.
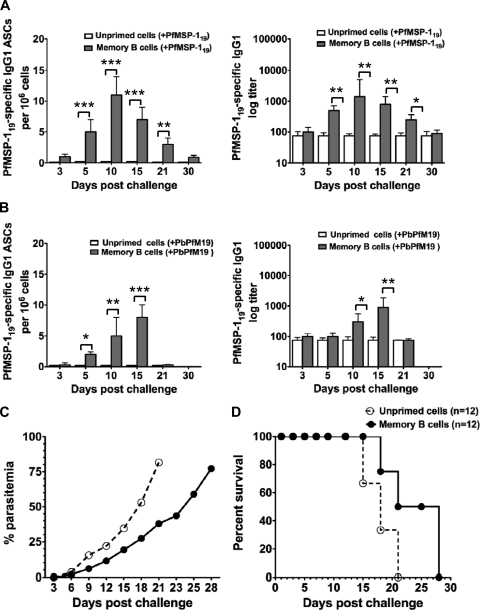
We next tried to confirm that PfMSP-119-induced B220+ CD27+ IgG1+ memory B cells were genuine memory cells. CD138+-depleted spleen or bone marrow cells isolated 8 weeks after last immunization from PfMSP-119-immune or adjuvant-treated control mice were adoptively transferred into nonirradiated naive recipient mice. A day later the recipients were either immunized with PfMSP-119 in IFA or infected with PbPfM19 parasites. Recipients with PfMSP-119-specific MBCs generated relatively low and transient memory PfMSP-119-specific IgG1 antibody and ASC responses, which peaked at day 10 and returned to baseline by approximately day 30 when challenged with recombinant PfMSP-119 (Fig. 5A). PfMSP-119-specific ASCs and antibody titers were negligible in mice that received unprimed donor cells from adjuvant-treated control mice, suggesting that PfMSP-119-specific MBCs were the source of responding B cells in the recipient mice. Collectively, these results indicate that the PfMSP-119-specific IgG response generated in recipient mice after antigenic challenge was likely the direct consequence of memory B cell activation, but the response was very short-lived.
Fig 5.
PfMSP-119-induced memory B cells offered no protection to infection in the adoptive transfer model. Eight weeks after the last immunization, splenocytes and bone marrow cells were isolated from PfMSP-119-primed (n = 6) and adjuvant-treated control (n = 6) mice were depleted of CD138+ cells and pooled. A total of 3 × 107 pooled cells were intravenously transferred into nonirradiated naive mice. For detecting response of transferred memory B cells, the recipient mice were bled at the indicated days after PfMSP-119 (Ag) challenge (n = 15) (A) and after PbPfM19 parasite challenge (n = 15) (B) to determine the anti-PfMSP-119-specific IgG1 response in serum and anti-PfMSP-119 IgG1 ASCs in spleen by ELISA and ELISPOT, respectively. (C and D) The course of parasitemia levels in blood and survival of mice to infection were monitored by examination of blood films stained with Giemsa reagent at the indicated times in the recipient mice with PfMSP-119-specific memory B donor cells or adjuvant-treated control unprimed donor cells (n = 12). *, P < 0.05; **, P < 0.02; ***, P < 0.001. (C) Data represent mean percentages of parasitemia ± standard errors of the mean of 12 mice per group. Values represent one of two independent experiments with similar findings. (D) Percent survival of recipient mice (n = 12) with PfMSP-119-specific memory B donor cells or adjuvant-treated control unprimed donor cells after intraperitoneal challenge with 104 pRBCs. The data are representative of two independent experiments.
In response to infection with PbPfM19, recipients with PfMSP-119-specific MBC donor cells showed a modest increase in PfMSP-119-specific IgG levels and ASCs between days 15 and 18 (Fig. 5B). We further compared the PfMSP-119-specific MBC response to either antigen or parasite challenge in recipient mice with MBC donor cells. We found that antibody titers and antibody-secreting cells were best maintained in the presence of antigen, whereas the initially increased antibody titers and ASC response disappeared rapidly by days 18 and 21 in mice challenged with PbPfM19 (Fig. 5A and B).
We finally investigated the protective efficacy of transferred memory B cells in the recipient mice infected with PbPfM19-pRBCs. The onset of patent parasitemia was delayed in mice that received PfMSP-119-specific MBCs compared with recipients of unprimed donor cells, but there was no statistically significant difference in the peak parasitemia between the two groups of infected mice (P > 0.05) (Fig. 5C). Though there was a delay in parasitemia, mice with MBC donor cells eventually showed relatively high parasitemia in blood and died between days 23 and 30 (Fig. 5C and D). Cumulatively, these data suggest that although memory B cells were generated by PfMSP-119 immunization, following transfer these cells failed to confer protection against natural infection.
DISCUSSION
Many successful vaccination regimes rely on the generation of protective antibodies that are produced by antibody-secreting B cells. With time, it is the long-lived plasma cells (LLPCs) that are responsible for the continuous maintenance of serum antibody levels, whereas memory B cells (MBCs) are responsible for driving secondary responses upon antigen reexposure (reviewed in references 14 and 30). Moreover, these memory B cells survive long term and replenish the pool of long-lived plasma cells to maintain antibody titers in the absence of antigen or pathogen. Although generation of protective antibodies is considered to be central to immunity against malaria, several studies indicate that humoral immunity to blood-stage malaria is short-lived; however, little is known about the B cell biology that underlies the inefficient acquisition of malaria-specific humoral immunity.
In this study we show that induction of a memory B cell response does not necessarily translate into a strong protective immunity in mice immunized with recombinant antigen PfMSP-119. As antibodies specific for PfMSP-119 were produced after three immunizations, we showed that a proportion of the B cells underwent phenotypic and functional changes that suggested appropriate B cell activation. Surface phenotype analysis of B cells from both the spleen and the bone marrow revealed that relatively few of the B cells had differentiated into germinal center B cells, plasmablasts, plasma cells, and memory B cells in response to PfMSP-119 immunization. In the primary response low levels of GC B cells with delayed kinetics were observed. B cells that form the GC can be recognized by increased expression of GL7 and a decrease in CD38 expression. The delay in the GC response (B220+ GL7+) induced by PfMSP-119 is striking. The duration of the germinal center reaction may vary depending on the nature of the antigen and may last for several weeks or months, implying that high-affinity antibodies and memory cells can be generated well after elimination of the pathogen (12). Classical antigens such as sheep red blood cells (SRBC), ovalbumin (OVA), and (4-hydroxy-3-nitrophenyl)acetyl (NP) induce immunization-specific GC reactions that are observed as early as days 4 to 6, peak on day 12, and dissipate by day 18 (17, 34, 39, 47). Unlike these classical antigens, in PfMSP-119-immunized mice we observed significantly reduced total numbers of B220+ GL7+ GC B cells at later phases of the primary immune response. However, after day 28, the GC B cell number returned to the background levels (as measured in naive mice), and little to no enhancement in the level of B220+ GL7+ GC B cell numbers was seen during a secondary response, suggesting that memory B cells failed to form structurally identifiable GCs (5). The GC reaction results in antibody affinity maturation, resulting in the production of IgG (7, 35, 40, 41). Thus, it is possible that the GC B cell response generated in response to PfMSP-119 primary immunization was not sufficient to drive the maintenance of serum antibodies long term, emphasizing the importance of GC differentiation and memory B cell formation.
Earlier it has been reported that plasma cell responses in the spleen decrease rapidly with a coincident increase in long-lived PC (LLPCs) in the bone marrow that have the potential to rapidly differentiate into ASCs during a secondary immune response (6, 18, 22, 37, 40, 41). These findings complement and help explain the increased frequency of observed PfMSP-119-induced plasma cells present predominantly in the bone marrow during the secondary immune response.
It is well established that the memory B cell response to the T-dependent Ag develops via a GC-dependent process culminating in the formation of Ag-experienced memory B cells and plasma cells (30). As the humoral immune response matures, the GC reaction recedes, and a proportion of activated B cells acquire a memory phenotype. The ability of memory B cells to survive depends on their germinal-center “history,” with the persistence of high-affinity B cell variants being favored (42). Both flow cytometry analysis and the in vitro culture system showed increases in the number of Ag-specific memory B cells in the bone marrow 15 days after the last immunization but these were not sustained long term, and their frequencies decreased rapidly by 4 weeks. Therefore, despite displaying a memory phenotype, the majority of MBCs in the bone marrow showed a limited life span. A continuous differentiation of memory B cells into plasma cells is apparently necessary to maintain long-term antibody titers (4, 16, 36). Also serum antibody levels correlate linearly with the frequencies of MBCs present in a host, indicating that the ASC compartment is genealogically linked to and is replenished by the MBC compartment (3, 8, 18, 31, 41). Our results support this hypothesis as decline in PfMSP-119-specific MBCs led to a decay of antigen-specific ASC numbers and serum antibody levels over time. In this context, the current study corroborates similar results of Crompton et al., who showed a positive correlation between the magnitude of the MSP142-C1 and AMA1-C1-specific MBC response and Ab titers (10). Therefore, it is possible that failure to maintain long-term PfMSP-119-specific antibody titers reflects a low level of differentiation of memory B cells into plasma cells.
Our transfer experiments gave further evidence that memory B cells become short-lived rather than long-lived plasma cells when reactivated with antigen because anti-PfMSP-119 titers in serum and PfMSP-119-specific ASCs in the spleen declined rapidly. Following parasite challenge there were even fewer PfMSP-119-specific memory B cells that differentiated into ASCs than after antigen challenge. It may be argued that recombinant protein in adjuvant induces a qualitatively and quantitatively better immune response than the parasite challenge. It could be that recombinant PfMSP-119 antigen initially induces a sufficiently high number of short-lived ASCs that were able to maintain high but rapidly declining levels of circulating IgG after protein immunization, whereas such potent ASC induction was not achieved after PbPfM19 transgenic parasite infection. Taken together, these findings support the idea that a majority of memory B cells, when activated either by antigen or parasite infection, become short-lived rather than long-lived plasma cells.
In natural human infections MSP-119-specific antibody responses have also been shown to be short-lived and comparatively low, despite repeated exposure to infection. One critical factor for effective humoral responses is the CD4+ T cell help for B cells. CD4+ T cell help is required for primary responding B cells to form germinal centers and produce high-affinity class-switched Abs. Memory B cells require CD4+ T cells for their activation and survival and activation (6, 28). The complex tertiary structure of MSP-119 stabilized by five or six disulfide bonds temporally hinders the processing of peptides for presentation on major histocompatibility complex (MHC) class II (24). These bonds impede antigen processing and, thereby, may affect the generation of CD4+ T cells providing help for B cells. But the resistance of native MSP-119 to proteolytic processing in vitro surprisingly did not appear to impede the development of CD4+ T cells specific for this antigen during a course of immunization in vivo (20). Another possibility is that the survival of PfMSP-119-specific CD4+ T cells may be compromised, and this affects the survival or activation of memory B cells (46, 50). Future experiments are aimed at analyzing the role of PfMSP-119-primed primary and memory CD4+ T cells on the differentiation and maintenance of PfMSP-119-specific memory B cells.
Memory B cells, per se, do not have antiparasite capacity since they represent a resting cell type. It is only upon activation by antigen/parasite that memory B cells attained their antiparasite function by producing antigen-specific antibody. Although immunization with PfMSP-119 induced memory antibody responses that reacted strongly with the native PfMSP-119 on the blood-stage parasite, antibodies were not effective at protecting mice against P. berghei transgenic parasite challenge infection. PfMSP-119-specific memory B cells failed to reduce the parasite load significantly and did not prolong the survival time of the infected mice, consistent with findings reported earlier (51). A previous study had shown that the significant reduction in numbers of Plasmodium yoelii MSP-119-specific ASCs was because MBCs either underwent early apoptosis or were not activated after antigen reexposure, which subsequently resulted in death of the MBCs (49). Similarly, another study had shown that parasite-induced B cell apoptosis in Trypanosoma brucei infections of C57/BL6 and BALB/c mice abolished the preestablished protective vaccine and antiparasite-induced MBC responses (37). It may also be true in the present study, and we are currently trying to determine whether this is indeed a possibility. Thus, the inability of PfMSP-119-primed mice to maintain an MBC population together with poor recall functions of the MBC population and relatively rapid decline in anamnestic IgG production suggests that memory B cell induction, persistence, and/or reactivation may have been suboptimal and was not adequate to ensure protection against parasite challenge. A better understanding of the factors that are crucial in the induction and maintenance of memory B cells cannot be overlooked in the development of a successful protein-based malaria vaccine.
ACKNOWLEDGMENTS
We thank Suman Mazumder for synthesizing recombinant PfMSP-119. We are grateful to Anmol Chandele for critical reading and editing of the manuscript.
This work is supported by a core grant from ICGEB.
We have no financial conflicts of interest
Footnotes
Published ahead of print 21 November 2011
REFERENCES
- 1. Achtman AH, Bull PC, Stephens R, Langhorne J. 2005. Longevity of the immune response and memory to blood-stage malaria infection. Curr. Top. Microbiol. Immunol. 297:71–102 [DOI] [PubMed] [Google Scholar]
- 2. Agematsu K, Hokibara S, Nagumo H, Komiyama A. 2000. CD27: a memory B-cell marker. Immunol. Today 21:204–206 [DOI] [PubMed] [Google Scholar]
- 3. Amanna IJ, Carlson NE, Slifka MK. 2007. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357:1903–1915 [DOI] [PubMed] [Google Scholar]
- 4. Anderson SM, Tomayko MM, Shlomchik MJ. 2006. Intrinsic properties of human and murine memory B cells. Immunol. Rev. 211:280–294 [DOI] [PubMed] [Google Scholar]
- 5. Benson MJ, et al. 2009. Distinction of the memory B cell response to cognate antigen versus bystander inflammatory signals. J. Exp. Med. 206:2013–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernasconi NL, Traggiai E, Lanzavecchi A. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199–2202 [DOI] [PubMed] [Google Scholar]
- 7. Blink EJ, et al. 2005. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J. Exp. Med. 201:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonsignori M, et al. 2009. HIV-1 envelope induces memory B cell responses that correlate with plasma antibody levels after envelope gp120 protein vaccination or HIV-1 infection. J. Immunol. 183:2708–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chauhan VS, Yazdani SS, Gaur D. 2010. Malaria vaccine development based on merozoite surface proteins of Plasmodium falciparum. Hum. Vaccine 6:757–762 [DOI] [PubMed] [Google Scholar]
- 10. Crompton PD, et al. 2009. The TLR9 ligand CpG promotes the acquisition of Plasmodium falciparum-specific memory B cells in malaria-naive individuals. J. Immunol. 182:3318–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Koning-Ward TF, et al. 2003. A new rodent model to assess blood stage immunity to the Plasmodium falciparum antigen merozoite surface protein 119 reveals a protective role for invasion inhibitory antibodies. J. Exp. Med. 198:869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dogan I, et al. 2009. Multiple layers of B cell memory with different effector functions, Nat. Immunol. 10:1292–1299 [DOI] [PubMed] [Google Scholar]
- 13. Drakeley CJ, et al. 2005. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc. Natl. Acad. Sci. U. S. A. 102:5108–5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elgueta R, de Vries VC, Noelle RJ. 2010. The immortality of humoral immunity. Immunol. Rev. 236:139–150 [DOI] [PubMed] [Google Scholar]
- 15. Fowkes FJ, Richards JS, Simpson JA, Beeson JG. 2010. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. PLoS Med. 7:e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gatto D, et al. 2007. Regulation of memory antibody levels: the role of persisting antigen versus plasma cell life span. J. Immunol. 178:67–76 [DOI] [PubMed] [Google Scholar]
- 17. Good-Jacobson KL, et al. 2010. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 11:535–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Good-Jacobson KL, Shlomchik MJ. 2010. Plasticity and heterogeneity in the generation of memory b cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. J. Immunol. 185:3117–3125 [DOI] [PubMed] [Google Scholar]
- 19. Hausl C, et al. 2004. Preventing restimulation of memory B cells in hemophilia A: a potential new strategy for the treatment of antibody-dependent immune disorders. Blood 104:115–122 [DOI] [PubMed] [Google Scholar]
- 20. Hirunpetcharat C, et al. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP119) of Plasmodium yoelii expressed in Saccharomyces cerevisiae. Correlation of protection with antigen-specific antibody titer, but not with effector CD4 T cells. J. Immunol. 159:3400–3411 [PubMed] [Google Scholar]
- 21. Hirunpetcharat C, et al. 1998. Intranasal immunization with yeast-expressed 19 kDa carboxyl-terminal fragment of Plasmodium yoelii merozoite surface protein-1 (yMSP119) induces protective immunity to blood stage malaria infection in mice. Parasite Immunol. 20:413–420 [DOI] [PubMed] [Google Scholar]
- 22. Höfer T, et al. 2006. Adaptation of humoral memory. Immunol. Rev. 211:295–302 [DOI] [PubMed] [Google Scholar]
- 23. Holder AA, et al. 1999. Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parassitologia 41:409–414 [PubMed] [Google Scholar]
- 24. Holder AA. 2009. The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria. Parasitology 136:1445–1456 [DOI] [PubMed] [Google Scholar]
- 25. Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. 2007. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar. J. 6:82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Langhorne J, Ndungu FM, Sponaas AM, Marsh K. 2008. Immunity to malaria: more questions than answers. Nat. Immunol. 9:725–732 [DOI] [PubMed] [Google Scholar]
- 27. MacLennan IC, Liu YJ, Johnson GD. 1992. Maturation and dispersal of B-cell clones during T cell-dependent antibody responses. Immunol. Rev. 126:143–161 [DOI] [PubMed] [Google Scholar]
- 28. MacLeod MKL, et al. 2011. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J. Immunol. 186:2889–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mazumdar S, et al. 2010. Plasmodium falciparum merozoite surface protein 1 (MSP-1)-MSP-3 chimeric protein: immunogenicity determined with human-compatible adjuvants and induction of protective immune response. Infect. Immun. 78:872–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McHeyzer-Williams LJ, McHeyzer-Williams MG. 2005. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23: 487–513 [DOI] [PubMed] [Google Scholar]
- 31. Nanan R, Heinrich D, Frosch M, Kreth HW. 2001. Acute and long-term effects of booster immunisation on frequencies of antigen-specific memory B-lymphocytes. Vaccine 20:498–504 [DOI] [PubMed] [Google Scholar]
- 32. Ndungu FM, et al. 2009. Functional memory B cells and long-lived plasma cells are generated after a single Plasmodium chabaudi infection in mice. PLoS Pathog. 5:e1000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ochsenbein AF, et al. 2000. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc. Natl. Acad. Sci. U. S. A. 97:13263–13268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Or-Guil M, Wittenbrink N, Weiser AA, Schuchhardt J. 2007. Recirculation of germinal center B cells: a multilevel selection strategy for antibody maturation. Immunol. Rev. 216:130–141 [DOI] [PubMed] [Google Scholar]
- 35. Przylepa J, Himes C, Kelsoe G. 1998. Lymphocyte development and selection in germinal center. Curr. Top. Microbiol. Immunol. 229:85–104 [DOI] [PubMed] [Google Scholar]
- 36. Radbruch A, et al. 2006. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 6: 741–750 [DOI] [PubMed] [Google Scholar]
- 37. Radwanska M, et al. 2008. Trypanosomiasis-induced B cell apoptosis results in loss of protective anti-parasite antibody responses and abolishment of vaccine-induced memory responses. PLoS Pathog. 4:e1000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sanderson RD, Lalor P, Bernfield M. 1989. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1:27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shinall SM, Gonzalez-Fernandez M, Noelle RJ, Waldschmidt TJ. 2000. Identification of murine germinal center B cell subsets defined by the expression of surface isotypes and differentiation antigens. J. Immunol. 164:5729–5738 [DOI] [PubMed] [Google Scholar]
- 40. Smith KG, Light A, Nossal GJ, Tarlinton DM. 1997. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 16:2996–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. 1998. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med. 187:885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tarlinton D. 2006, B-cell memory: are subsets necessary? Nat. Rev. Immunol. 6:785–790 [DOI] [PubMed] [Google Scholar]
- 43. Toellner KM, Gulbranson-Judge A, Taylor DR, Sze DM, MacLennan IC. 1996. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J. Exp. Med. 183:2303–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weiss GE, et al. 2010. The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog. 6:e1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wipasa J, et al. 2010. Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 6:e1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wipasa J, Xu H, Stowers A, Good MF. 2001. Apoptotic deletion of Th cells specific for the 19-kDa carboxyl-terminal fragment of merozoite surface protein 1 during malaria infection. J. Immunol. 167:3903–3909 [DOI] [PubMed] [Google Scholar]
- 47. Wolniak KL, Noelle RJ, Waldschmidt TJ. 2006. Characterization of (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific germinal center B cells and antigen-binding B220− cells after primary NP challenge in mice. J. Immunol. 177:2072–2079 [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization 2009. World malaria report 2009. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2009/9789241563901_eng.PDF. [Google Scholar]
- 49. Wykes MN, Zhou YH, Liu XQ, Good MF. 2005. Plasmodium yoelii can ablate vaccine-induced long-term protection in mice. J. Immunol. 175:2510–2516 [DOI] [PubMed] [Google Scholar]
- 50. Xu H, et al. 2002. The mechanism and significance of deletion of parasite-specific CD4+ T cells in malaria infection. J. Exp. Med. 195:881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yoshida S, et al. 2010. Plasmodium berghei circumvents immune responses induced by merozoite surface protein 1- and apical membrane antigen 1-based vaccines. PLoS One 5:e13727. [DOI] [PMC free article] [PubMed] [Google Scholar]