Abstract
Invasion of intestinal epithelial cells is a critical step in Salmonella infection and requires the expression of genes located in Salmonella pathogenicity island 1 (SPI-1). A key factor for SPI-1 expression is DNA adenine (Dam) methylation, which activates synthesis of the SPI-1 transcriptional activator HilD. Dam-dependent regulation of hilD is postranscriptional (and therefore indirect), indicating the involvement of unknown cell functions under Dam methylation control. A genetic screen has identified the std fimbrial operon as the missing link between Dam methylation and SPI-1. We show that all genes in the std operon are part of a single transcriptional unit, and describe three previously uncharacterized ORFs (renamed stdD, stdE, and stdF). We present evidence that two such loci (stdE and stdF) are involved in Dam-dependent control of Salmonella SPI-1: in a Dam− background, deletion of stdE or stdF suppresses SPI-1 repression; in a Dam+ background, constitutive expression of StdE and/or StdF represses SPI-1. Repression of SPI-1 by products of std operon explains the invasion defect of Salmonella Dam− mutants, which constitutively express the std operon. Dam-dependent repression of std in the ileum may be required to permit invasion, as indicated by two observations: constitutive expression of StdE and StdF reduces invasion of epithelial cells in vitro (1,000 fold) and attenuates Salmonella virulence in the mouse model (>60 fold). In turn, crosstalk between std and SPI-1 may play a role in intestinal infections by preventing expression of SPI-1 in the caecum, an intestinal compartment in which the std operon is known to be expressed.
Introduction
Salmonella enterica is a Gram-negative bacterium that causes intestinal and systemic diseases in a variety of animal hosts [1]. Salmonella is a typical foodborne pathogen, and infection usually starts by the ingestion of contaminated food or water [2]. Salmonella has the ability to penetrate epithelial cells in the small intestine, a process known as invasion [3], [4]. After invasion, the infection can remain localized in the intestine, producing gastroenteritis. In specific serovar-host combinations, however, Salmonella can cross the intestinal epithelial barrier and disseminate inside the host, producing a systemic, life-threatening infection (e. g., typhoid fever in humans). It has been estimated that >90 million cases of Salmonella-associated gastroenteritis and >20 million cases of typhoid fever occur per year worldwide, resulting in 155,000 and 200,000 deaths respectively [5], [6].
Salmonella and Escherichia are close relatives, and it has been estimated that genus divergence occurred 120–160 million years ago [7]. Salmonella pathogenicity has evolved by sequential acquisition of genetic elements, each contributing to distinct aspects of virulence [8], [9]. Amongst those elements are the Salmonella pathogenicity islands (SPIs), which are clusters of virulence genes located in the chromosome. More than 10 SPIs have been described [10], although some of them are serotype-specific. These regions are absent in the chromosome of other enterics and usually have a G+C content different from that of the Salmonella chromosome, suggesting that they have been acquired by horizontal transfer [9], [11]. This view is supported by two additional lines of evidence. First, SPIs are frequently inserted at tRNA genes [10], which are hotspots for the integration of foreign DNA elements [12], [13]. Second, some SPIs contain putative homologs of genes encoding integrases or transposases, and are flanked by direct repeats [8], [10].
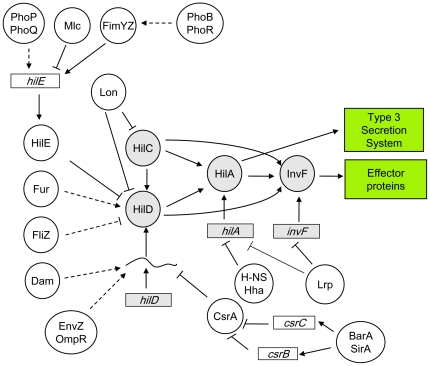
Salmonella infection requires coordinated expression of virulence genes. During evolution, SPIs have been integrated into pre-existing regulatory networks, thus resulting in crosstalk between the Salmonella core genome and horizontally-acquired genetic elements [8]. One of the best characterized SPIs is the Salmonella pathogenicity island 1 (SPI-1), which is necessary for invasion of epithelial cells in the animal intestine. SPI-1 encodes a type 3 secretion system (TTSS) as well as effector proteins that are translocated into the eukaryotic cell cytoplasm [14]–[16]. SPI-1 expression is controlled by four SPI-1-encoded transcriptional activators: HilA, HilC, HilD, and InvF [14], [17]–[19]. These regulators form a regulatory network that incorporates regulatory inputs from global regulators. For instance, the leucine-responsive regulatory protein, Lrp, reduces SPI-1 expression by repressing transcription of hilA and invF [20]. The nucleoid-associated proteins H-NS and Hha repress hilA expression by direct binding to regions located upstream and downstream the hilA promoter [21], [22]. HilC and HilD are substrates for the ATP-dependent Lon protease [23], which contributes to SPI-1 repression after invasion of epithelial cells [24]. HilE is a negative regulator of SPI-1 [25] and may interfere with HilD function by direct protein-protein interaction [26]. Transcription of hilE is activated by the fimbrial regulator FimYZ [27], and is repressed by the PTS-dependent regulator Mlc [28], thus transmitting inputs to SPI-1 through HilD. In addition, the two-component systems PhoP/PhoQ and PhoB/PhoR may activate hilE expression [18], [19]. SP1-1 is also regulated by the Csr system [29]. Overexpression of csrA represses SPI-1 expression [29], [30]. CsrA binds to a region in hilD mRNA that overlaps with the ribosome-binding sequence, likely preventing translation and accelerating mRNA decay [30]. The two-component regulatory system BarA/SirA activates SPI-1 expression through the Csr pathway, activating transcription of the csrB and csrC genes, which encode CsrA antagonists [31]. Another activator of SPI-1 is the ferric uptake regulator, Fur, and the mechanism of regulation is controversial [32]–[34]. The EnvZ/OmpR two-component system activates SPI-1, likely by controlling hilD expression at the postranscriptional level [18], [35]. Furthermore, a recent report shows that FliZ, an RpoS inhibitor [36], activates SPI-1 expression by controlling HilD activity [37]. A diagram that summarizes SPI-1 regulation is shown in Figure 1 .
Figure 1. Diagram of SPI-1 regulation.
Boxes represent genes, and circles represent proteins. Grey boxes and grey circles indicate SPI-1-encoded regulators. White circles indicate regulators encoded outside SPI-1.
In previous studies, we showed that DNA adenine (Dam) methylation is necessary to sustain a high level of SPI-1 expression [38]–[40]. Genetic analysis indicated that Dam-dependent regulation of SPI-1 is transmitted via HilD [39]. However, Dam-dependent regulation of hilD is not transcriptional but postranscriptional [39], and several lines of evidence suggest that a postranscriptional regulator whose synthesis is Dam-dependent may control hilD mRNA stability [39]. In this study, we show that Dam-dependent postranscriptional regulation of hilD is exerted by products encoded on another horizontally-acquired genetic element, the std fimbrial cluster. Std fimbriae belong to the chaperone-usher-dependent assembly class [41], [42]. StdA constitutes the major fimbrial subunit [43], [44], while StdB and StdC are a putative outer membrane usher protein and a putative periplasmic chaperone, respectively [45]. We have characterized 3 additional genes of unknown function in the std cluster, and have renamed them stdD, stdE, and stdF. Products of two such genes, stdE and stdF, turn out to be the molecular link between Dam methylation and SPI-1: lack of Dam methylation permits expression of the std operon, and the StdE and StdF products directly or indirectly downregulate hilD mRNA. These findings contribute to understand the invasion defect of Salmonella Dam− mutants [40], [46] and the relief of virulence attenuation observed in Dam− Std− mutants [47]. Furthermore, we show that constitutive expression of StdE and StdF reduces invasion of epithelial cells and impairs S. enterica virulence in the mouse model. StdEF-mediated repression of SPI-1 may be an example of coordination in the control of virulence genes, preventing synthesis of the invasion machinery in enviroments which are not appropriate for invasion. One such compartment may be the caecum, an intestinal section in which Std fimbriae are known to be produced [48], [49].
Materials and Methods
Bacterial strains, bacteriophages, and standard strain construction
All the Salmonella enterica strains listed in Table S1 belong to serovar Typhimurium, and derive from the mouse virulent strain ATCC 14028. For simplicity, Salmonella enterica serovar Typhimurium is often abbreviated as S. enterica. Targeted gene disruption was achieved using pKD4 or pKD13 [50]. Antibiotic resistance cassettes introduced during strain construction were excised by recombination with plasmid pCP20 [50]. The oligonucleotides used for disruption (labeled “UP” and “DO”) are listed in Table S2, together with the oligonucleotides (labeled “E”) used for allele verification by the polymerase chain reaction. For the construction of transcriptional and translational lac fusions in the Salmonella chromosome, FRT sites generated by excision of Kmr cassettes [50] were used to integrate either pCE37 or pCE40 [51]. Addition of a 3xFLAG epitope tag to protein-coding DNA sequences was carried out using plasmid pSUB11 (Kmr, 3xFLAG) [52]. Transductional crosses using phage P22 HT 105/1 int201 ([53] and G. Roberts, unpublished) were used for strain construction operations involving chromosomal markers. The transduction protocol was described elsewhere [54]. To obtain phage-free isolates, transductants were purified by streaking on green plates. Phage sensitivity was tested by cross-streaking with the clear-plaque mutant P22 H5.
Growth conditions
Luria-Bertani (LB) broth was used as standard liquid medium. Solid media contained 1.5% agar. When needed, kanamycin sulfate (Km), chloramphenicol (Cm), or ampicillin (Ap) was added to LB at a final concentration of 50 µg/ml, 20 µg/ml and 100 µg/ml, respectively. Green plates were prepared according to Chan and co-workers [55], except that methyl blue (Sigma Chemical Co., St. Louis, MO) substituted for aniline blue. Plate tests for monitoring ß-galactosidase activity used 5-bromo-4-chloro-3-indolyl-ß-D-galactopyranoside (“X-gal”, Sigma Chemical Co.) as indicator. To monitor expression of SPI-1 genes by ß-galactosidase assays, Western blotting, or Northern blotting, saturated cultures were diluted 1∶50 in LB and incubated at 37°C with shaking (200 r. p. m.). Samples were taken when the cultures had reached the stationary phase (O.D.600 = 2.0−2.5).
Construction of a pBR328-based plasmid library of Salmonella genome
Genomic DNA from Salmonella enterica serovar Typhimurium ATCC 14028 was partially digested with Sau3A. DNA fragments 7–11 kb long were ligated to the pBR328 vector, previously digested with BamHI and dephosphorylated. Salmonella strain TR5878 was transformed with the ligation products, and ampicillin-resistant colonies were selected on LB+Ap plates. Pools of ∼1,000 independent transformants were collected and lysed with phage P22 HT 105/1 int201. As a quality control, we tested the ability of the library pools to complement null mutations in araA (required for growth with L-arabinose as the sole carbon source) or xylA (required for growth with D-xylose as the sole carbon source). Lysates that permitted sucessful complementation were stored and used for plasmid delivery to recipient strains in subsequent genetic screens.
Construction of relevant strains
PLtetO -stdEF and PLtetO -stdF constructions were engineered by inserting the PLtetO promoter [56] upstream stdE and stdF (respectively) on the Salmonella chromosome. In both constructions, PLtetO insertion removed the upstream genes in the std operon and the native promoter upstream stdA. A fragment containing the cat gene and the PLtetO promoter was amplified by PCR using pXG1 as template [57]. The primers were labelled PLtetOUP and PLtetODO (Table S2). The PCR product was treated with DpnI to remove template traces. The construction was inserted in the chromosome by Lambda Red recombinase-mediated recombination [50], and Cmr colonies were selected. Insertion of the construct was verified by PCR, using a pair of primers specific for the cat gene and the target gene (Table S2).
Protein extracts and Western blot analysis
Total protein extracts were prepared from bacterial cultures grown at 37°C in LB until stationary phase (final O.D.600∼2.5). Bacterial cells were collected by centrifugation (16,000 g, 2 min) and suspended in 100 µl of Laemmli sample buffer [1.3% SDS, 10% (v/v) glycerol, 50 mM Tris-HCl, 1.8% ß-mercaptoethanol, 0.02% bromophenol blue, pH 6.8]. Proteins were resolved by Tris-Tricine-PAGE (12%). Conditions for protein transfer have been described elsewhere [47]. Optimal dilutions of primary antibodies were as follows: anti-FLAG M2 monoclonal antibody (Sigma Chemical Co.), 1∶5,000; anti-GroEL polyclonal antibody (Sigma Chemical Co.), 1∶20,000. Goat anti-mouse horseradish peroxidase-conjugated antibody (1∶5,000, BioRad, Hercules, CA) or Goat anti-rabbit horseradish peroxidase conjugated antibody (1∶20,000, Santa Cruz Biotechnology, Heidelberg, Germany) were used as secondary antibodies. Proteins recognized by the antibodies were visualized by chemoluminescence using luciferin-luminol reagents in a LAS 3000 Mini Imaging System (Fujifilm, Tokyo, Japan). For quantification, the intensity of the bands was determined using MultiGauge software (Fujifilm). GroEL was used as loading control.
Co-transcription analysis of std genes
RNA used for retrotranscription was extracted from S. enterica cultures grown in LB to stationary phase (O.D.600∼2.5) using the SV total RNA isolation system (Promega Co., Madison, WI) as described at http://www.ifr.ac.uk/safety/microarrays/protocols.html. The quality of the preparation and the concentration of RNA were determined using a ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). To avoid genomic DNA contamination, the preparation was treated twice with DNase I (Turbo DNA free, Applied Biosystems/Ambion, Austin, TX), following manufacturer's instructions. A 0.6 µg aliquot of DNase I-treated RNA was used for cDNA synthesis using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). One µl of retrotranscribed cDNA was used as template for PCR with primer pairs specific for contiguous std ORFs (Table S2). Non-retrotranscribed RNA and genomic DNA were used as negative and positive controls, respectively.
RNA extraction and Northern analysis
A 2 ml aliquot from a stationary culture (O.D.600∼2) was centrifuged at 16,000 g, 4°C, during 5 min. The pellet was resuspended in 100 µl of a solution of lysozyme (Sigma Chemical Co.), 3 mg/ml. Cell lysis was facilitated by three consecutive freeze-thaw cycles. After lysis, RNA was extracted using 1 ml of Trizol reagent (Invitrogen Co, Carlsbad, CA), according to manufacter's instructions. Lastly, total RNA was resuspended in 30 µl of RNase-free water. The quality of the preparation and the RNA concentration were determined using a ND-1000 spectrophotometer (NanoDrop Technologies). For Northern blot analysis, 10 µg of total RNA was loaded per well and electrophoresed in denaturing 1% agarose formaldehyde gels. Vaccum transfer and fixation to Hybond-N+ membranes (GE Healthcare, Little Chalfont, UK) were performed using 0.05 M NaOH. Filters were then hybridized using an internally labelled [(32P)UTP] riboprobe specific for the upstream (5′) 300 nucleotides of the hilD coding sequence. Hybridization was carried out at 65°C. As a control of RNA loading and transfer efficiency, the filters were hybridized with a riboprobe for the RNase P mRNA gene (rnpB). Images of radioactive filters were obtained with a FLA-5100 imaging system (Fujifilm), and quantification was performed using MultiGauge software (Fujifilm).
ß-galactosidase assays
Levels of ß-galactosidase activity were assayed using the CHCl3-sodium dodecyl sulfate permeabilization procedure [58]. ß-galactosidase activity data are the averages and standard deviations from ≥3 independent experiments. The Student's t test was used to determine if the differences in ß-galactosidase activities were statistically significant.
Virulence assays in mice
Groups of 5 eight-weeks-old female BALB/c mice (Charles River Laboratories, Santa Perpetua de Mogoda, Spain) were inoculated with a 1∶1 ratio of two strains, one carrying the PLtetO -stdEF construction and another carrying the PLtetO -stdEF ΔstdEF construction. To differentiate between the two strains, a MudJ transposon carrying a lacZ gene was inserted in the trg locus in the chromosome of the PLtetO -stdEF ΔstdEF strain. The trg::MudJ allele has been shown to be neutral for Salmonella virulence ([59]; F. Ramos-Morales, personal communication). For oral inoculation, bacterial cultures were grown overnight at 37°C in LB without shaking. Oral inoculation was performed by feeding the mice with 25 µl of saline containing 0.1% lactose and 108 bacterial CFU. A competitive index (CI) for each mutant was calculated as the ratio between the mutant and the wild type strain in the output (bacteria recovered from the murine spleen after infection) divided by their ratio in the input (initial inoculum) [60], [61]. The Student's t test was used to determine whether the output ratio was or not significantly different from the input ratio.
Ethics statement
Animal research adhered to the principles mandatory in the European Union, as established in the Legislative Act 86/609 CEE (November 24, 1986), and followed the specific protocols established by the Royal Decree 1201/2005 of the Government of Spain (October 10, 2005). The protocols employed in the study were reviewed by the Comité Ético de Experimentación de la Universidad de Sevilla, and were approved on January 16, 2010 (permit number 59-A-2010). All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Invasion assays
HeLa cells (ATCC CCL2) were cultured in tissue culture medium (Dulbecco's modified essential medium supplemented with 10% fetal calf serum and 2 mM L-glutamine). For routine cultivation, 60 µg/ml penicillin and 100 µg/ml streptomycin were added to the culture medium. The day before infection, approximately 1.5×105 HeLa cells were seeded, using 24-well plates (Costar, Corning, New York, NY). Each well contained 1 ml of tissue culture medium without antibiotics. Cells were grown at 37°C, 5% CO2 to obtain 80% confluency. One hour before infection, the culture medium was removed and replaced by 0.5 ml fresh tissue culture medium without antibiotics. Bacteria were grown overnight at 37°C in LB with shaking, diluted into fresh medium (1∶50), and incubated at 37°C without shaking up to O.D.600 0.6–0.8 (overnight). Bacteria were added to reach a multiplicity of infection (MOI) of 50∶1 bacteria/HeLa cell. HeLa cells were infected for 30 min, washed 3 times with PBS, incubated in fresh tissue culture medium containing 100 µg/ml gentamicin for 1.5 hours, and washed 3 times with PBS. Numbers of viable intracellular bacteria were obtained by lysing infected cells with 1% Triton X-100 (prepared in PBS) and subsequent plating. Invasion rates were determined as the ratio between viable intracellular bacteria and viable bacteria added to infect the HeLa cells. Data are averages and standard deviations of 3 independent experiments. The Student's t test was used to determine the statistical significance of the differences observed.
Results
Genetic screen for regulators of hilD expression using a multicopy plasmid library of Salmonella enterica
We previously reported that regulation by Dam methylation is transmitted to SPI-1 via HilD [39]. However, Dam methylation was found to regulate hilD expression at the postranscriptional level, suggesting the involvement of additional, unknown regulators under Dam methylation control. The view that Dam-dependent regulation of hilD is indirect is further supported by the absence of GATC sites in the hilD promoter and upstream regulatory region, and by the observation that elimination of GATC sites in the hilD coding sequence does not abrogate Dam-dependent regulation (Figure S1). To search for Dam-dependent regulators of hilD, we considered two alternative possibilities: either Dam+ hosts might produce a factor that upregulates hilD or Dam− hosts might produce a factor that downregulates hilD. We also considered the possibility that overexpression of the hypothetical regulator might render SPI-1 expression Dam-independent: namely, that overproduction of a repressor might downregulate SPI-1 in a Dam+ background, while overproduction of an activator might upregulate SPI-1 in Dam− background. On these grounds, we devised a genetic screen for SPI-1 regulators in Dam+ and Dam− backgrounds, using a pBR328-based multicopy plasmid library of the Salmonella enterica genome. As a reporter, we used a fusion (hilD::lac930) that bears the lacZ gene inserted immediately downstream the hilD stop codon and shows Dam-dependent expression (Figure S2).
Dam+ and Dam− isogenic strains carrying the hilD::lac930 fusion were transduced with 9 pools of the plasmid library, each containing around 1,000 independent clones. Transductants were selected on LB plates containing chloramphenicol and X-gal. Colonies with reduced ß-galactosidase activity (white) were sought in the Dam+ background, and colonies with increased ß-galactosidase activity (deep blue) were sought in the Dam− background. Relevant results from these trials were as follows:
Twelve independent candidates with increased ß-galactosidase activity were chosen amongst blue colonies obtained in the Dam− screen. The fragments contained in the library plasmids were sequenced using specific primers flanking the insertion site (Table S2). DNA sequencing revealed that all pBR328 derivatives carried the same cloned fragment, which contained the rtsA gene amongst other genes (Figure S3A). Because RtsA is known to activate hilD transcription [35], we concluded that increased hilD::lac930 expression was due to overproduction of RtsA. However, neither rtsA nor the other genes contained in the plasmid (STM4310, STM4312, STM4313, STM4316, STM4317 and STM4318) are regulated by Dam methylation [38]. Hence, we ruled out that RtsA might be the Dam-dependent factor that controls hilD expression.
In the Dam+ screen, five different plasmids were found to reduce hilD::lac930 activity (Figure S3B). One such plasmid contained the std fimbrial gene cluster, amongst other neighboring genes (Figure S3B). The latter finding, together with transcriptomic data indicating that std mRNA is >100-fold more abundant in a Dam− background [38], suggested that the std gene cluster might encode regulator(s) involved in SPI-1 repression in Dam− mutants. None of the other loci present in the plasmids that reduced hilD::lac930 activity (Figure S3B) is known to be under Dam methylation control [38]. Hence, further work was centered upon std.
All genes in the std gene cluster are overexpressed in Dam− mutants
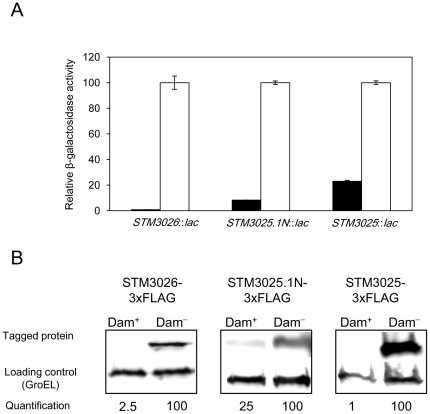
Transcriptomic analysis had shown that stdA, stdB, stdC, and the uncharacterized putative genes STM3026 and STM3025 are all repressed by Dam methylation. Dam-dependent expression of stdA, stdB, and stdC had been confirmed by independent methods [38], [47]. However, the effect of Dam methylation on STM3026 and STM3025 expression had not been further analyzed. On the other hand, DNA sequence analysis in silico had indicated the existence of a sixth, uncharacterized open reading frame (ORF), designed STM3025.1N, in the intergenic region between STM3026 and STM3025 [45]. To update and complete previous data on Dam-dependent control of std, we compared the expression of STM3026, STM3025.1N, and STM3025 in Dam+ and Dam− backgrounds by two independent methods: (i) analysis of ß-galactosidase activity using STM3026::lac, STM3025.1N::lac, and STM3025::lac translational fusions ( Figure 2A ); and (ii) determination of STM3026, STM3025.1N, and STM3025 protein levels in protein extracts from Dam+ and Dam− hosts, using protein variants tagged with the 3xFLAG epitope ( Figure 2B ). ß-galactosidase assays and Western blot analyses show that STM3026, STM3025.1N, and STM3025 are expressed in a Dam− background. Detection of the proteins by Western blot indicates that the three ORFs encode proteins indeed. Detection of Std proteins in a Dam− background is consistent with previous observations indicating that the std operon is repressed under laboratory conditions and becomes derepressed in Dam− mutants [38], [47]. To simplify and rationalize gene designations, STM3026, STM3025.1N, and STM3025 have been renamed stdD, stdE, and stdF, respectively.
Figure 2. Expression of STM3026 (stdD), STM3025.1N (stdE), and STM3025 (stdF) in Dam+ and Dam− backgrounds.
A. ß-galactosidase activity of STM3026::lac, STM3025.1N::lac, and STM3025::lac translational fusions in a Dam+ background (black histograms) and in a Dam− background (white histograms). ß-galactosidase activity has been relativized to 100 in the Dam− background. Student's t test indicates that the differences observed in the ß-galactosidase activities of the fusions in a Dam+ and Dam− backgrounds are statistically significant (P<0.005 in every case). B. Levels of STM3026 (StdD), STM3025.1N (StdE), and STM3025 (StdF) proteins in extracts from Dam+ and Dam− hosts. 3xFLAG-tagged proteins were detected by Western blotting using anti-FLAG antibodies. GroEL was used as loading control. For quantification, the ratio tagged protein/GroEL was relativized to 100 in the Dam− background.
stdA, stdB, stdC, stdD, stdE, and stdF constitute a polycistronic transcriptional unit
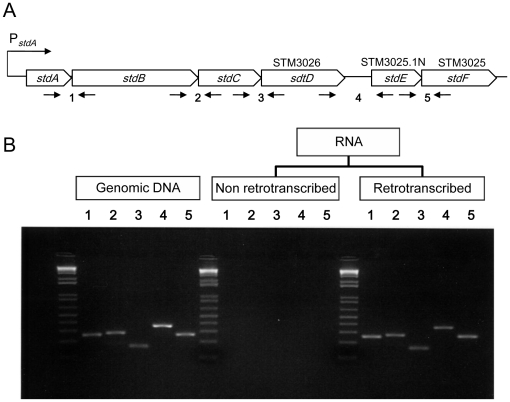
Expression of stdA is known to be driven by a promoter whose transcription is Dam-dependent [47]. The fact that expression of all the genes in the std cluster is upregulated in a Dam− background suggested that the entire cluster might constitute a polycistronic unit transcribed from the stdA promoter. This hypothesis received experimental support from co-transcription analysis of contiguous ORFs by retrotranscription and PCR amplification. For this purpose, total RNA was extracted from a Dam− mutant, and traces of DNA were removed by treatment with DNase I. The RNA sample was split in two fractions, one of which was retrotranscribed to cDNA using random primers; the other fraction underwent the same treatment but water was added instead of retrotranscriptase. Next, we performed PCR amplification with primer pairs specific for contiguous ORFs ( Figure 3A , Table S2) in the presence of the following templates: Salmonella genomic DNA as positive control, nonretrotranscribed RNA as negative control, and cDNA as the experimental query. The PCR products were resolved in a 2% agarose gel with 0.5 µg/ml ethidium bromide, and visualized under UV light. As shown in Figure 3B , PCR products of the expected sizes were obtained using either genomic DNA or cDNA. However, no fragment was observed when RNA was used as template. These results indicate that the six genes in the std cluster constitute a polycistronic operon, transcribed from the promoter previously identified upstream stdA ([47]; Figure 3A ). Our results, however, do not rule out the possibility that internal promoters may also exist.
Figure 3. Evidence that stdA, stdB, stdC, stdD, stdE, and stdF constitute a polycistronic transcriptional unit.
A. Diagram of the std operon. Opposite arrows below the diagram represent primer pairs used to examine co-transcription of contiguous coding sequences. B. Co-transcription of contiguous coding sequences in the std operon. PCR fragments generated with primer pairs specific for contiguous coding sequences were resolved in a 2% agarose gel, stained with ethidium bromide, and visualized with UV light. The 1 kb+ DNA ladder (Innovaplex, Sugar Land, TX) was used as size marker.
The stdE and stdF gene products are functional links between Dam methylation and SPI-1
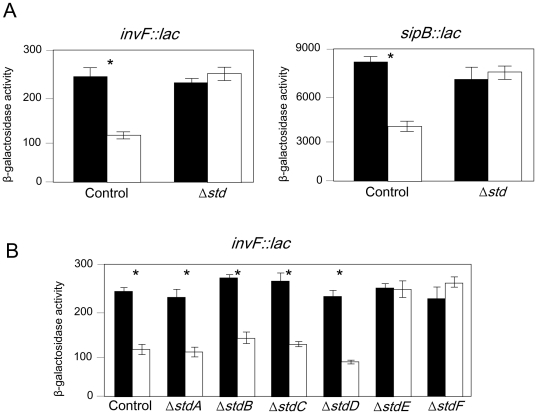
If overexpression of the std operon was the cause of SPI-1 repression in Dam− mutants (Figure S3B), we reasoned, SPI-1 repression in a Dam− background should be suppressed by deletion of the std operon. On these grounds, we compared the expression of invF::lac and sipB::lac fusions in isogenic Dam+ and Dam− strains that contained either an intact std operon or a complete deletion of std. As shown in Figure 4A , the ß-galactosidase activities of the invF::lac and sipB::lac fusions were reduced in a Dam− background in the presence of a functional std operon. However, in a strain lacking the std operon, both fusions displayed similar ß-galactosidase activities in Dam+ and Dam− backgrounds. These results support the hypothesis that one or more proteins encoded in the std operon are involved in the transmission of Dam-dependent regulation to SPI-1. In an attempt to identify such protein(s), Dam-dependent regulation of an invF::lac fusion was monitored in a set of mutants carrying in-frame, non-polar deletions in individual std genes. Figure 4B shows that invF::lac expression remains Dam-dependent in strains lacking stdA, stdB, stdC, and stdD, suggesting that these genes are not required for Dam-dependent control of SPI-1. However, repression of invF::lac in a Dam− background is suppressed in strains lacking either stdE or stdF, suggesting that the products of both genes are necessary for SPI-1 repression in Dam− mutants.
Figure 4. Identification of std operon products involved in SPI-1 repression.
A. ß-galactosidase activities of invF::lac and sipB::lac fusions in a strain containing an intact std operon (control) and in a isogenic strain lacking the entire std operon (Δstd). Black and white histograms represent ß-galactosidase activities in Dam+ and Dam− backgrounds, respectively. Asterisks above columns indicate that the differences observed are statistically significant (P<0.005). B. Regulation of an invF::lac fusion by Dam methylation in strains carrying in-frame deletions in individual std genes. Histograms represent ß-galactosidase activities in a Dam+ background (black) and in a Dam− background (white). Asterisks above columns indicate that the differences observed are statistically significant (P<0.005).
StdE and StdF independently repress SPI-1 expression
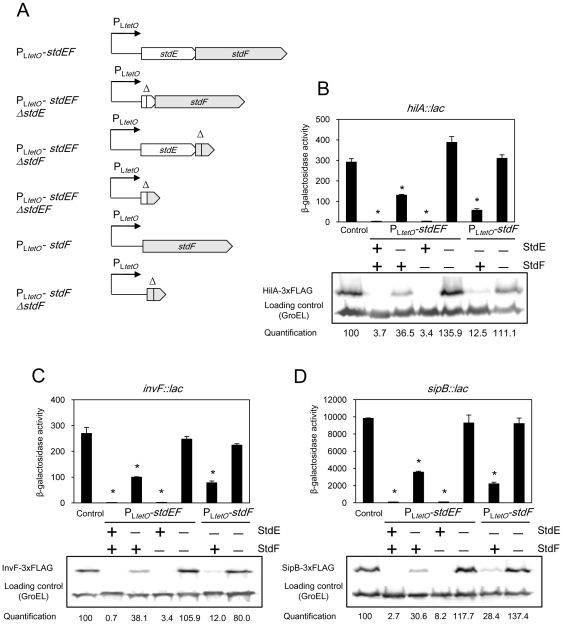
The above experiments provide a tentative explanation for the SPI-1 expression defect observed in the absence of Dam methylation [38], [39]: in a Dam− background, the std operon is expressed, and the stdE and stdF gene products repress SPI-1. If this model was correct, we reasoned, constitutive expression of stdE and stdF should repress SPI-1 expression in a Dam+ background. To test this hypothesis, we placed stdE and stdF under the control of the PLtetO promoter [56] to achieve moderate, Dam-independent expression of stdE and stdF. To avoid potential artefacts (e. g., caused by autogenous regulation of the std operon), the native stdA promoter and all the std genes upstream stdE were deleted. Two basic constructions were made: (i) PLtetO -stdEF in which PLtetO was placed upstream stdE on the chromosome, thus permitting constitutive expression of both stdE and stdF; (ii) PLtetO -stdF, in which PLtetO was inserted right upstream stdF, thus expressing stdF only. As controls, we used derivatives of the same strains which carry in-frame deletions in stdE, stdF, or in both genes ( Figure 5A ). Production of StdE and StdF in strains carrying the PLtetO -stdEF or PLtetO -stdF constructions was monitored by Western blotting, using protein variants tagged with the 3xFLAG epitope (Figure S4).
Figure 5. Downregulation of SPI-1 by StdE and StdF.
A. Diagram representing PLtetO -stdEF and PLtetO -stdF constructions, and their respective deletion controls lacking stdE, stdF, or both. B–D. Expression of hilA, invF, and sipB in strains carrying a native std operon (control), PLtetO -stdEF, PLtetO -stdF, and their respective control constructs. Histograms represent ß-galactosidase activities of hilA::lac, invF::lac, and sipB::lac fusions. An asterisk indicates a statistically significant difference (P<0.005) compared to the control. HilA-3XFLAG, InvF-3xFLAG, and SipB-3xFLAG levels were determined by Western blotting using anti-FLAG antibodies. GroEL was used as loading control. For quantification, the ratio tagged protein/GroEL was relativized to 100 in control samples. The symbols “+” and “−”indicate the presence or the absence of StdE or StdF.
The effect of constitutive expression of stdE and stdF on the expression of SPI-1 genes hilA, invF, and sipB was monitored in strains carrying PLtetO-stdEF, PLtetO-stdF and the appropriate deletion controls. Two independent methods were used: (i) ß-galactosidase activities were measured in strains carrying hilA::lac, invF::lac, and sipB::lac fusions; (ii) HilA, InvF, and SipB protein levels were monitored by Western blotting, using protein variants tagged with the 3xFLAG epitope. The results obtained with both methods were congruent ( Figure 5 , panels B–D), and can be summarized as follows:
Expression of hilA, invF, and sipB is strongly downregulated when PLtetO is inserted upstream stdE.
Downregulation is partially relieved when stdE is deleted. However, deletion of stdF alone does not restore SPI-1 expression. Deletion of both genes completely restores SPI-1 expression to wild type level, suggesting that SPI-1 downregulation is due to expression of both stdE and stdF.
Insertion of PLtetO upstream stdF downregulates the expression of hilA, invF, and sipB, but less efficiently than PLtetO insertion upstream stdE.
Deletion of stdF completely suppresses SPI-1 downregulation.
Altogether, these observations provide evidence that both StdE and StdF downregulate SPI-1 expression. In addition, both gene products downregulate SPI-1 independently: each is able to downregulate SPI-1 in the absence of the other.
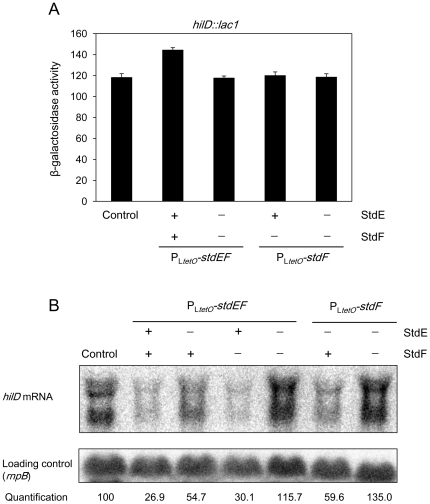
StdE and StdF regulate hilD expression at the postranscriptional level
We previously reported that regulation of hilD by Dam methylation is postranscriptional [39]. After finding that Dam-dependent hilD regulation depended on StdE and StdF, it seemed logical to expect that StdE and StdF would repress hilD expression at the postranscriptional level. To test this hypothesis, we examined hilD expression using a transcriptional fusion (hilD::lac1) in which lacZ is inserted exactly at the hilD transcription start site. The hilD::lac1 fusion faithfully reproduces transcriptional regulation of hilD, as indicated by the observation that the fusion is upregulated in the presence of a multicopy plasmid that expresses RtsA, a transcriptional activator of hilD [35] (Figure S5). The expression level of hilD::lac1 was determined in the wild type as well as in the PLtetO -stdEF, PLtetO -stdEF ΔstdEF, PLtetO -stdF, and PLtetO -stdF ΔstdF backgrounds ( Figure 5A , Figure 6A ). The ß-galactosidase activities were similar in all strains, suggesting that StdE and StdF do not regulate hilD transcription initiation.
Figure 6. Postranscriptional regulation of hilD expression by StdE and StdF.
A. ß-galactosidase activity of a hilD::lac fusion in a strain with a native std operon (control), and in strains carrying PLtetO -stdEF, PLtetO -stdEF ΔstdEF, PLtetO -stdF, or PLtetO -stdF ΔstdF constructions. The symbols “+” and “−”indicate the presence or the absence of StdE and StdF. None of the backgrounds shows ß-galactosidase activity significantly lower than the control. B. Level of hilD mRNA in RNA extracts from the wild type, PLtetO -stdEF, PLtetO -stdF, and their respective control strains. Levels of hilD mRNA were monitored by Northern blotting, using a riboprobe specific for the first (5′) 300 nucleotides of the hilD coding sequence. The symbols “+” and “−”indicate the presence or the absence of StdE and StdF.
The possibility that StdE and StdF downregulate hilD expression at the postranscriptional level was examined by analyzing hilD mRNA levels by Northern blot in the following backgrounds: wild type, PLtetO -stdEF, PLtetO -stdEF ΔstdE, PLtetO -stdEF ΔstdF, PLtetO -stdEF ΔstdEF, PLtetO -stdF, and PLtetO -stdF ΔstdF. As shown in Figure 6B , the level of hilD mRNA is reduced around 4 fold in the PLtetO -stdEF strain. Deletion of stdE partially restores the hilD mRNA level, and simultaneous deletion of stdE and stdF completely restores hilD mRNA to wild type level. Furthermore, the amount of hilD mRNA is reduced twofold in the PLtetO -stdF background, and this reduction is completely abolished by an stdF deletion. Altogether, these observations support the view that StdE and StdF downregulate hilD expression at the postranscriptional level.
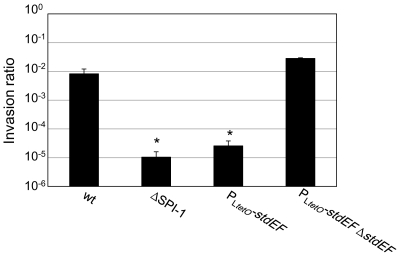
Expression of stdE and stdF inhibits Salmonella invasion
Because constitutive expression of stdE and stdF represses SPI-1 ( Figure 5 ; Figure 6 ) and SPI-1 is essential for Salmonella invasion, we examined the invasiveness of S. enterica ser. Typhimurium upon constitutive expression of stdE and stdF. For this purpose, we compared the ability of Salmonella strains carrying either the PLtetO -stdEF or PLtetO -stdEF ΔstdEF constructions to invade epithelial HeLa cells in vitro. As a positive control, we used the wild type strain. As a negative control, we used a strain with a deletion of SPI-1 (ΔSPI-1). As shown in Figure 7 , the ΔSPI-1 strain is approximately 1,000 fold less invasive than the wild type. Constitutive expression of stdE and stdF (PLtetO -stdEF) produces an invasion defect similar to deletion of SPI-1 ( Figure 7 ), and this defect is suppressed by mutation of stdE and stdF (PLtetO -stdEF ΔstdEF) ( Figure 7 ). Hence, constitutive expression of stdE and stdF inhibits Salmonella invasion.
Figure 7. Effect of stdE and stdF expression on Salmonella invasion in vitro.
The invasion ratios of epithelial HeLa cells by four Salmonella strains are represented: the wild type (wt), a strain with a deletion of SPI-1 (ΔSPI-1), a strain carrying the PLtetO -stdEF construction, and a strain carrying the PLtetO -stdEF ΔstdEF construction. Histograms represent averages and standard deviations of 3 independent experiments. An asterisk indicates a statistically significant difference (P<0.05) compared with the wild type.
Expression of stdE and stdF attenuates Salmonella virulence in mice
The observation that expression of stdE and stdF inhibits Salmonella invasion in vitro led us to speculate that StdE and StdF might also inhibit invasion of epithelial cells in the animal intestine. If such was the case, we reasoned, a Salmonella strain which constitutively expresses stdE and stdF should be attenuated in mice infected by the oral route. To test this hypothesis, we compared the virulence of Salmonella strains that expressed or not stdE and stdF. Five BALB/c mice were orally inoculated with a 1∶1 mixture of a strain that constitutively expressed stdE and stdF (PLtetO -stdEF) and an isogenic strain carrying a deletion of both stdE and stdF (PLtetO -stdEF ΔstdEF). To distinguish the two strains on plates, we inserted a MudJ transposon at the trg chromosomal locus of the PLtetO -stdEF ΔstdEF strain. The trg::MudJ allele has been shown to be neutral for Salmonella virulence ([59]; F. Ramos-Morales, personal communication). The competitive index of PLtetO -stdEF strain (expressing stdE and stdF) versus the PLtetO -stdEF ΔstdEF strain (lacking stdE and stdF) was 0.016 ( Table 1 ), indicating that constitutive expression of stdE and stdF attenuates Salmonella virulence by the oral route more than 60 fold.
Table 1. Effect of constitutive expression of stdE and stdF in oral competition assays.
| Strain number | Relevant genotype | CI (A/B) | P | |
| Strain A | SV6503 | PLtetO-stdEF | 0.016 | <0.0001 |
| Strain B | SV6901 | PLtetO-stdEF ΔstdEF |
Discussion
Evolutionary acquisition of genetic modules has provided Salmonella with new abilities to interact with eukaryotic cells and to exploit a variety of niches [8], [11]. The std gene cluster is conserved amongst Salmonella serovars and absent in closely related species [11], suggesting horizontal acquisition. A critical requirement of modular evolution is to coordinate expression of the genetic modules, which in some cases carry regulatory genes that provide connections with the core genome [8]. In addition, examples of crosstalk between horizontally acquired modules have been described: HilD, a regulator encoded by SPI-1, activates SPI-2 expression during late stationary phase [62]; expression of SPI-4 genes is activated by SprB, a transcriptional regulator encoded on SPI-1 [63]; HilE, a SPI-1 negative regulator, is encoded on a region of Salmonella chromosome that has been proposed to be a pathogenicity island [26]; SPI-1 and SPI-2 transcriptional regulators control the synthesis of effector proteins encoded outside the islands [64], [65], often in horizontally-acquired DNA fragments [66], [67]. In this study, we describe a connection between std and SPI-1 which may be viewed as a novel example of crosstalk between horizontally-acquired, virulence-related genes.
We have characterized three putative ORFs in the std gene cluster, previously annotated as STM3026, STM3025.1N, and STM3025. Western blot analyses have confirmed the existence of three proteins, which have been renamed StdD (STM3026), StdE (STM3025.1N), and StdF (STM3025) respectively. In silico analysis indicates that StdE and StdF may be cytoplasmic proteins, while StdD may be an outer membrane protein.
Evidence that stdA, stdB, stdC, stdD, stdE, and stdF may constitute a polycistronic transcriptional unit is provided by simultaneous upregulation of all six genes in a Dam− background ( Figure 2 ; [38], [47]), and by retrotranscription and PCR amplification showing that the six genes are co-transcribed ( Figure 3 ). Transcription of std is driven by a promoter located upstream stdA [47]. Transcription from PstdA is activated by binding of HdfR, a LysR-like factor, to a regulatory region upstream the promoter. However, methylation of two GATC sites in the regulatory region prevents binding of HdfR, thus repressing std expression ([47], Jakomin et al., in preparation). Altogether, these observations suggest that all std genes may be coordinately regulated by Dam methylation upon transcription from PstdA. However, internal promoters may also exist.
Dam− mutants of Salmonella enterica are attenuated in the mouse model and present a plethora of virulence-related defects both at the intestinal stage of the infection and during systemic infection [40], [46], [68]. A relevant defect during intestinal infection is reduced SPI-1 expression, which is a consequence of hilD mRNA instability in Dam− mutants [38], [39]. Genetic screens and subsequent experiments described in this study have identified the std fimbrial operon as the missing link between Dam methylation and SPI-1: (i) a multicopy plasmid containing the std operon downregulates hilD expression (Figure S3B); (ii) std genes are upregulated in Dam− background ( Figure 2 ; [38], [47]); and (iii) SPI-1 regulation by Dam methylation is completely suppressed in a strain lacking the std operon ( Figure 4A ). Altogether, these results suggest that expression of std in Dam− mutants leads to SPI-1 repression. Our observations may also explain the intriguing observation that the extreme attenuation of S. enterica Dam− mutants upon oral infection [40], [46] is partially suppressed by deletion of std [47].
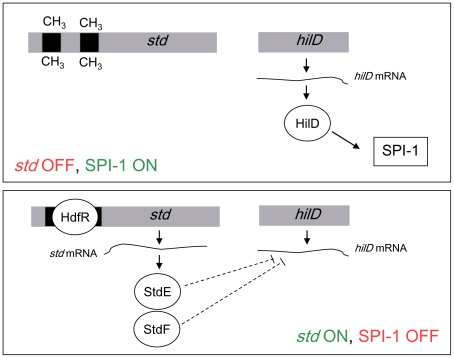
Epistasis analysis indicates that Dam-dependent control of SPI-1 requires both StdE and StdF. This conclusion is further supported by the following observations: (i) constitutive expression of stdE and stdF in a Dam+ background represses SPI-1 expression ( Figure 5 ); (ii) StdE and StdF are overproduced in Dam− background ( Figure 2 ); (iii) Dam methylation, StdE, and StdF regulate SPI-1 expression through HilD ( Figure 6 ; [39]); and (iv) as in the case of Dam methylation, StdE and StdF do not regulate hilD transcription but control the level of hilD mRNA ( Figure 6 ). However, it remains to be established whether StdE and StdF directly control the hilD mRNA level or the regulatory mechanism involves intermediaries. A tentative model to explain SPI-1 regulation by Dam methylation is depicted in Figure 8: in a Dam+ background, GATC sites in the PstdA regulatory region are methylated, thus preventing binding of HdfR and subsequent activation of std transcription. In the absence of Dam methylation, HdfR activates transcription from PstdA and all the proteins encoded in the operon are produced. StdE and StdF then repress hilD expression at the postranscriptional level. As a consequence, the entire SPI-1 is downregulated.
Figure 8. Model for crosstalk between the std operon and SPI-1.
Lack of std expression (e. g., under laboratory conditions and in the ileum) permits HilD synthesis and subsequent expression of SPI-1. Transcription of std (e. g., in Dam− mutants and in the caecum) yields StdE and StdF products, resulting in postranscriptional inhibition of HilD synthesis, and subsequent SPI-1 repression.
A corollary from the above results is that Salmonella invasion can be expected to be inhibited whenever the std operon is expressed ( Figure 8 ). This expectation was fulfilled by invasion assays in vitro showing that expression of StdE and StdF causes a >1,000 fold reduction in HeLa cell invasion ( Figure 7 ). Furthermore, expression of StdE and StdF was found to attenuate Salmonella virulence about 60 fold upon infection of BALB/c mice ( Table 1 ).
Under laboratory growth conditions, the std operon is tightly repressed [38], [43], [44], [47]. However, several observations indicate that Std fimbriae are produced in the animal intestine: (i) mice infected with serovar Typhimurium seroconvert to StdA, the major fimbrial component of Std fimbriae [43]; (ii) std deletion reduces the ability of Salmonella to colonize and persist in the caecum of infected mice, while it has no consequence for colonization of the small intestine [48]; and (iii) Std fimbriae bind α(1,2)fucose residues, which are abundant in the cecal mucosa [49]. In turn, Salmonella invasion takes place preferentially in the ileum [3] and is inhibited in the caecum [69]. Hence, it is tempting to speculate that StdE and StdF may play a role in SPI-1 expression inhibition in the caecum, an intestinal compartment which is not appropriate for invasion.
StdE shares 40–50% identity with the transcriptional activators GrlA and CaiF from E. coli and Enterobacter cloacae, respectively [70], [71]. Interestingly, StdF is related to an uncharacterized protein encoded immediately downstream CaiF in the E. cloacae chromosome [70], [71], which is part of a hypothetical fimbrial gene cluster whose genetic organization is reminiscent of the std operon [72]. StdF is also related to the SPI-1 Salmonella protein SprB, a transcriptional regulator that represses the hilD promoter and activates the siiA promoter [63]. Even though StdE and StdF are similar to known transcriptional regulators, they do not regulate hilD at the transcriptional level but at the postranscriptional level. Thus, these proteins may have either acquired the ability to control Salmonella gene expression at the postranscriptional level or may regulate transcription of a postranscriptional regulator of hilD. If the latter view is correct, crosstalk between std and SPI-1 may turn out to be more complex than described in this study, perhaps involving elements of the Salmonella core genome.
Supporting Information
Elimination of the GATC sites in the hilD coding sequence does not alter the control of SPI-1 expression by Dam methylation. A. Diagram showing the distribution of GATCs within hilD; each CH3 represents a GATC. B. Site-directed mutagenesis of hilD GATCs. A single nucleotide exchange was introduced at each GATC site, generating a synonimous codon (shown in red). C. ß-galactosidase activity of an invF::lac fusion in Dam+ (black histograms) and Dam− (white histograms) isogenic backgrounds. Measurements were performed in a strain that contained the 3 GATCs in the hilD coding sequence (control), and in a strain in which the 3 GATCs had been mutated (GATC*123). The differences observed between Dam+ and Dam− are statistically significant in both strains (P<0.005).
(TIF)
ß-galactosidase activity of the hilD::lac930 fusion in a Dam+ background (black histogram), and in a Dam− background (white histogram). The differences observed are statistically significant (P<0.005).
(TIF)
ß-galactosidase activity of the hilD::lac930 fusion in control strains (carrying pBR328), in candidates showing increased ß-galactosidase activity in a Dam− background (A) and in candidates showing reduced ß-galactosidase activity in a Dam+ background (B). Diagrams representing the fragments harbored by the plasmids present in the candidates are also shown.
(TIF)
Production of StdE and StdF in strains carrying PLtetO -stdEF and PLtetO -stdF constructions A. Diagrams of strain construction and StdE and StdF tagging. B. Levels of StdE-3xFLAG and StdF-3xFLAG in protein extracts from the wild type, Dam−, PLtetO -stdEF, and PLtetO -stdF strains. 3xFLAG-tagged proteins were detected by Western blotting using a commercial anti-FLAG antibody. GroEL was used as loading control. For quantification, the ratio tagged protein/GroEL was relativized to 100 in the Dam− background.
(TIF)
ß-galactosidase activity of the hilD::lac1 fusion in a strain carrying pBR328 (black histogram), and in a strain carrying a pBR328 derivative that contains the rtsA gene (white histogram). The differences observed are statistically significant (P<0.005).
(TIF)
Strain list.
(DOC)
Oligonucleotides used in this study (5′→3′).
(DOC)
Acknowledgments
We thank Elena Puerta-Fernández and Francisco Ramos-Morales for advice, Meritxell García-Quintanilla for help in mouse infections, and Modesto Carballo, Laura Navarro, and Cristina Reyes of the Servicio de Biología (CITIUS, Universidad de Sevilla) for help in experiments performed at the facility. Strain SV6062 was constructed by Fernando Baisón. Strain TR5878 was kindly provided by John R. Roth, University of California Davis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by grant BIO2010-15023 from the Spanish Ministry of Science and Innovation and the European Regional Fund, and grant CSD2008-00013 from the Consolider-Ingenio 2010 Program of the Spanish Ministry of Science and Innovation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Falkow S. The evolution of pathogenicity in Escherichia, Shigella, and Salmonella. In: Neidhardt FC, editor. Escherichia coli and Salmonella. Cellular and Molecular Biology. Washington: ASM Press; 1996. pp. 2723–2729. [Google Scholar]
- 2.Ellermeier CD, Slauch JM. The genus Salmonella. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, editors. The prokaryotes. New York, USA: Springer Science; 2006. pp. 123–158. [Google Scholar]
- 3.Carter PB, Collins FM. The route of enteric infection in normal mice. J Exp Med. 1974;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the peyer's patches. J Exp Med. 1994;180(1):15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82(5):346–353. [PMC free article] [PubMed] [Google Scholar]
- 6.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50(6):882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 7.Ochman H, Wilson AC. Evolution in bacteria: Evidence for a universal substitution rate in cellular genomes. J Mol Evol. 1987;26(1–2):74–86. doi: 10.1007/BF02111283. [DOI] [PubMed] [Google Scholar]
- 8.Groisman EA, Ochman H. How Salmonella became a pathogen. Trends Microbiol. 1997;5(9):343–349. doi: 10.1016/S0966-842X(97)01099-8. [DOI] [PubMed] [Google Scholar]
- 9.Kelly BG, Vespermann A, Bolton DJ. The role of horizontal gene transfer in the evolution of selected foodborne bacterial pathogens. Food Chem Toxicol. 2009;47(5):951–968. doi: 10.1016/j.fct.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Hensel M. Evolution of pathogenicity islands of Salmonella enterica. Int J Med Microbiol. 2004;294(2–3):95–102. doi: 10.1016/j.ijmm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Porwollik S, McClelland M. Lateral gene transfer in Salmonella. Microbes Infect. 2003;5(11):977–989. doi: 10.1016/s1286-4579(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 12.Groisman EA, Ochman H. Pathogenicity islands: Bacterial evolution in quantum leaps. Cell. 1996;87(5):791–794. doi: 10.1016/s0092-8674(00)81985-6. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt H, Hensel M. Pathogenicity islands in bacterial pathogenesis. Clin Microbiol Rev. 2004;17(1):14–56. doi: 10.1128/CMR.17.1.14-56.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lostroh CP, Lee CA. The Salmonella pathogenicity island-1 type III secretion system. Microbes Infect. 2001;3(14–15):1281–1291. doi: 10.1016/s1286-4579(01)01488-5. [DOI] [PubMed] [Google Scholar]
- 15.Darwin KH, Miller VL. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin Microbiol Rev. 1999;12(3):405–428. doi: 10.1128/cmr.12.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohl ME, Miller SI. Salmonella: A model for bacterial pathogenesis. Annu Rev Med. 2001;52:259–274. doi: 10.1146/annurev.med.52.1.259. [DOI] [PubMed] [Google Scholar]
- 17.Altier C. Genetic and environmental control of Salmonella invasion. J Microbiol. 2005;43 Spec No:85–92. [PubMed] [Google Scholar]
- 18.Ellermeier JR, Slauch JM. Adaptation to the host environment: Regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol. 2007;10(1):24–29. doi: 10.1016/j.mib.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Jones BD. Salmonella invasion gene regulation: A story of environmental awareness. J Microbiol. 2005;43 Spec No:110–117. [PubMed] [Google Scholar]
- 20.Baek CH, Wang S, Roland KL, Curtiss R., 3rd Leucine-responsive regulatory protein (Lrp) acts as a virulence repressor in Salmonella enterica serovar Typhimurium. J Bacteriol. 2009;191(4):1278–1292. doi: 10.1128/JB.01142-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olekhnovich IN, Kadner RJ. Crucial roles of both flanking sequences in silencing of the hilA promoter in Salmonella enterica. J Mol Biol. 2006;357(2):373–386. doi: 10.1016/j.jmb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Queiroz MH, Madrid C, Paytubi S, Balsalobre C, Juarez A. Integration host factor alleviates H-NS silencing of the Salmonella enterica serovar Typhimurium master regulator of SPI1, hilA. Microbiology. 2011;157(9):2504–2514. doi: 10.1099/mic.0.049197-0. [DOI] [PubMed] [Google Scholar]
- 23.Takaya A, Kubota Y, Isogai E, Yamamoto T. Degradation of the HilC and HilD regulator proteins by ATP-dependent Lon protease leads to downregulation of Salmonella pathogenicity island 1 gene expression. Mol Microbiol. 2005;55(3):839–852. doi: 10.1111/j.1365-2958.2004.04425.x. [DOI] [PubMed] [Google Scholar]
- 24.Boddicker JD, Jones BD. Lon protease activity causes down-regulation of Salmonella pathogenicity island 1 invasion gene expression after infection of epithelial cells. Infect Immun. 2004;72(4):2002–2013. doi: 10.1128/IAI.72.4.2002-2013.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahlen TF, Mathur N, Jones BD. Identification and characterization of mutants with increased expression of hilA, the invasion gene transcriptional activator of Salmonella typhimurium. FEMS Immunol Med Microbiol. 2000;28(1):25–35. doi: 10.1111/j.1574-695X.2000.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 26.Baxter MA, Fahlen TF, Wilson RL, Jones BD. HilE interacts with HilD and negatively regulates hilA transcription and expression of the Salmonella enterica serovar Typhimurium invasive phenotype. Infect Immun. 2003;71(3):1295–1305. doi: 10.1128/IAI.71.3.1295-1305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baxter MA, Jones BD. The fimYZ genes regulate Salmonella enterica serovar Typhimurium invasion in addition to type 1 fimbrial expression and bacterial motility. Infect Immun. 2005;73(3):1377–1385. doi: 10.1128/IAI.73.3.1377-1385.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim S, Yun J, Yoon H, Park C, Kim B, et al. Mlc regulation of Salmonella pathogenicity island I gene expression via hilE repression. Nucleic Acids Res. 2007;35(6):1822–1832. doi: 10.1093/nar/gkm060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altier C, Suyemoto M, Lawhon SD. Regulation of Salmonella enterica serovar Typhimurium invasion genes by CsrA. Infect Immun. 2000;68(12):6790–6797. doi: 10.1128/iai.68.12.6790-6797.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez LC, Yakhnin H, Camacho MI, Georgellis D, Babitzke P, et al. Integration of a complex regulatory cascade involving the SirA/BarA and Csr global regulatory systems that controls expression of the Salmonella SPI-1 and SPI-2 virulence regulons through HilD. Mol Microbiol. 2011;80(6):1637–1656. doi: 10.1111/j.1365-2958.2011.07674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fortune DR, Suyemoto M, Altier C. Identification of CsrC and characterization of its role in epithelial cell invasion in Salmonella enterica serovar Typhimurium. Infect Immun. 2006;74(1):331–339. doi: 10.1128/IAI.74.1.331-339.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellermeier JR, Slauch JM. Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J Bacteriol. 2008;190(2):476–486. doi: 10.1128/JB.00926-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teixido L, Carrasco B, Alonso JC, Barbe J, Campoy S. Fur activates the expression of Salmonella enterica pathogenicity island 1 by directly interacting with the hilD operator in vivo and in vitro. PLoS One. 2011;6(5):e19711. doi: 10.1371/journal.pone.0019711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Troxell B, Sikes ML, Fink RC, Vazquez-Torres A, Jones-Carson J, et al. Fur negatively regulates hns and is required for the expression of HilA and virulence in Salmonella enterica serovar Typhimurium. J Bacteriol. 2011;193(2):497–505. doi: 10.1128/JB.00942-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellermeier CD, Ellermeier JR, Slauch JM. HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol. 2005;57(3):691–705. doi: 10.1111/j.1365-2958.2005.04737.x. [DOI] [PubMed] [Google Scholar]
- 36.Pesavento C, Becker G, Sommerfeldt N, Possling A, Tschowri N, et al. Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli. Genes Dev. 2008;22(17):2434–2446. doi: 10.1101/gad.475808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chubiz JE, Golubeva YA, Lin D, Miller LD, Slauch JM. FliZ regulates expression of the Salmonella pathogenicity island 1 invasion locus by controlling HilD protein activity in Salmonella enterica serovar Typhimurium. J Bacteriol. 2010;192(23):6261–6270. doi: 10.1128/JB.00635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balbontin R, Rowley G, Pucciarelli MG, Lopez-Garrido J, Wormstone Y, et al. DNA adenine methylation regulates virulence gene expression in Salmonella enterica serovar Typhimurium. J Bacteriol. 2006;188(23):8160–8168. doi: 10.1128/JB.00847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Garrido J, Casadesus J. Regulation of Salmonella enterica pathogenicity island 1 (SPI-1) by DNA adenine methylation. Genetics. 2010;184(3):637–649. doi: 10.1534/genetics.109.108985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Del Portillo F, Pucciarelli MG, Casadesus J. DNA adenine methylase mutants of Salmonella typhimurium show defects in protein secretion, cell invasion, and M cell cytotoxicity. Proc Natl Acad Sci U S A. 1999;96(20):11578–11583. doi: 10.1073/pnas.96.20.11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Townsend SM, Kramer NE, Edwards R, Baker S, Hamlin N, et al. Salmonella enterica serovar Typhi possesses a unique repertoire of fimbrial gene sequences. Infect Immun. 2001;69(5):2894–2901. doi: 10.1128/IAI.69.5.2894-2901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thanassi DG, Saulino ET, Hultgren SJ. The chaperone/usher pathway: A major terminal branch of the general secretory pathway. Curr Opin Microbiol. 1998;1(2):223–231. doi: 10.1016/s1369-5274(98)80015-5. [DOI] [PubMed] [Google Scholar]
- 43.Humphries A, Deridder S, Baumler AJ. Salmonella enterica serotype Typhimurium fimbrial proteins serve as antigens during infection of mice. Infect Immun. 2005;73(9):5329–5338. doi: 10.1128/IAI.73.9.5329-5338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Humphries AD, Raffatellu M, Winter S, Weening EH, Kingsley RA, et al. The use of flow cytometry to detect expression of subunits encoded by 11 Salmonella enterica serotype Typhimurium fimbrial operons. Mol Microbiol. 2003;48(5):1357–1376. doi: 10.1046/j.1365-2958.2003.03507.x. [DOI] [PubMed] [Google Scholar]
- 45.Chaudhuri RR, Loman NJ, Snyder LA, Bailey CM, Stekel DJ, et al. xBASE2: A comprehensive resource for comparative bacterial genomics. Nucleic Acids Res. 2008;36(Database issue):D543–6. doi: 10.1093/nar/gkm928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heithoff DM, Sinsheimer RL, Low DA, Mahan MJ. An essential role for DNA adenine methylation in bacterial virulence. Science. 1999;284(5416):967–970. doi: 10.1126/science.284.5416.967. [DOI] [PubMed] [Google Scholar]
- 47.Jakomin M, Chessa D, Baumler AJ, Casadesus J. Regulation of the Salmonella enterica std fimbrial operon by DNA adenine methylation, SeqA, and HdfR. J Bacteriol. 2008;190(22):7406–7413. doi: 10.1128/JB.01136-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weening EH, Barker JD, Laarakker MC, Humphries AD, Tsolis RM, et al. The Salmonella enterica serotype Typhimurium lpf, bcf, stb, stc, std, and sth fimbrial operons are required for intestinal persistence in mice. Infect Immun. 2005;73(6):3358–3366. doi: 10.1128/IAI.73.6.3358-3366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chessa D, Winter MG, Jakomin M, Baumler AJ. Salmonella enterica serotype Typhimurium Std fimbriae bind terminal alpha(1,2)fucose residues in the cecal mucosa. Mol Microbiol. 2009;71(4):864–875. doi: 10.1111/j.1365-2958.2008.06566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97(12):6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellermeier CD, Janakiraman A, Slauch JM. Construction of targeted single copy lac fusions using lambda red and FLP-mediated site-specific recombination in bacteria. Gene. 2002;290(1–2):153–161. doi: 10.1016/s0378-1119(02)00551-6. [DOI] [PubMed] [Google Scholar]
- 52.Uzzau S, Figueroa-Bossi N, Rubino S, Bossi L. Epitope tagging of chromosomal genes in Salmonella. Proc Natl Acad Sci U S A. 2001;98(26):15264–15269. doi: 10.1073/pnas.261348198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- 54.Garzon A, Cano DA, Casadesus J. Role of Erf recombinase in P22-mediated plasmid transduction. Genetics. 1995;140(2):427–434. doi: 10.1093/genetics/140.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan RK, Botstein D, Watanabe T, Ogata Y. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. properties of a high-frequency-transducing lysate. Virology. 1972;50(3):883–898. doi: 10.1016/0042-6822(72)90442-4. [DOI] [PubMed] [Google Scholar]
- 56.Lutz R, Bujard H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1–I2 regulatory elements. Nucleic Acids Res. 1997;25(6):1203–1210. doi: 10.1093/nar/25.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urban JH, Vogel J. Translational control and target recognition by Escherichia coli small RNAs in vivo. Nucleic Acids Res. 2007;35(3):1018–1037. doi: 10.1093/nar/gkl1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller JH. Experiments in molecular genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 59.Segura I, Casadesus J, Ramos-Morales F. Use of mixed infections to study cell invasion and intracellular proliferation of Salmonella enterica in eukaryotic cell cultures. J Microbiol Methods. 2004;56(1):83–91. doi: 10.1016/j.mimet.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freter R, Allweiss B, O'Brien PC, Halstead SA, Macsai MS. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: In vitro studies. Infect Immun. 1981;34(1):241–249. doi: 10.1128/iai.34.1.241-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bustamante VH, Martinez LC, Santana FJ, Knodler LA, Steele-Mortimer O, et al. HilD-mediated transcriptional cross-talk between SPI-1 and SPI-2. Proc Natl Acad Sci U S A. 2008;105(38):14591–14596. doi: 10.1073/pnas.0801205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saini S, Rao CV. SprB is the molecular link between Salmonella pathogenicity island 1 (SPI1) and SPI4. J Bacteriol. 2010;192(9):2459–2462. doi: 10.1128/JB.00047-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Darwin KH, Miller VL. Type III secretion chaperone-dependent regulation: Activation of virulence genes by SicA and InvF in Salmonella typhimurium. EMBO J. 2001;20(8):1850–1862. doi: 10.1093/emboj/20.8.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knodler LA, Celli J, Hardt WD, Vallance BA, Yip C, et al. Salmonella effectors within a single pathogenicity island are differentially expressed and translocated by separate type III secretion systems. Mol Microbiol. 2002;43(5):1089–1103. doi: 10.1046/j.1365-2958.2002.02820.x. [DOI] [PubMed] [Google Scholar]
- 66.Hardt WD, Urlaub H, Galan JE. A substrate of the centisome 63 type III protein secretion system of Salmonella typhimurium is encoded by a cryptic bacteriophage. Proc Natl Acad Sci U S A. 1998;95(5):2574–2579. doi: 10.1073/pnas.95.5.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood MW, Jones MA, Watson PR, Hedges S, Wallis TS, et al. Identification of a pathogenicity island required for Salmonella enteropathogenicity. Mol Microbiol. 1998;29(3):883–891. doi: 10.1046/j.1365-2958.1998.00984.x. [DOI] [PubMed] [Google Scholar]
- 68.Marinus MG, Casadesus J. Roles of DNA adenine methylation in host-pathogen interactions: Mismatch repair, transcriptional regulation, and more. FEMS Microbiol Rev. 2009;33(3):488–503. doi: 10.1111/j.1574-6976.2008.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lawhon SD, Maurer R, Suyemoto M, Altier C. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol Microbiol. 2002;46(5):1451–1464. doi: 10.1046/j.1365-2958.2002.03268.x. [DOI] [PubMed] [Google Scholar]
- 70.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 71.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lucas S, Copeland A, Lapidus A, Cheng JF, Bruce D, et al. Complete sequence of Enterobacter cloacae SCF1. 2010. NCBI Genome Database, entry NC_014618.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Elimination of the GATC sites in the hilD coding sequence does not alter the control of SPI-1 expression by Dam methylation. A. Diagram showing the distribution of GATCs within hilD; each CH3 represents a GATC. B. Site-directed mutagenesis of hilD GATCs. A single nucleotide exchange was introduced at each GATC site, generating a synonimous codon (shown in red). C. ß-galactosidase activity of an invF::lac fusion in Dam+ (black histograms) and Dam− (white histograms) isogenic backgrounds. Measurements were performed in a strain that contained the 3 GATCs in the hilD coding sequence (control), and in a strain in which the 3 GATCs had been mutated (GATC*123). The differences observed between Dam+ and Dam− are statistically significant in both strains (P<0.005).
(TIF)
ß-galactosidase activity of the hilD::lac930 fusion in a Dam+ background (black histogram), and in a Dam− background (white histogram). The differences observed are statistically significant (P<0.005).
(TIF)
ß-galactosidase activity of the hilD::lac930 fusion in control strains (carrying pBR328), in candidates showing increased ß-galactosidase activity in a Dam− background (A) and in candidates showing reduced ß-galactosidase activity in a Dam+ background (B). Diagrams representing the fragments harbored by the plasmids present in the candidates are also shown.
(TIF)
Production of StdE and StdF in strains carrying PLtetO -stdEF and PLtetO -stdF constructions A. Diagrams of strain construction and StdE and StdF tagging. B. Levels of StdE-3xFLAG and StdF-3xFLAG in protein extracts from the wild type, Dam−, PLtetO -stdEF, and PLtetO -stdF strains. 3xFLAG-tagged proteins were detected by Western blotting using a commercial anti-FLAG antibody. GroEL was used as loading control. For quantification, the ratio tagged protein/GroEL was relativized to 100 in the Dam− background.
(TIF)
ß-galactosidase activity of the hilD::lac1 fusion in a strain carrying pBR328 (black histogram), and in a strain carrying a pBR328 derivative that contains the rtsA gene (white histogram). The differences observed are statistically significant (P<0.005).
(TIF)
Strain list.
(DOC)
Oligonucleotides used in this study (5′→3′).
(DOC)