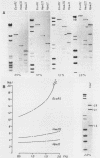
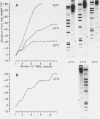
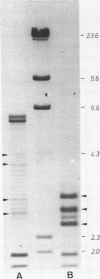
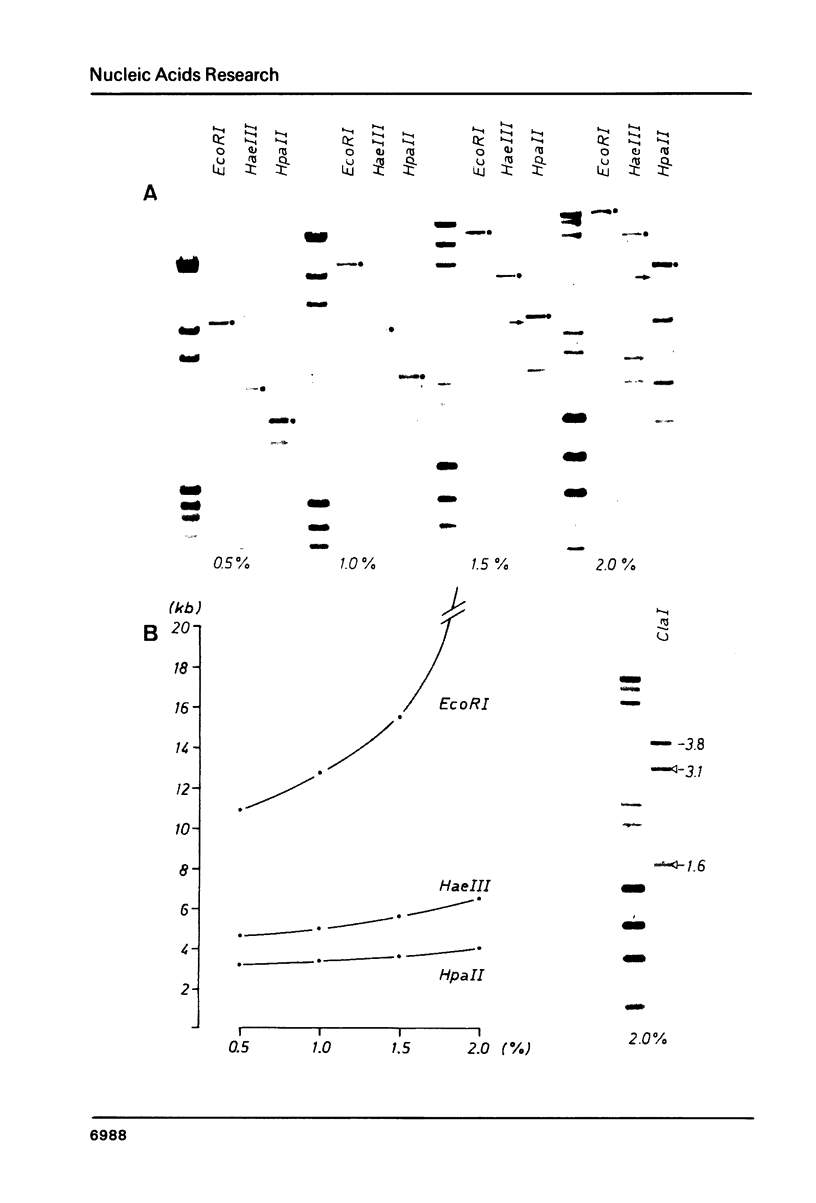
Abstract
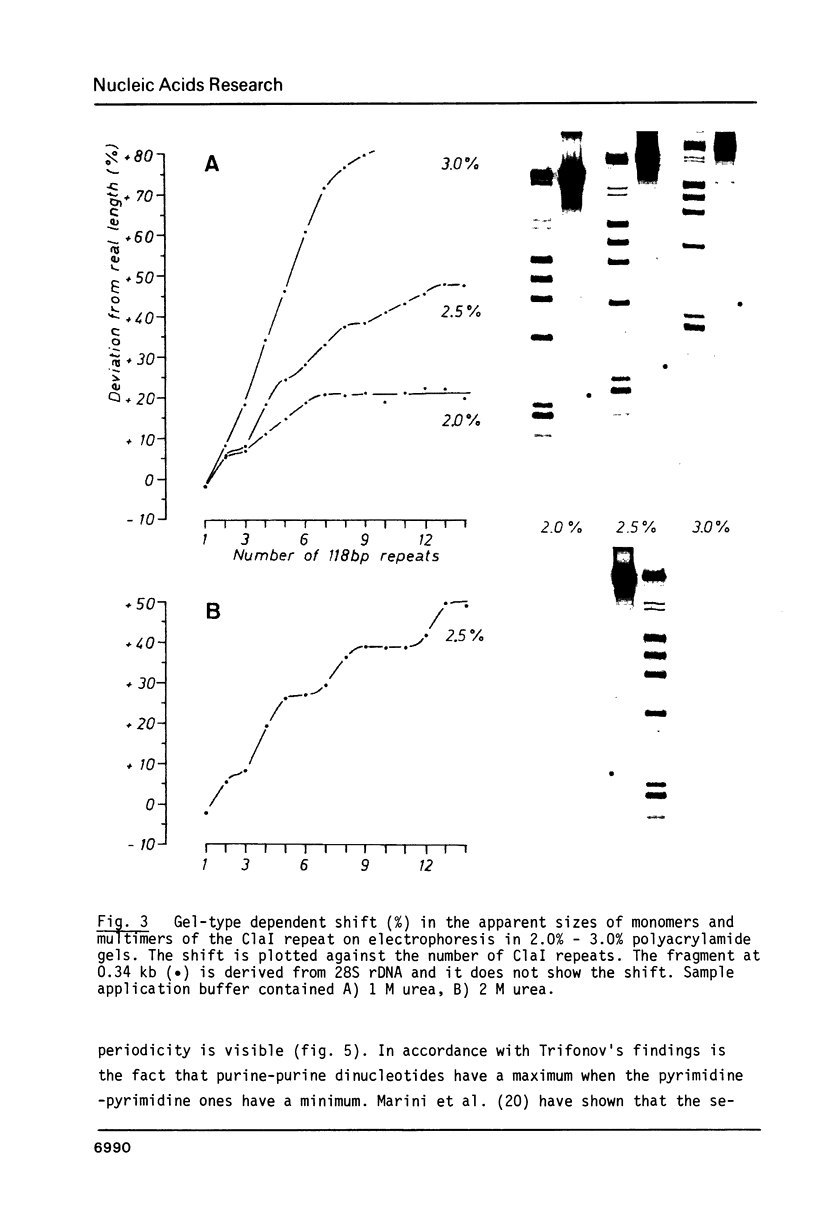
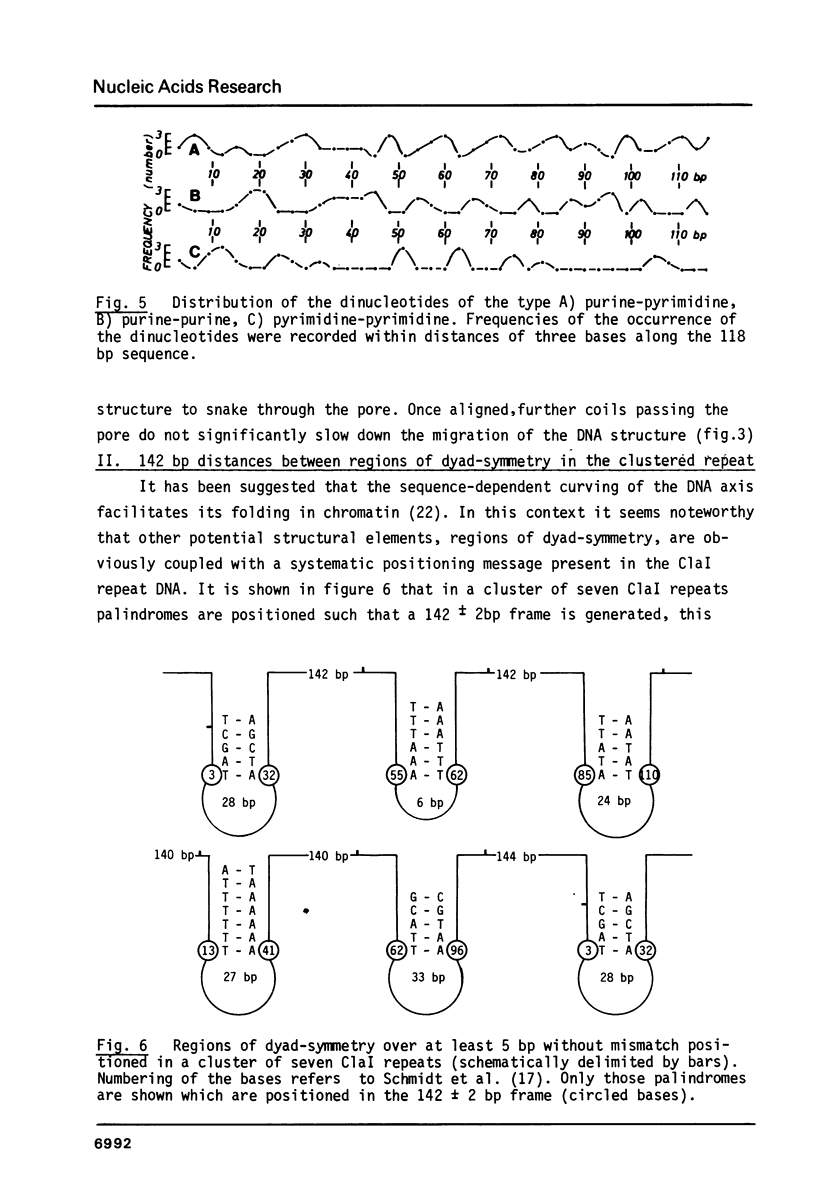
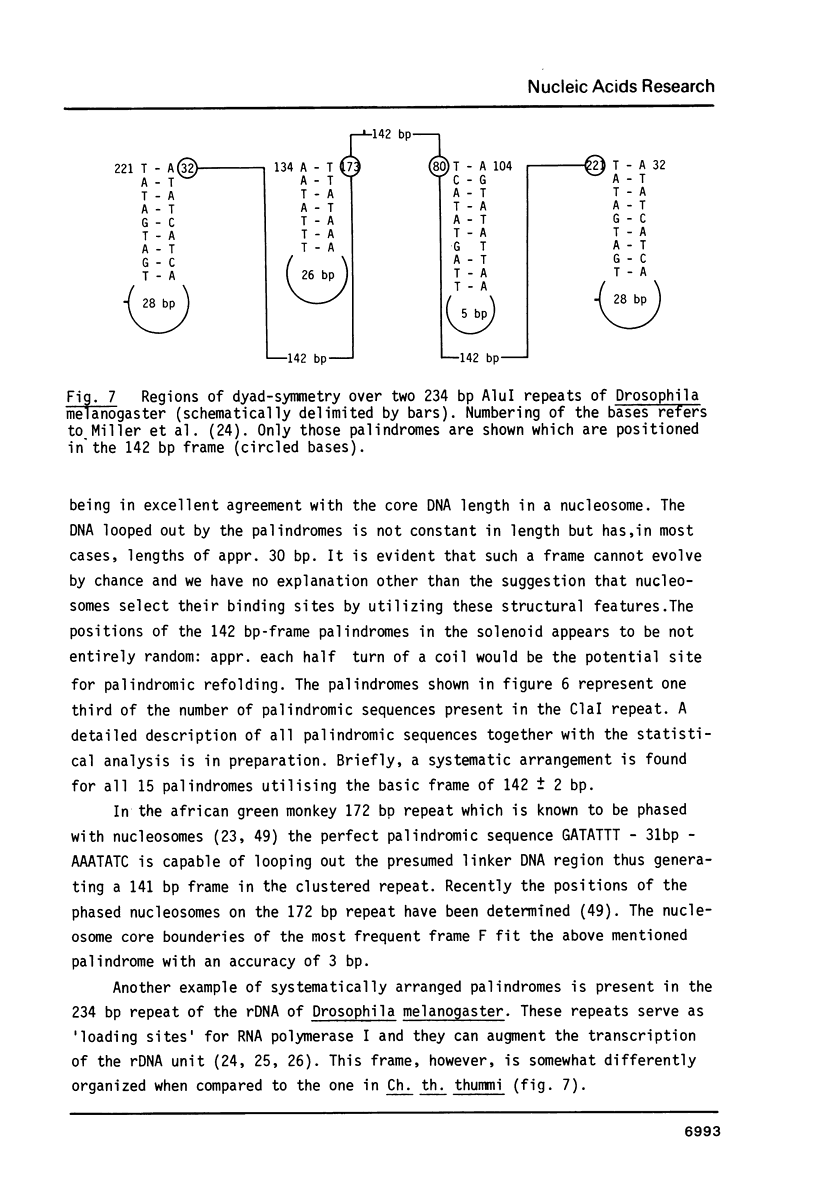
Chironomus thummi thummi contains a repetitive AT-rich 118 bp sequence mainly in the centromere regions and elsewhere in the genome (1). A large cluster of repeats is regularly present in the non-transcribed spacer of rDNA. Dimers and multimers of the repeat migrate slower in small pore gels than would be expected from their size. The results indicate a solenoidal structure with a coil girth of appr. 350 bp. This structure is most probably due to a highly periodic positioning of di-nucleotides of the type purine - purine or pyrimidine-pyrimidine with distances of appr. 10 bases. In a cluster of 118 bp repeats, regions of dyad-symmetry are positioned such that a 142 +/- 2 bp palindrome-frame is generated. Evidence is presented favouring the assumption that the repeat functions primarily in sister chromatid exchange.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleaver J. E. Correlations between sister chromatid exchange frequencies and replicon sizes. A model for the mechanism of SCE production. Exp Cell Res. 1981 Nov;136(1):27–30. doi: 10.1016/0014-4827(81)90034-3. [DOI] [PubMed] [Google Scholar]
- Coen E. S., Dover G. A. Multiple Pol I initiation sequences in rDNA spacers of Drosophila melanogaster. Nucleic Acids Res. 1982 Nov 11;10(21):7017–7026. doi: 10.1093/nar/10.21.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff N. V., Brown D. D. The nucleotide sequence of the repeating unit in the oocyte 5S ribosomal DNA of Xenopus laevis. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1195–1200. doi: 10.1101/sqb.1978.042.01.120. [DOI] [PubMed] [Google Scholar]
- Grindley N. D., Lauth M. R., Wells R. G., Wityk R. J., Salvo J. J., Reed R. R. Transposon-mediated site-specific recombination: identification of three binding sites for resolvase at the res sites of gamma delta and Tn3. Cell. 1982 Aug;30(1):19–27. doi: 10.1016/0092-8674(82)90007-1. [DOI] [PubMed] [Google Scholar]
- Hennig W. Molecular hybridization of DNA and RNA in situ. Int Rev Cytol. 1973;36:1–44. doi: 10.1016/s0074-7696(08)60214-4. [DOI] [PubMed] [Google Scholar]
- Hägele K. Differential staining of polytene chromosome bands in Chironomus by Giemsa banding methods. Chromosoma. 1977 Feb 3;59(3):207–216. doi: 10.1007/BF00292778. [DOI] [PubMed] [Google Scholar]
- Israelewski N., Schmidt E. R. Spacer size heterogeneity in ribosomal DNA of Chironomus thummi is due to a 120 bp repeat homologous to a predominantly centromeric repeated sequence. Nucleic Acids Res. 1982 Dec 11;10(23):7689–7700. doi: 10.1093/nar/10.23.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John B. The cytogenetic systems of grasshoppers and locusts. II. The origin and evolution of supernumerary segments. Chromosoma. 1973 Nov 21;44(2):123–146. doi: 10.1007/BF00329114. [DOI] [PubMed] [Google Scholar]
- Kato H. Spontaneous and induced sister chromatid exchanges as revealed by the BUdR-labeling method. Int Rev Cytol. 1977;49:55–97. doi: 10.1016/s0074-7696(08)61947-6. [DOI] [PubMed] [Google Scholar]
- Keyl H. G. Duplikationen von Untereinheiten der Chromosomalen DNS während der Evolution von Chironomus thummi. Chromosoma. 1965;17(2):139–180. doi: 10.1007/BF00330079. [DOI] [PubMed] [Google Scholar]
- Keyl H. G. Lampbrush chromosomes in spermatocytes of Chironomus. Chromosoma. 1975;51(1):75–91. doi: 10.1007/BF00285810. [DOI] [PubMed] [Google Scholar]
- Kohorn B. D., Rae P. M. Nontranscribed spacer sequences promote in vitro transcription of Drosophila ribosomal DNA. Nucleic Acids Res. 1982 Nov 11;10(21):6879–6886. doi: 10.1093/nar/10.21.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhotia S. C., Mishra A. Fluorescence patterns of heterochromatin in mitotic and polytene chromosomes in seven members of three sub-groups of the melanogaster species group of Drosophila. Chromosoma. 1980;81(1):137–150. doi: 10.1007/BF00292428. [DOI] [PubMed] [Google Scholar]
- Liu L. S., Lark K. G. The Red function of phage lambda mediates the alteration of an interspersed repeated DNA sequence from the kangaroo rat Dipodomys ordii. Mol Gen Genet. 1982;188(1):27–36. doi: 10.1007/BF00332992. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Marini J. C., Levene S. D., Crothers D. M., Englund P. T. Bent helical structure in kinetoplast DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7664–7668. doi: 10.1073/pnas.79.24.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklos G. L., Nankivell R. N. Telomeric satellite DNA functions in regulating recombination. Chromosoma. 1976 Jun 30;56(2):143–167. doi: 10.1007/BF00293113. [DOI] [PubMed] [Google Scholar]
- Miller J. R., Hayward D. C., Glover D. M. Transcription of the 'non-transcribed' spacer of Drosophila melanogaster rDNA. Nucleic Acids Res. 1983 Jan 11;11(1):11–19. doi: 10.1093/nar/11.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau J., Marcaud L., Maschat F., Kejzlarova-Lepesant J., Lepesant J. A., Scherrer K. A + T-rich linkers define functional domains in eukaryotic DNA. Nature. 1982 Jan 21;295(5846):260–262. doi: 10.1038/295260a0. [DOI] [PubMed] [Google Scholar]
- Moreau J., Matyash-Smirniaguina L., Scherrer K. Systematic punctuation of eukaryotic DNA by A+T-rich sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1341–1345. doi: 10.1073/pnas.78.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musich P. R., Brown F. L., Maio J. J. Nucleosome phasing and micrococcal nuclease cleavage of African green monkey component alpha DNA. Proc Natl Acad Sci U S A. 1982 Jan;79(1):118–122. doi: 10.1073/pnas.79.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D. Unequal meiotic recombination within tandem arrays of yeast ribosomal DNA genes. Cell. 1980 Mar;19(3):765–774. doi: 10.1016/s0092-8674(80)80052-3. [DOI] [PubMed] [Google Scholar]
- Schaefer J., Schmidt E. R. Different repetition frequencies of a 120 base-pair DNA-element and its arrangement in Chironomus thummi thummi and Chironomus thummi piger. Chromosoma. 1981;84(1):61–66. doi: 10.1007/BF00293363. [DOI] [PubMed] [Google Scholar]
- Schmidt E. R., Godwin E. A., Keyl H. G., Israelewski N. Cloning and analysis of ribosomal DNA of Chironomus thummi piger and Chironomus thummi thummi. The nontranscribed spacer of Ch. th. thummi contains a highly repetitive DNA sequence. Chromosoma. 1982;87(4):389–407. doi: 10.1007/BF00327181. [DOI] [PubMed] [Google Scholar]
- Schmidt E. R., Godwin E. A. The nucleotide sequence of an unusual non-transcribed spacer and its ancestor in the rDNA in Chironomus thummi. EMBO J. 1983;2(7):1177–1183. doi: 10.1002/j.1460-2075.1983.tb01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. R., Stern H. Repeated DNA synthesized during pachytene in Lilium henryi. Nat New Biol. 1973 Sep 19;245(142):94–96. doi: 10.1038/newbio245094a0. [DOI] [PubMed] [Google Scholar]
- Sun Y. L., Xu Y. Z., Chambon P. A simple and efficient method for the separation and detection of small DNA fragments by electrophoresis in formamide containing agarose gels and Southern blotting to DBM-paper. Nucleic Acids Res. 1982 Oct 11;10(19):5753–5763. doi: 10.1093/nar/10.19.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Wu R. Unequal crossing over in the ribosomal DNA of Saccharomyces cerevisiae. Nature. 1980 Apr 3;284(5755):426–430. doi: 10.1038/284426a0. [DOI] [PubMed] [Google Scholar]
- Trifonov E. N. Sequence-dependent deformational anisotropy of chromatin DNA. Nucleic Acids Res. 1980 Sep 11;8(17):4041–4053. doi: 10.1093/nar/8.17.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifonov E. N., Sussman J. L. The pitch of chromatin DNA is reflected in its nucleotide sequence. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3816–3820. doi: 10.1073/pnas.77.7.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF W. El problema da la etiología de la obesidad. Rev Iber Endocrinol. 1957 Mar-Apr;4(20):169–180. [PubMed] [Google Scholar]
- Westergaard M., von Wettstein D. The synaptinemal complex. Annu Rev Genet. 1972;6:71–110. doi: 10.1146/annurev.ge.06.120172.000443. [DOI] [PubMed] [Google Scholar]
- Wolff S., Bodycote J., Painter R. B. Sister chromatid exchanges induced in Chinese hamster cells by UV irradiation of different stages of the cell cycle: the necessity for cells to pass through S. Mutat Res. 1974 Oct;25(1):73–81. doi: 10.1016/0027-5107(74)90220-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto M. Interchromosomal effects of heterochromatic deletions on recombination in Drosophila melanogaster. Genetics. 1979 Oct;93(2):437–448. doi: 10.1093/genetics/93.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Miklos G. L. Genetic studies on heterochromatin in Drosophila melanogaster and their implications for the functions of satellite DNA. Chromosoma. 1978 Mar 22;66(1):71–98. doi: 10.1007/BF00285817. [DOI] [PubMed] [Google Scholar]
- Zhang X. Y., Fittler F., Hörz W. Eight different highly specific nucleosome phases on alpha-satellite DNA in the African green monkey. Nucleic Acids Res. 1983 Jul 11;11(13):4287–4306. doi: 10.1093/nar/11.13.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]