Abstract
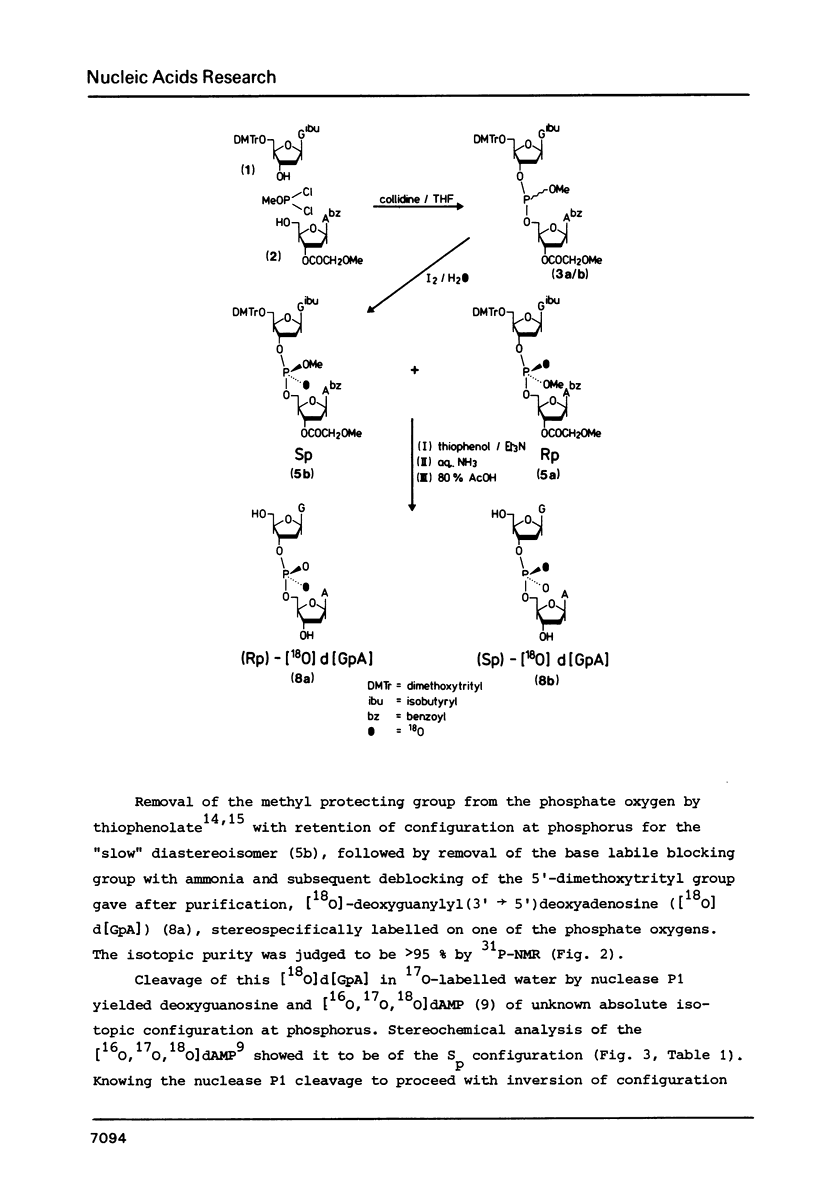
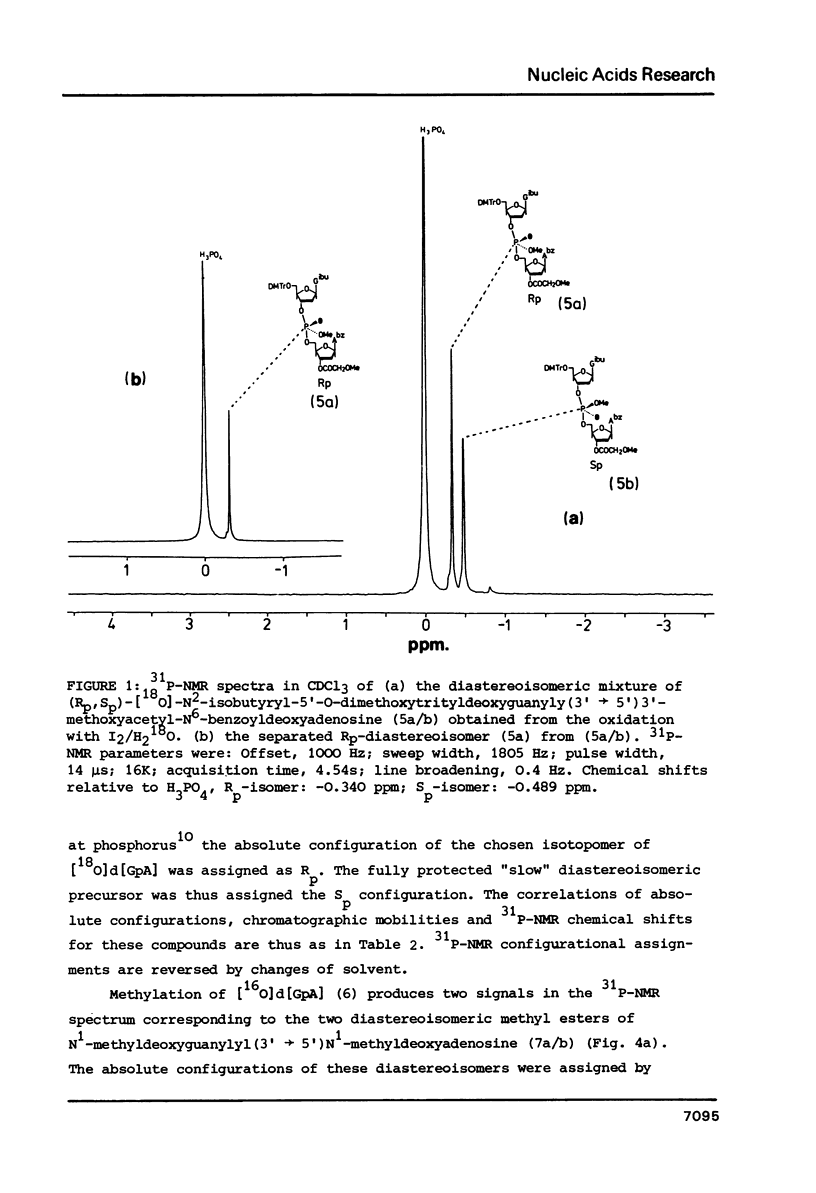
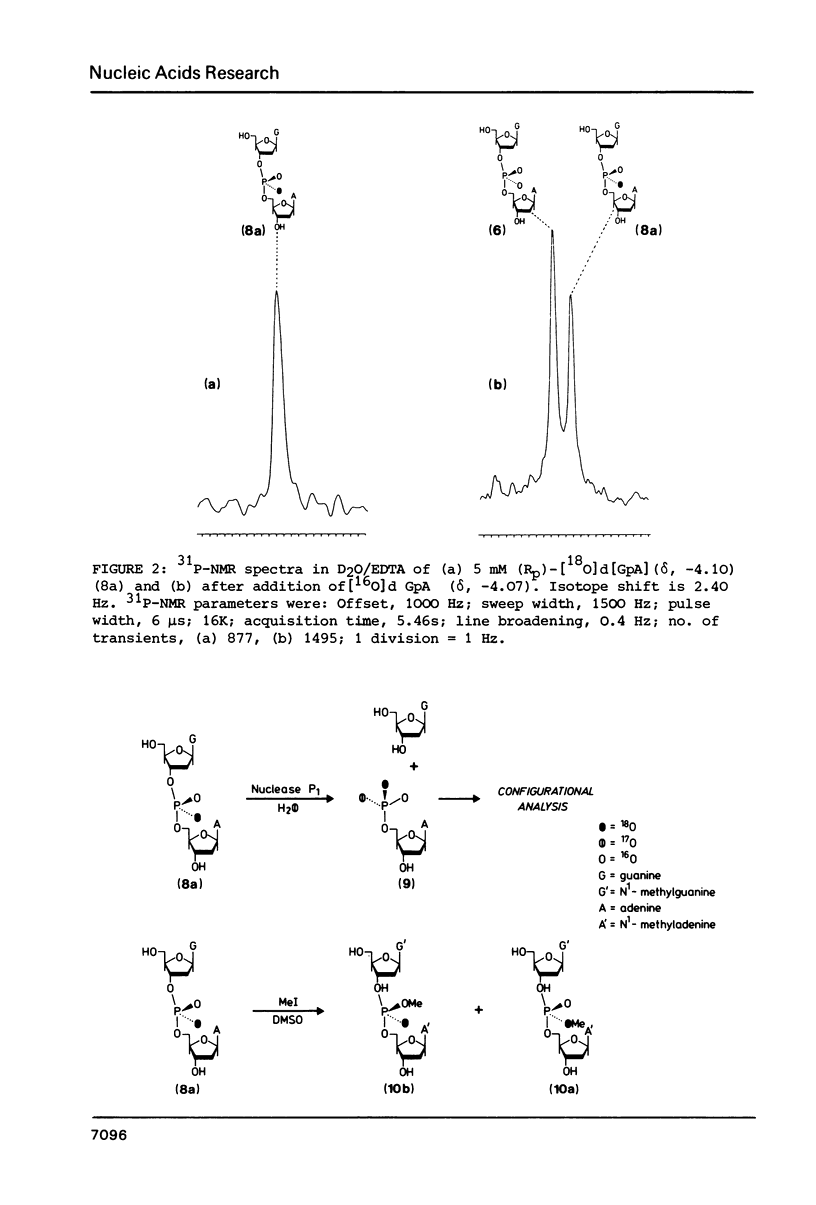
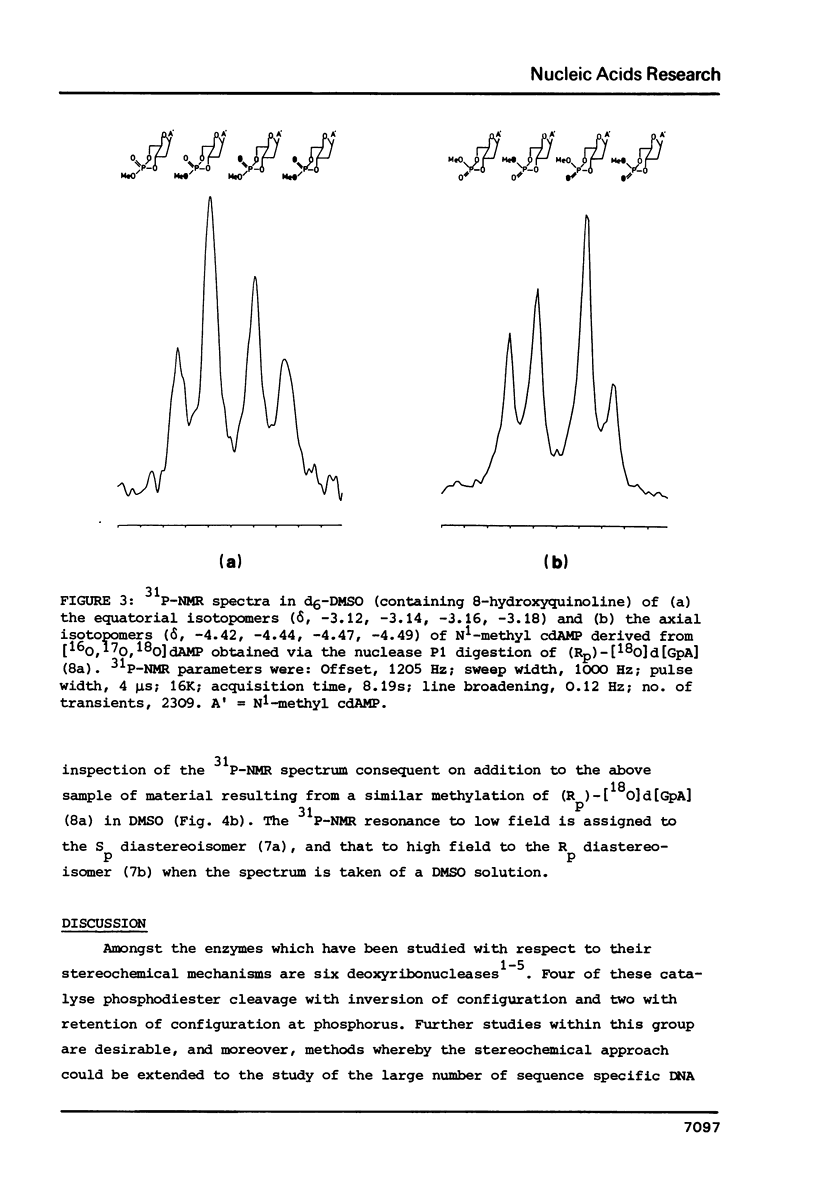
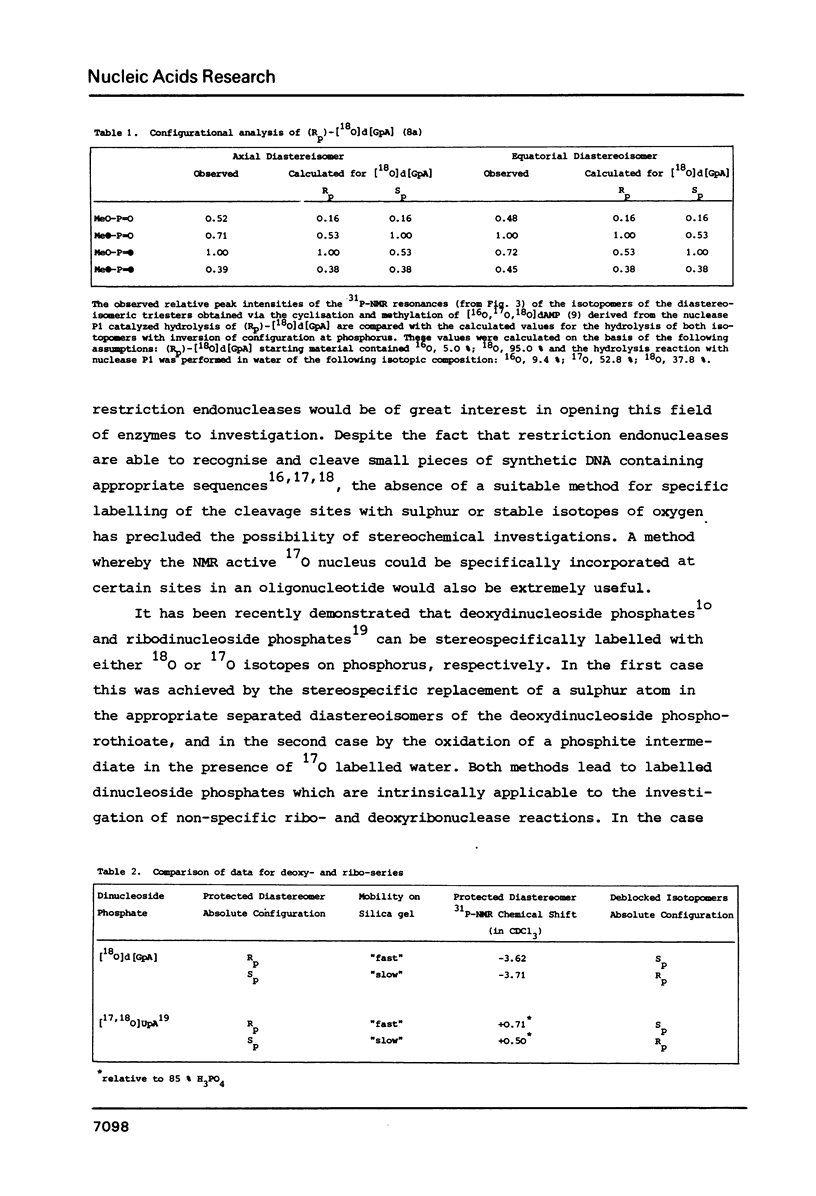
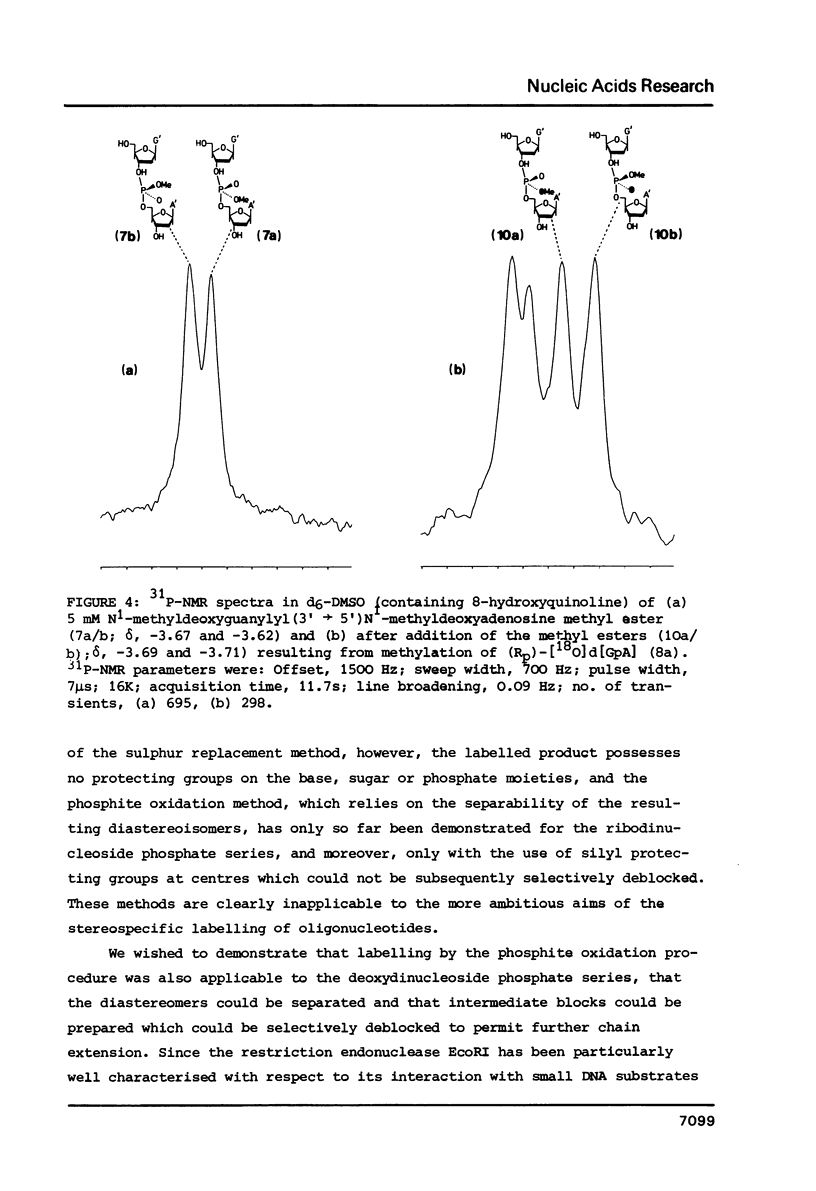
Fully protected diastereoisomers of deoxyguanylyl (3' leads to 5') deoxyadenosine stereospecifically labelled on phosphorus with oxygen-18 have been synthesized by oxidation of phosphite triester intermediates in the presence of 18O-labelled water. The diastereoisomers have been chromatographically separated and their absolute configuration at phosphorus determined. (Rp)-[18O]deoxyguanylyl (3' leads to 5')deoxyadenosine has been prepared by complete deprotection of the parent diastereoisomer of the Sp configuration. Methylation of the former compound permits assignment of the absolute configurations of the methyl esters of N1-methyldeoxyguanylyl (3' leads to 5') N1-methyldeoxyadenosine.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daub G. W., van Tamelen E. E. Synthesis of oligoribonucleotides based on the facile cleavage of methyl phosphotriester intermediates. J Am Chem Soc. 1977 May 11;99(10):3526–3528. doi: 10.1021/ja00452a069. [DOI] [PubMed] [Google Scholar]
- Dwyer-Hallquist P., Kézdy F. J., Agarwal K. L. Interaction of the HpaI endonuclease with synthetic oligonucleotides. Biochemistry. 1982 Sep 14;21(19):4693–4700. doi: 10.1021/bi00262a027. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Romaniuk P. J., Connolly B. A. Stereochemistry of enzymic phosphoryl and nucleotidyl transfer. Methods Enzymol. 1982;87:197–212. doi: 10.1016/s0076-6879(82)87015-8. [DOI] [PubMed] [Google Scholar]
- Goppelt M., Pingoud A., Maass G., Mayer H., Köster H., Frank R. The interaction of the EcoRI restriction endonuclease with its substrate. A physico-chemical study employing natural and synthetic oligonucleotides and polynucleotides. Eur J Biochem. 1980 Feb;104(1):101–107. doi: 10.1111/j.1432-1033.1980.tb04405.x. [DOI] [PubMed] [Google Scholar]
- Greene P. H., Poonian M. S., Nussbaum A. L., Tobias L., Garfin D. E., Boyer H. W., Goodman H. M. Restriction and modification of a self-complementary octanucleotide containing the EcoRI substrate. J Mol Biol. 1975 Dec 5;99(2):237–261. doi: 10.1016/s0022-2836(75)80143-4. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem. 1980;49:877–919. doi: 10.1146/annurev.bi.49.070180.004305. [DOI] [PubMed] [Google Scholar]
- Letsinger R. L., Lunsford W. B. Synthesis of thymidine oligonucleotides by phosphite triester intermediates. J Am Chem Soc. 1976 Jun 9;98(12):3655–3661. doi: 10.1021/ja00428a045. [DOI] [PubMed] [Google Scholar]
- Potter B. V., Connolly B. A., Eckstein F. Synthesis and configurational analysis of a dinucleoside phosphate isotopically chiral at phosphorus. Stereochemical course of Penicillium citrum nuclease P1 reaction. Biochemistry. 1983 Mar 15;22(6):1369–1377. doi: 10.1021/bi00275a008. [DOI] [PubMed] [Google Scholar]


