Abstract
The intracellular protozoan Toxoplasma gondii is well known for its skill at invading and living within host cells. New discoveries are now also revealing the astounding ability of the parasite to inject effector proteins into the cytoplasm to seize control of the host cell. This review summarizes recent advances in our understanding of one such secretory protein called ROP16. This molecule is released from rhoptries into the host cell during invasion. The ROP16 molecule acts as a kinase, directly activating both signal transducer and activator of transcription 3 (STAT3) and STAT6 signaling pathways. In macrophages, an important and preferential target cell of parasite infection, the injection of ROP16 has multiple consequences, including downregulation of proinflammatory cytokine signaling and macrophage deviation to an alternatively activated phenotype.
INTRODUCTION
Intracellular microorganisms, whether bacterial, fungal, or protozoal, are faced with special challenges to achieve a productive infection. They must survive in a potentially hostile intracellular environment, and to do so they employ strategies of host evasion, active interference with host cell machinery, or a combination of both. The payoff of this high-risk intracellular lifestyle is that the microorganism gains access to the nutrient-rich environment of the host cytosol and at the same time avoids extracellular effectors of host immunity. Unraveling how intracellular pathogens achieve this intracellular ecological niche is not only fascinating from a purely biological perspective but can also provide us with new targets to control infection. In addition, understanding mechanisms developed by intracellular pathogens to manipulate the host cell internal environment may provide new insights into controlling mammalian cell behavior.
Nowhere is this more the case than for the apicomplexan protozoan Toxoplasma gondii, a parasite that has emerged in recent years as the model obligate intracellular eukaryotic pathogen. Toxoplasma is transmitted by ingestion of infectious cysts as a result of carnivorism or predation. In the intestine of cats, T. gondii undergoes sexual reproduction, resulting in fecal shedding of highly infectious oocysts (22). While normally asymptomatic, Toxoplasma may cause severe disease in immunocompromised populations and during congenital infection (63). The astonishingly widespread geographical and biological distribution of T. gondii is a dramatic indication of the success of this parasite in living with its host and achieving successful transmission to new hosts.
Toxoplasma tachyzoites (the rapidly replicating form of the parasite responsible for acute toxoplasmosis) enter host cells through a well-studied process of active invasion involving parasite actin-based motility and establishment of a moving junction at the interface between host and parasite membranes (57, 77, 85). During invasion, the parasite creates a specialized parasitophorous vacuole that resists acidification and lysosomal fusion (58). The vacuole membrane consists of parasite and host lipids and a subset of parasite proteins but is largely devoid of host cell proteins (57, 80). The parasitophorous vacuole membrane (PVM) serves as a molecular sieve through which T. gondii scavenges host cell nutrients, including certain amino acids, nucleic acid precursors, and lipids, such as cholesterol (12, 16, 24, 25, 74). During invasion and creation of the PVM, apically oriented organelles (hence the term “apicomplexan” for this group of protozoa) called micronemes and rhoptries are discharged, followed later by release of dense granules (11, 35). Regulated secretion of parasite proteins originating from these organelles mediates adhesion, invasion, and creation of the mature PVM. Of direct relevance to the present review, it is also now clear that some of these secreted molecules are injected directly into the host cell cytoplasm during invasion (32). Moreover, some injected parasite molecules are directed to the host cell nucleus (6, 31). One of these parasite proteins is ROP16, a specialized kinase that hacks into host cell signaling cascades to modify the behavior of the parasite-infected host cell (71).
Contained within the parasitophorous vacuole, Toxoplasma evades elimination by the immune system. The parasite actively deploys an infection strategy that keeps the host alive to allow establishment of long-lasting latent infection, promoting the likelihood of transmission to new hosts. The latent phase of infection is characterized by the formation of quiescent cysts in the brain and skeletal muscle tissue. To prevent host death, T. gondii triggers a robust Th1 response characterized by early interleukin 12 (IL-12) production by cells, such as macrophages, dendritic cells (DC), and neutrophils (5, 29, 67). Early IL-12 production is followed by emergence of gamma interferon (IFN-γ)-producing CD4+ and CD8+ T lymphocytes, and these cell types are required for surviving acute infection and ultimately preventing potentially lethal reactivation events during chronic infection (20, 26, 28, 82). Both IL-12 and IFN-γ production are essential for the host to survive infection (72, 73, 81). Absence of either cytokine results in the inability to control parasite replication and dissemination, resulting in massive tissue necrosis and host death. Yet, parasite-induced production of both IL-12 and IFN-γ must also be tightly regulated to prevent cytokine-mediated mortality (the so-called “cytokine storm”). Control of these proinflammatory mediators is achieved initially by direct parasite-mediated suppression (further detailed below) and later by induction of anti-inflammatory cytokines such as IL-10. Evidence for the importance of the latter cytokine was revealed by now classic studies demonstrating that without IL-10, mice rapidly succumb to Toxoplasma infection as a result of dysregulated proinflammatory cytokines rather than loss of an ability to control the parasite itself (30, 83).
Recent years have witnessed the identification of several Toxoplasma effector molecules that directly interact with cells of the immune system to mediate many of the effects described above (Table 1). Early reports suggested a role for parasite cyclophilin-18 interacting with chemokine receptor CCR5 to induce IL-12 (1). This was followed by identification of Toxoplasma profilin as a ligand for Toll-like receptor 11 (TLR11) driving DC IL-12 induction (90). Recently, release of dense granule protein GRA15 into the host cell cytoplasm was found to mediate NF-κB activation and initiate IL-12 synthesis (69). The rhoptry kinase ROP18 was identified as a virulence determinant that phosphorylates and deactivates host immunity-related p47 GTPase (IRG) proteins that mediate IFN-γ-dependent destruction of the PVM (23, 79). A rhoptry pseudokinase, ROP5, is a major virulence determinant, although the host target molecule is not yet known (3, 65). Similarly, ROP38 has been identified as a rhoptry protein influencing host cell signaling (62). Finally, the ROP16 kinase is another determinant of virulence (71). As we review here, the ROP16 molecule is highly complex, because it directly activates at least two distinct transcription factors in the classical Janus kinase (JAK)/signal transducer and activator of transcription (STAT) cytokine signaling pathway.
Table 1.
Toxoplasma molecules that interact with components of host defense
| Parasite molecule | Host target/ligand | Major effect on host | Reference(s) |
|---|---|---|---|
| Profilin | TLR11 | Induction of IL-12 | 90 |
| Cyclophilin-18 | CCR5 | Induction of IL-12 | 1 |
| GPI moeities | TLR2, TLR4 | Induction of IL-12, TNF-α | 19 |
| ROP16 | STAT3, STAT6 | IL-12 downregulation, arginase 1 induction | 8, 36, 61, 71, 89 |
| ROP18 | IRG proteins, ATF6β | PVM destruction, promotion of CD8 responses | 23, 79 |
| ROP5 | Unknown | Contributes to virulence | 3, 65 |
| ROP38 | Unknown | Suppression of MAPK signaling | 62 |
| GRA15 | NF-κB | IL-12 induction | 36, 69 |
LINKING TOXOPLASMA-DEPENDENT IMMUNOSUPPRESSION TO STAT3 ACTIVATION AND FUNCTION
Although T. gondii is known for its ability to infect diverse host cell types, macrophages, DC, and neutrophils, major effectors of innate immunity, are preferentially targeted during in vivo infection (4, 14, 17). This is of keen biological interest, because these cell types are well known for their ability to produce cytokines and proinflammatory antimicrobial effector molecules. How does Toxoplasma survive within these masters of antimicrobial defense? Groundbreaking advances in recent years are for the first time yielding fascinating molecular insight into how this eukaryotic parasitic pathogen lives within these cells. In particular, Toxoplasma seizes control of intracellular signaling pathways during invasion, specifically those transduction pathways involved in the JAK/STAT pathway of cytokine production.
While in vivo infection stimulates strong type 1 cytokine-based immunity in the host, closer examination of the response unexpectedly reveals another side to this host-parasite interaction. For example, from within the infected cell, the parasite efficiently blocks TLR-linked signaling pathways that originate via extracellular signals, such as lipopolysaccharide (LPS) (9, 21, 43). Similarly, parasite-infected cells become largely nonresponsive to IFN-γ activation (39, 40, 50). This results in their defective ability to activate T cells (51). DC infected with Toxoplasma are also nonresponsive to TLR activation, and cells are likewise defective in T cell activation (56). Importantly, DC targeted for infection in vivo are also nonresponsive to ex vivo TLR activation (4). Early on, it was recognized that suppression of host responses by T. gondii required live parasites and an ability to actively invade host cells (7). The studies collectively suggest that Toxoplasma has developed mechanisms to downregulate antimicrobial effector mechanisms despite (or perhaps because of) being immersed in an overwhelmingly proinflammatory cytokine environment. Many different mechanisms have been proposed to explain these downregulatory effects. These include inhibition of NF-κB and STAT1 nuclear translocation (9, 52, 76), degradation of STAT1 (92), inhibition of mitogen-activated protein kinase (MAPK) signaling (37), induction of suppressor of eytokine synthesis-1 (SOCS1) (92), and most recently interference with chromatin remodeling (45, 46). While each of these phenomena may indeed contribute to parasite-mediated suppression, a unifying mechanism of suppression is not apparent.
Insight into how Toxoplasma interferes with host signal transduction came from the observation that the parasite triggers rapid, strong, and sustained activation of STAT3 during infection (10). The response requires live parasites and is restricted to infected cells rather than noninfected bystander cells. Importantly, the ability of T. gondii to mediate suppression of TLR responsiveness in STAT3-null macrophages was severely curtailed, suggesting that the parasite actively exploits this transcription factor to inhibit proinflammatory responses.
As a member of the JAK/STAT signaling family, the canonical model of STAT3 function is that cytokines such as IL-10 and IL-6 bind to their heterodimeric receptors, resulting in tyrosine phosphorylation of receptor-associated JAK kinases, in turn resulting in STAT3 recruitment (66). After JAK-dependent STAT3 tyrosine phosphorylation, STAT3 dimerizes and translocates to the nucleus as a functional transcription factor. Full activation of STAT3 and other STAT molecules also requires MAPK-mediated serine phosphorylation. Nevertheless, this model JAK/STAT pathway has also been called into question, and alternate models involving constitutive shuttling of STAT between the nucleus and cytoplasm have been recently proposed (75).
The effects of STAT3 activation are complex. On the one hand, IL-10 activation of STAT3 leads to anti-inflammatory responses and attenuation of cytokines such as tumor necrosis factor alpha (TNF-α) in response to LPS (41, 84). On the other hand, IL-6, a proinflammatory cytokine that triggers the acute-phase response, also acts though activation of STAT3 (2). What determines whether STAT3 is a pro- or anti-inflammatory transcription factor depends upon the activity of suppressor of cytokine synthesis 3 (SOCS3) during IL-6 signaling (18, 42, 91). Thus, in the absence of SOCS3, IL-6-stimulated STAT3 activation is prolonged and the cytokine acts like IL-10 and downregulates TNF-α and IL-12. Because the suppression of proinflammatory responses mediated by T. gondii is dependent on STAT3 and parasite invasion, the parasite appears to be exploiting the IL-10 signaling pathway. Nevertheless, it was recently found that parasite-induced STAT3 activation is enhanced in the absence of SOCS3 in a manner similar to that of IL-6 (86). The implication of this interesting result is that there is additional complexity to Toxoplasma-mediated STAT3 activation that we have yet to understand.
RHOPTRY PROTEIN ROP16 ACTIVATES STAT3 AND STAT6
In Europe and North America, Toxoplasma occurs predominantly as 3 clonal lineages (types I, II, and III) that display differences in disease pathogenesis in humans and animals (78). Thus, type I strains are highly virulent in mice, whereas types II and III are considerably less virulent and cause less pathology during infection. The stage was set for a major leap in understanding Toxoplasma-host cell interactions by the observation that type II but not type I or type III parasite strains triggered large amounts of macrophage IL-12 and that this was accompanied by strain-specific translocation of NF-κB (38, 68). By genetic examination of F1 progeny between type II and type III parasite strains, virulence loci were identified, and in this way several secretory rhoptry proteins emerged as important parasite effectors that controlled virulence (6). In particular, ROP16 was identified as a virulence determinant, and pathway analysis suggested that interaction with STAT signaling was required for the activity of this rhoptry protein (70, 71). Type I and type III ROP16 were found to be involved in activation of both STAT3 and STAT6, whereas type II ROP16 was defective in this regard. The tyrosine and serine phosphorylation stimulated by ROP16 occur on the same amino acid residues as that induced by cytokine-mediated signaling.
The ROP16 molecule contains a nuclear localization sequence (NLS), and during invasion it is injected into the host cell cytoplasm followed by rapid translocation into the nucleus. As such, ROP16 is one of a growing number of rhoptry proteins and dense granule molecules now known to gain access to the intracellular environment during invasion. The exact mechanism by which ROP16 is introduced into the host cytoplasm is not known. Although ROP16 contains an NLS which is required for ROP16 to enter the nucleus, NLS-mutated ROP16 is still capable of activating STAT6 and also probably STAT3, suggesting that this rhoptry molecule acts in the cytoplasm to phosphorylate STAT proteins (71). This raises the interesting possibility that ROP16 may also possess STAT3- and STAT6-independent activities that provide additional functions within the host nucleus.
The ROP16 protein was originally identified as a putative serine-threonine kinase, but more recently it was shown by in vitro assays to directly tyrosine phosphorylate STAT3 and STAT6 (61, 89). Parenthetically, the recent observation that virus and nucleic acids activate STAT molecules through endoplasmic reticulum IFN stimulator (ERIS or STING)-dependent recruitment to the endoplasmic reticulum provides another possible model for STAT3 and STAT6 activation by ROP16 (13). Consistent with the role of STAT3 in downregulating proinflammatory signaling, ROP16 knockout parasites induce larger amounts of IL-12 than wild-type parasites, and cells infected with ROP16 knockout parasites display a markedly reduced ability to suppress cellular responses triggered by LPS and IFN-γ (8).
Interestingly, using ROP16 knockout parasites, we found that immediate-early activation of STAT3 was normal in the absence of ROP16, but sustained phosphorylation of STAT3 required this rhoptry protein (8). In contrast, activation of STAT6 was completely dependent upon ROP16. The parasite or host molecules required for the immediate-early ROP16-independent STAT3 activation are not currently known. Although inhibitors of JAK signaling can block the immediate-early STAT3 response, interpretation of this result is difficult, because such inhibitors may also act on parasite kinases (61). Regardless, the observation that the kinetics of ROP16-dependent STAT3 and STAT6 activation are not directly parallel is further indication of the complexity of this rhoptry protein in the host-parasite interaction.
ROLE OF ROP16-DEPENDENT STAT6 ACTIVATION
Activation of STAT6 is normally associated with signaling driven by IL-4 or IL-13. In macrophages, this can lead to an M2 (or alternatively activated) phenotype that is associated with the production of anti-inflammatory mediators. In contrast, M1 (classically activated) macrophages are generated by proinflammatory cytokines and TLR ligands, such as LPS. One of the main products of M2 macrophages is arginase 1. This enzyme converts arginine into ornithine, which is a precursor of polyamines and collagen, in turn contributing to extracellular matrix generation. Thus, one of the main functions of alternatively activated macrophages is believed to be tissue repair, and for this reason these cells are also called wound-healing macrophages (59).
Type I ROP16 is a potent inducer of arginase 1, consistent with a specific role of ROP16 in sustained activation of STAT6 (8). In fact, an interesting antagonistic relationship has been proposed in which type I and type III parasites trigger ROP16-dependent and STAT6-dependent M2 activation, whereas type II parasites induce GRA15-dependent and NF-κB-dependent M1 activation (36). Two biologically opposing outcomes are possible following induction of arginase 1 in infected cells. On the one hand, Toxoplasma is a polyamine auxotroph; therefore, induction of arginase 1 could promote growth of type 1 parasites by supplying essential polyamine nutrients, as is seen in some cases (15, 36). Alternatively, the parasite is also an arginine auxotroph, and therefore induction of arginase 1 could act to limit growth of parasites by limiting the availability of this essential amino acid, which is also seen in some cases (8, 25). Possibly, use of different host cell types or amounts of arginine available underlies these disparate results. During in vivo infection, ROP16 knockout parasites display a growth advantage over ROP16 competent parasites that depends on ROP16-dependent and STAT6-dependent host cell expression of arginase 1. The in vivo growth advantage observed in ROP16 knockout parasites suggests that arginine availability can become limiting during infection (8). Parasite control of host signaling pathways can regulate nutrient availability, perhaps allowing the parasite to regulate its own growth rate. This is a particularly interesting nutritional adaptation for ROP16, because Toxoplasma is unusual in lacking metabolic pathways for either synthesis of arginine (25), or for the degradation of this amino acid via a parasite-encoded arginase (24).
ROP16-mediated STAT6 activation and induction of arginase 1 may also decrease the availability of arginine as a substrate for inducible nitric oxide synthase (iNOS). Therefore, in addition to the potential of ROP16 to reduce arginine availability for parasite growth, ROP16 may also enhance parasite survival by decreasing the production of nitric oxide (NO). While iNOS and NO do not appear to be essential for the control of acute infection, these host responses are necessary for the control of chronic infection (72).
Additional functions for parasite-induced STAT6 activation are also possible. The chemokines CCL17 and CCL22 (whose receptor is CCR4) are strongly upregulated in macrophages infected with type I but not type II T. gondii (44). The ccl17 gene is known to possess functional STAT6 binding sites, and it therefore seems likely that this chemokine is under the control of type I ROP16 (47, 87). Studies in Ccr4−/− mice suggest that this receptor and its chemokine ligands are involved in several aspects of innate immunity as well as in Th2-type adaptive immunity (60). It remains to be determined if ROP16-mediated induction of these chemokines plays a role in disease pathogenesis or initiation of immunity in vivo.
UNDERSTANDING ROP16 AND IN VIVO STAT3 AND STAT6 ACTIVATION
Studies on the function of ROP16 are revealing the complexity of this rhoptry molecule in the host-parasite interaction. STAT3-driven activation by type I ROP16 plays a role in downregulating inflammatory cytokines, an effect that is apparent in vivo as decreased IL-12 responses (8, 10). For the parasite, this could be a means to prevent or delay immune recognition and elimination. We found that mouse astrocytes and microglial cells produce microbicidal nitric oxide (NO) following IFN-γ activation but that this response was suppressed by Toxoplasma in a ROP16-dependent manner (8). The finding that ROP16 knockout parasites do not persist in activated astrocytes coupled with older reports showing that NO is an important defense mechanism against T. gondii in the brain suggests that ROP16-dependent STAT3 activation is a way to promote parasite persistence during chronic infection (27, 72). Alternatively, ROP16-dependent downregulation of proinflammatory responses could be a means to avoid proinflammatory cytokine pathology during infection. It is well known that Toxoplasma can cause lethal Th1 cytokine overproduction dependent on the host genotype and parasite dose (30, 49). This is not a desired outcome for the parasite, which seeks to establish latent infection to maximize chances of transmission to a new host. ROP16 could function to dampen down the inflammatory response enough to avoid pathology without leading to uncontrolled parasite replication and host death.
Evidence for an anti-inflammatory function for type I ROP16 comes from models of oral infection. High-dose type II Toxoplasma infection of C57BL/6 mice results in lesions in the small intestine mediated by overproduction of IL-12, TNF-α, and IFN-γ (48, 49). The parasite seems to act as a trigger, causing tissue damage, emergence of enteroadhesive Escherichia coli, and bacterial subepithelial translocation culminating in fulminant pathology (33, 34). However, type II parasites expressing type I ROP16 appear to cause less inflammation in the intestine (36). This might be seen as evidence for an anti-inflammatory effect of type I ROP16, but it is also possible that this is due to type I ROP16-dependent limitation in parasite replication.
An important role for ROP16 may be its capacity to influence nutrient acquisition by Toxoplasma. The parasite relies on the host for many nutrients, including amino acids and nucleic acid precursors. In particular, arginine is an essential amino acid that T. gondii must scavenge from the host cell (25). Limiting arginine availability through increased arginase 1 expression could be a host's defense response to limit infection. Alternatively, by limiting parasite replication, ROP16-driven STAT6-dependent arginase 1 induction could allow cells to carry parasites longer, effectively functioning as Trojan horses to spread infection. Yet, it has also been proposed that induction of arginase 1 could have the opposite effect, promoting production of polyamines that the parasite also requires for survival (36). In this respect, ROP16 might function to increase parasite dissemination by promoting increased cycles of infection and lysis. However, the observation that type I ROP16-null parasites expand more rapidly in vivo tends to argue against this (8).
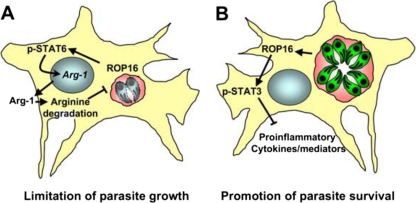
We do not fully understand the role of ROP16 during infection, but clearly the biology of this rhoptry kinase is complex (Fig. 1). ROP16 appears to be a central regulator of the host-parasite interaction that controls inflammatory cytokine production, suppression of TLR and IFN-γ responses in infected cells, and nutrient availability that determines both parasite growth rate and host ability to produce nitric oxide. This complexity is not surprising given that ROP16 activates (at least) two distinct transcription factors with their own independent activities. Determining whether ROP16 mediates its effects through STAT3 or STAT6 in vivo is complicated by the lethality of global STAT3 knockout, as well as bystander STAT activation due to the parasite-induced cytokine response. The predominant activity of ROP16 may depend on the cell type hosting the parasite, amount of arginine available in the host microenvironment, or on the parasite life cycle stage. The concept that a single rhoptry molecule may have multiple distinct functions has recent precedent, inasmuch as rhoptry kinase ROP18 deactivates IRG proteins to prevent destruction of the PVM while at the same time targeting transcription factor ATF6β for proteasomal degradation that affects DC antigen presentation to CD8+ T lymphocytes (23, 79, 88). The multifunctional activities of these secreted parasite effector molecules that function within infected cells provide new challenges to understanding their functions in cells and animals.
Fig 1.
ROP16 is a multifunctional kinase with diverse effects on the host. (A) ROP16 activates STAT6, resulting in arginase-1 induction. Degradation of arginine limits replication of Toxoplasma, which is auxotrophic for this amino acid. Limiting parasite growth is beneficial for the host. It could also assist in the spread of the parasite, which uses cells such as macrophages and dendritic cells in dissemination during in vivo infection. (B) ROP16 and tyrosine phosphorylate STAT3. This transcription factor can have proinflammatory or anti-inflammatory activity, depending on the context of infection. During Toxoplasma infection, the predominant activity appears to be anti-inflammatory, although the exact nuclear targets of STAT3 are not known. For the parasite, this may be a way to evade antimicrobial immunity. Anti-inflammatory STAT3 function may also be a means to downmodulate harmful proinflammatory pathology. This benefits the host but is also advantageous to Toxoplasma, which seeks to keep its host alive to permit establishment of latent infection that is required for parasite transmission to new hosts. Which of these activities predominates may depend upon host cell type and life cycle stage of the parasite.
PROTOZOAN TYROSINE KINASES—EVOLUTIONARY CONSIDERATIONS
Protein tyrosine kinases are an evolutionary innovation that accompanied emergence of multicellular organisms. They function to create high-affinity binding sites for proteins containing Src homology-2 (SH2) domains, and they are regulated by protein tyrosine phosphatases. With the exception of choanoflagellates (which are believed to be the most closely related of unicellular organisms to metazoans), there are few recognizable tyrosine kinases, protein tyrosine phosphatases, or SH2 domain-containing proteins in the protozoa (54, 55, 64). Then how is it that ROP16 can be a tyrosine kinase for this signaling module? ROP16 was originally identified as a putative serine-threonine kinase, molecules that are abundant throughout evolution (53). There is evidence in some fungi that serine-threonine kinases can also mediate inefficient tyrosine phosphorylation (64). The function of ROP16 would be consistent with a view that this was originally a functional serine-threonine kinase that acquired tyrosine kinase activity. It is therefore possible that ROP16 is a promiscuous kinase, maintaining functional serine-threonine activity on still-to-be-discovered host or parasite protein targets while also maintaining functional tyrosine kinase activity on host STAT3 and STAT6 signaling cascades. The unusual adaptation of ROP16 into a functional tyrosine kinase in mammalian cells suggests that ROP16 is important for the successful global expansion of this parasite.
FUTURE DIRECTIONS
Research on T. gondii is leading the way in our understanding of how intracellular eukaryotes manipulate the invaded host cell, but the field is still in its infancy. The consequences of ROP16 intracellular injection in other host species, such as humans and cats (the definitive host), are not yet known. We do not completely understand how ROP16 affects the mucosal immune response during initiation of infection, nor do we know if this molecule plays a role during establishment of chronic infection or during toxoplasmic encephalitis. Because of the promiscuous nature of T. gondii, the consequences of ROP16-mediated STAT activation need to be considered in other cells that the parasite invades. It has been estimated based on phylogenomic analysis that there are at least 44 rhoptry kinase family genes (62). We understand something of the function of two (ROP16, ROP18). The functions of ROP5 and ROP38 kinases, in particular, can be expected to be revealed in the near future. It seems certain that other important rhoptry molecules that interact with the host cell await discovery.
ACKNOWLEDGMENTS
Our work on ROP16 is supported by NIH grants AI50617 (E.Y.D.) and AI073142 (D.J.B.).
Footnotes
Published ahead of print 21 November 2011
REFERENCES
- 1. Aliberti J, et al. 2003. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat. Immunol. 4:485–490 [DOI] [PubMed] [Google Scholar]
- 2. Alonzi T, et al. 2001. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation [correction of activation] in the liver. Mol. Cell. Biol. 21:1621–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Behnke MS, et al. 2011. Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proc. Natl. Acad. Sci. U. S. A. 108:9631–9636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bierly AL, Shufesky WJ, Sukhumavasi W, Morelli A, Denkers EY. 2008. Dendritic cells expressing plasmacytoid marker PDCA-1 are Trojan horses during Toxoplasma gondii infection. J. Immunol. 181:8445–8491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bliss SK, Marshall AJ, Zhang Y, Denkers EY. 1999. Human polymorphonuclear leukocytes produce IL-12, TNF-α, and the chemokines macrophage-inflammatory protein-1a and -1b in response to Toxoplasma gondii antigens. J. Immunol. 162:7369–7375 [PubMed] [Google Scholar]
- 6. Boothroyd JC, Dubremetz JF. 2008. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat. Rev. Microbiol. 6:79–88 [DOI] [PubMed] [Google Scholar]
- 7. Butcher BA, Denkers EY. 2002. Mechanism of entry determines ability of Toxoplasma gondii to inhibit macrophage proinflammatory cytokine production. Infect. Immun. 70:5216–5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butcher BA, et al. 2011. Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog. 7:e1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butcher BA, Kim L, Johnson PF, Denkers EY. 2001. Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NFkB. J. Immunol. 167:2193–2201 [DOI] [PubMed] [Google Scholar]
- 10. Butcher BA, et al. 2005. Cutting edge: IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-α in host macrophages. J. Immunol. 174:3148–3152 [DOI] [PubMed] [Google Scholar]
- 11. Carruthers VB, Sibley LD. 1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73:114–123 [PubMed] [Google Scholar]
- 12. Charron AJ, Sibley LD. 2002. Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J. Cell Sci. 115:3049–3059 [DOI] [PubMed] [Google Scholar]
- 13. Chen H, et al. 2011. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell 147:436–446 [DOI] [PubMed] [Google Scholar]
- 14. Chtanova T, et al. 2008. Dynamics of neutrophil migration in lymph nodes during infection. Immunity 29:487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cook T, et al. 2007. Divergent polyamine metabolism in the Apicomplexa. Microbiology 153:1123–1130 [DOI] [PubMed] [Google Scholar]
- 16. Coppens I, Sinai AP, Joiner KA. 2000. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J. Cell Biol. 149:167–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Courret N, et al. 2006. CD11c and CD11b expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Croker BA, et al. 2003. SOCS3 negatively regulates IL-6 signaling in vivo. Nat. Immunol. 4:540–545 [DOI] [PubMed] [Google Scholar]
- 19. Debierre-Grockiego F, et al. 2007. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 179:1129–1137 [DOI] [PubMed] [Google Scholar]
- 20. Denkers EY, Gazzinelli RT. 1998. Regulation and function of T cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Denkers EY, Kim L, Butcher BA. 2003. In the belly of the beast: subversion of macrophage proinflammatory signaling cascades during Toxoplasma gondii infection. Cell. Micro. 5:75–83 [DOI] [PubMed] [Google Scholar]
- 22. Dubey JP. 2007. The history and life-cycle of Toxoplasma gondii, p 1–17 In Weiss LM, Kim K. (ed), Toxoplasma gondii. The model apicomplexan: perspective and methods. Academic Press, San Diego, CA [Google Scholar]
- 23. Fentress SJ, et al. 2010. Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe 8:484–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fox BA, Chaudhary K, Bzik DJ. 2007. Nucleotides and amino acids, p 265–387 In Ajioka JW, Soldati D. (ed), Toxoplasma: molecular and cellular biology. Horizon Bioscience, Norfolk, VA [Google Scholar]
- 25. Fox BA, Gigley JP, Bzik DJ. 2004. Toxoplasma gondii lacks the enzymes required for de novo arginine biosynthesis and arginine starvation triggers cyst formation. Int. J. Parasitol. 34:323–331 [DOI] [PubMed] [Google Scholar]
- 26. Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149:175–180 [PubMed] [Google Scholar]
- 27. Gazzinelli RT, Eltoum I, Wynn TA, Sher A. 1993. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J. Immunol. 151:3672–3681 [PubMed] [Google Scholar]
- 28. Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated T. gondii vaccine. J. Immunol. 146:286–292 [PubMed] [Google Scholar]
- 29. Gazzinelli RT, Hieny S, Wynn T, Wolf S, Sher A. 1993. IL-12 is required for the T-cell independent induction of IFN-γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. U. S. A. 90:6115–6119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gazzinelli RT, et al. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent upon CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J. Immunol. 157:798–805 [PubMed] [Google Scholar]
- 31. Gilbert LA, Ravindran S, Turetzky JM, Boothroyd JC, Bradley PJ. 2007. Toxoplasma gondii targets a protein phosphatase 2C to the nuclei of infected host cells. Eukaryot. Cell 6:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hakansson S, Charron AJ, Sibley LD. 2001. Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 20:3132–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heimesaat MM, et al. 2006. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 177:8785–8795 [DOI] [PubMed] [Google Scholar]
- 34. Heimesaat MM, et al. 2007. Exacerbation of murine ileitis by Toll-like receptor 4 meditated sensing of lipopolysaccharide from commensal Escherichia coli. Gut 56:941–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huynh M-H, et al. 2003. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 22:2082–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jensen KD, et al. 2011. Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe 9:472–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim L, Butcher BA, Denkers EY. 2004. Toxoplasma gondii interferes with lipopolysaccharide-induced mitogen-activated protein kinase activation by mechanisms distinct from endotoxin tolerance. J. Immunol. 172:3003–3010 [DOI] [PubMed] [Google Scholar]
- 38. Kim L, et al. 2006. Toxoplasma gondii genotype determines MyD88-dependent signaling in infected macrophages. J. Immunol. 177:2584–2591 [DOI] [PubMed] [Google Scholar]
- 39. Kim SK, Fouts AE, Boothroyd JC. 2007. Toxoplasma gondii dysregulates IFN-gamma-inducible gene expression in human fibroblasts: insights from a genome-wide transcriptional profiling. J. Immunol. 178:5154–5165 [DOI] [PubMed] [Google Scholar]
- 40. Lang C, Gross U, Luder CG. 2007. Subversion of innate and adaptive immune responses by Toxoplasma gondii. Parasitol. Res. 100:191–203 [DOI] [PubMed] [Google Scholar]
- 41. Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. 2002. Shaping gene expression in activated and resting primary macrophages by IL-10. J. Immunol. 169:2253–2263 [DOI] [PubMed] [Google Scholar]
- 42. Lang R, et al. 2003. SOCS regulates the plasticity of gp130 signaling. Nat. Immunol. 4:546–550 [DOI] [PubMed] [Google Scholar]
- 43. Lee CW, Bennouna S, Denkers EY. 2006. Screening for Toxoplasma gondii regulated transcriptional responses in LPS-activated macrophages. Infect. Immun. 74:1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee CW, Sukhumavasi W, Denkers EY. 2007. Phosphoinositide-3-kinase-dependent, MyD88-independent induction of CC-type chemokines characterizes the macrophage response to Toxoplasma gondii strains with high virulence. Infect. Immun. 75:5788–5797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leng J, Butcher BA, Egan CE, Abdallah DS, Denkers EY. 2009. Toxoplasma gondii prevents chromatin remodeling initiated by TLR-triggered macrophage activation. J. Immunol. 182:489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leng J, Denkers EY. 2009. Toxoplasma gondii inhibits covalent modification of histone H3 at the IL-10 promoter in infected macrophages. PLoS One 4:e7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liddiard K, et al. 2006. Interleukin-4 induction of the CC chemokine TARC (CCL17) in murine macrophages is mediated by multiple STAT6 sites in the TARC gene promoter. BMC Mol. Biol. 7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liesenfeld O, et al. 1999. TNF-α, nitric oxide and IFN-γ are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 21:365–376 [DOI] [PubMed] [Google Scholar]
- 49. Liesenfeld O, Kosek J, Remington JS, Suzuki Y. 1996. Association of CD4+ T cell-dependent, IFN-γ-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luder CGK, Algner M, Lang C, Bleicher N, Gross U. 2003. Reduced expression of the inducible nitric oxide synthase after infection with Toxoplasma gondii facilitates parasite replication in activated murine macrophages. Internat. J. Parasitol. 33:833–844 [DOI] [PubMed] [Google Scholar]
- 51. Luder CGK, Lang T, Beurle B, Gross U. 1998. Down-regulation of MHC class II molecules and inability to up-regulate class I molecules in murine macrophages after infection with Toxoplasma gondii. Clin. Exp. Immunol. 112:308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luder CGK, Walter W, Beuerle B, Maeurer MJ, Gross U. 2001. Toxoplasma gondii down-regulates MHC class II gene expression and antigen presentation by murine macrophages via interference with nuclear translocation of STAT1a. Eur. J. Immunol. 31:1475–1484 [DOI] [PubMed] [Google Scholar]
- 53. Manning G, Plowman GD, Hunter T, Sudarsanam S. 2002. Evolution of protein kinase signaling from yeast to man. Trends Biochem. Sci. 27:514–520 [DOI] [PubMed] [Google Scholar]
- 54. Manning G, Young SL, Miller WT, Zhai Y. 2008. The protist, Monosiga brevicollis, has a tyrosine kinase signaling network more elaborate and diverse than found in any known metazoan. Proc. Natl. Acad. Sci. U. S. A. 105:9674–9679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mayer BJ. 2008. Clues to the evolution of complex signaling machinery. Proc. Natl. Acad. Sci. U. S. A. 105:9453–9454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McKee AS, Dzierszinski F, Boes M, Roos DS, Pearce EJ. 2004. Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. J. immunol. 173:2632–2640 [DOI] [PubMed] [Google Scholar]
- 57. Mordue DG, Dessai N, Dustin M, Sibley LD. 1999. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J. Exp. Med. 190:1783–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mordue DG, Hakansson S, Niesman I, Sibley LD. 1999. Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp. Parasitol. 92:87–99 [DOI] [PubMed] [Google Scholar]
- 59. Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8:958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ness TL, Ewing JL, Hogaboam CM, Kunkel SL. 2006. CCR4 is a key modulator of innate immune responses. J. Immunol. 177:7531–7539 [DOI] [PubMed] [Google Scholar]
- 61. Ong YC, Reese ML, Boothroyd JC. 2010. Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. J. Biol. Chem. 285:28731–28740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Peixoto L, et al. 2010. Integrative genomic approaches highlight a family of parasite-specific kinases that regulate host responses. Cell Host Microbe 8:208–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Peterson E, Liesenfeld O. 2007. Clinical disease and diagnostics, p 81–100 In Weiss LM, Kim K. (ed), Toxoplasma gondii. The model apicomplexan: perspectives and methods. Academic Press, Amsterdam, Netherlands [Google Scholar]
- 64. Pincus D, Letunic I, Bork P, Lim WA. 2008. Evolution of the phospho-tyrosine signaling machinery in premetazoan lineages. Proc. Natl. Acad. Sci. U. S. A. 105:9680–9684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP. 2011. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc. Natl. Acad. Sci. U. S. A. 108:9625–9630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Reich NC, Liu L. 2006. Tracking STAT nuclear traffic. Nat. Rev. Immunol. 6:602–612 [DOI] [PubMed] [Google Scholar]
- 67. Reis e Sousa C, et al. 1997. In vivo microbial stimulation induces rapid CD40L-independent production of IL-12 by dendritic cells and their re-distribution to T cell areas. J. Exp. Med. 186:1819–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Robben PM, et al. 2004. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J. Immunol. 172:3686–3694 [DOI] [PubMed] [Google Scholar]
- 69. Rosowski EE, et al. 2011. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J. Exp. Med. 208:195–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Saeij JP, et al. 2006. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314:1780–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Saeij JP, et al. 2007. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445:324–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Scharton-Kersten T, Yap G, Magram J, Sher A. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 185:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Scharton-Kersten TM, et al. 1996. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 157:4045–4054 [PubMed] [Google Scholar]
- 74. Schwab JC, Beckers CJ, Joiner KA. 1994. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. U. S. A. 91:509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sehgal PB. 2008. Paradigm shifts in the cell biology of STAT signaling. Semin. Cell Dev. Biol. 19:329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shapira SS, Speirs K, Gerstein A, Caamano J, Hunter CA. 2002. Suppression of NF-kB activation by infection with Toxoplasma gondii. J. Infect. Dis. 185:S66–S72 [DOI] [PubMed] [Google Scholar]
- 77. Sibley LD. 2003. Toxoplasma gondii: perfecting an intracellular life style. Traffic 4:581–588 [DOI] [PubMed] [Google Scholar]
- 78. Sibley LD, Ajioka JW. 2008. Population structure of Toxoplasma gondii: clonal expansion driven by infrequent recombination and selective sweeps. Annu. Rev. Microbiol. 62:329–351 [DOI] [PubMed] [Google Scholar]
- 79. Steinfeldt T, et al. 2010. Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol. 8:e1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Suss-Toby E, Zimmerberg EJ, Ward GE. 1996. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fusion pore. Proc. Natl. Acad. Sci. U. S. A. 93:8413–8418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Suzuki Y, Orellana MA, Schreiber RD, Remington JS. 1988. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518 [DOI] [PubMed] [Google Scholar]
- 82. Suzuki Y, Remington JS. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt1+, L3T4+ T cells in mice. J. Immunol. 140:3943–3946 [PubMed] [Google Scholar]
- 83. Suzuki Y, et al. 2000. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J. Immunol. 164:5375–5382 [DOI] [PubMed] [Google Scholar]
- 84. Takeda K, et al. 1999. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of stat3 in macrophages and neutrophils. Immunity 10:39–49 [DOI] [PubMed] [Google Scholar]
- 85. Tonkin ML, et al. 2011. Host cell invasion by apicomplexan parasites: insights from the co-structure of AMA1 with a RON2 peptide. Science 333:463–467 [DOI] [PubMed] [Google Scholar]
- 86. Whitmarsh RJ, et al. 2011. A critical role for SOCS3 in innate resistance to Toxoplasma gondii. Cell Host Microbe 10:224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wirnsberger G, Hebenstreit D, Posselt G, Horejs-Hoeck J, Duschl A. 2006. IL-4 induces expression of TARC/CCL17 via two STAT6 binding sites. Eur. J. Immunol. 36:1882–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yamamoto M, et al. 2011. ATF6{beta} is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J. Exp. Med. doi:10.1084/jem.20101660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yamamoto M, et al. 2009. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J. Exp. Med. 206:2747–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yarovinsky F, et al. 2005. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308:1626–1629 [DOI] [PubMed] [Google Scholar]
- 91. Yasukawa H, et al. 2003. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat. Immunol. 4:551–556 [DOI] [PubMed] [Google Scholar]
- 92. Zimmermann S, Murray PJ, Heeg K, Dalpke AH. 2006. Induction of suppressor of cytokine signaling-1 by Toxoplasma gondii contributes to immune evasion in macrophages by blocking IFN-{gamma} signaling. J. Immunol. 176:1840–1847 [DOI] [PubMed] [Google Scholar]