Abstract
Trypanosoma brucei, a eukaryotic pathogen that causes African sleeping sickness in humans and nagana in cattle, depends on the enzyme acetyl-CoA carboxylase (ACC) for full virulence in mice. ACC produces malonyl-CoA, the two carbon donor for fatty acid synthesis. We assessed the effect of haloxyfop, an aryloxyphenoxypropionate herbicide inhibitor of plastid ACCs in many plants as well as Toxoplasma gondii, on T. brucei ACC activity and growth in culture. Haloxyfop inhibited TbACC in cell lysate (EC50 67 μM), despite the presence of an amino acid motif typically associated with resistance. Haloxyfop also reduced growth of bloodstream and procyclic form parasites (EC50 of 0.8 mM and 1.2 mM). However, the effect on growth was likely due to off-target effects because haloxyfop treatment had no effect on fatty acid elongation or incorporation into complex lipids in vivo.
Keywords: Trypanosoma brucei, acetyl-CoA carboxylase, fatty acid synthesis, haloxyfop, aryloxyphenoxypropionate, inhibitor
1. Introduction
Trypanosoma brucei is a protozoan parasite and the etiological agent of human African trypanosomiasis, also known as African sleeping sickness. The disease causes significant morbidity and mortality across its range in sub-Saharan Africa. The World Health Organization estimates that 60 million people are at risk of contracting sleeping sickness (WHO, 2006). Livestock and working animals are also susceptible to infection, and the resulting disease, nagana, is estimated to cause 4.5 billion dollars in trypanosome-related agricultural losses each year (FAO, 2007).
The public health consequences and enormous economic burden caused by T. brucei highlight the desperate need for new chemotherapeutic treatments for these diseases. Currently, available drugs have substantial negative side effects, and parasite drug resistance is an ever-present concern (Burri, 2010). Vaccine development is not a viable option. This strategy is confounded by the parasite’s ability to change its glycoprotein surface coat through a process called antigenic variation (Horn and McCulloch, 2010).
Previously, we reported that T. brucei acetyl-CoA carboxylase (TbACC1) is required to efficiently establish and maintain an infection in a mouse model (Vigueira and Paul, 2011). Knockdown of TbACC by RNA interference (RNAi) nearly doubled the mean time until death, suggesting TbACC is a suitable candidate for investigation as a drug target. In T. brucei, TbACC exists as a single cytoplasmically-disposed isoform. TbACC is a large multidomain enzyme, consisting of biotin carboxylase, biotin-carboxyl carrier protein (BCCP), and carboxyl-transferase (CT) domains. ACC catalyses the first committed step in fatty acid synthesis (FAS): the ATP-dependent carboxylation of acetyl-CoA to make malonyl-CoA, the two-carbon donor for FAS (Tong and Harwood, 2006). In lieu of a conventional fatty acid synthase, the parasite utilizes a series of microsomal elongases (ELO) for the bulk of FAS (Lee et al., 2006). See (Lee et al., 2007) for review of T. brucei FAS.
ACC has long been recognized as a useful target for chemical intervention in crop management. The aryloxyphenoxypropionates (FOPs) and the cyclohexanediones (DIMs) are ACC inhibitors commonly used to control grass weeds affecting a number of agricultural crops (e.g. leaf vegetables, onion, strawberry). The FOPs and DIMs target the plastid ACCs of grasses by binding the CT domain and causing conformational changes that prevent transfer of the carboxyl group from the BCCP domain to the acetyl-CoA substrate (Delye et al., 2003, Zhang et al., 2004, Xiang et al., 2009).
Research into weed FOP- and DIM-resistance mechanisms has identified two amino acid residues in ACC that appear important in determining enzyme resistance status. In the yeast, Saccharomyces cerevisiae, these residues are L1705 and V1967, and according to the crystal structure, these residues lie in the haloxyfop binding pocket of the CT domain (Zhang et al., 2004). In rye grass, Lolium rigidum, a single change from the native I at either of these important residues is sufficient to confer enzyme resistance to FOPs, specifically haloxyfop (Zagnitko et al., 2001, Delye et al., 2003). However, a growing body of evidence suggests that these residues are likely just two of multiple potential residues in the highly conserved CT domain capable of influencing sensitivity of ACC enzymes to FOPs (Zhang et al., 2004, Zhang and Powles, 2006a, Zhang and Powles, 2006b, Liu et al., 2007).
ACC and lipid metabolism have also been identified as a potential drug targets for treating parasitic protozoan infections (Surolia and Surolia, 2001, Roberts et al., 2003, Paul et al., 2004, Singh et al., 2009). In particular, haloxyfop has been demonstrated to inhibit the apicoplast-localized ACC of the Apicomplexan parasite Toxoplasma gondii (Zuther et al., 1999). Here, we report the sensitivity of a second protozoan ACC to haloxyfop. Despite possessing the amino acid sequence motif typically associated with haloxyfop resistance, TbACC is inhibited by haloxyfop. We demonstrate that haloxyfop kills insect midgut stage, procyclic form (PF) and mammalian bloodstream form (BF) parasites in vitro. However, in vivo lipid metabolism is not detectably influenced upon treatment, suggesting that the toxicity of haloxyfop to T. brucei cannot be entirely attributed to TbACC inhibition.
2. Materials and methods
2.1. Reagents
All chemicals and reagents were purchased from Thermo Fisher Scientific and Sigma, except: Serum Plus (JRH Biosciences) and streptavidin-conjugated horseradish peroxidase (SA-HRP) (Pierce). Minimum Essential Medium, Iscove’s Modified Dulbecco’s Medium, and goat anti-mouse-HRP IgG antibody were from Invitrogen. [14C]NaHCO3 and 3H-labeled fatty acids were from American Radiolabeled Chemicals. Silica Gel 60 and C18 reverse phase thin layer chromatography (TLC) plates were from Analtech. The mouse anti-tubulin antibody (clone B-5-1-2) was from Sigma. Haloxyfop (CAS-No: 69806-34-4), quizalofop (CAS-No: 94051-08-8) and sethoxydim (CAS-No: 74051-80-2) were from Sigma. Fluazifop (CAS-No: 69335-91-7) was from Wako.
2.2. Trypanosome strains and media
Wild-type (WT) strain 427 PF and BF T. brucei were provided by Dr. Paul Englund (Johns Hopkins School of Medicine). BF parasites were grown in HMI-9 medium (Hirumi and Hirumi, 1989) containing 10% heat-inactivated FBS/10% Serum Plus. PF parasites were grown in SDM-79 medium (Brun and Schonenberger, 1979) containing 10% heat inactivated FBS and supplemented with 7.5 mg/L hemin.
2.3. ACC enzyme activity
We assayed ACC activity as described (Vigueira and Paul, 2011). Inhibitors were prepared in filter-sterilized dimethyl sulfoxide (DMSO) and used as 100X stocks. Lysates were incubated with inhibitors for 30 min on ice prior to the addition of reaction components. The final reaction volume of 100 μl contained 5 mM ATP, 1 mM acetyl-CoA, 1% v/v DMSO and 5 mM [14C]NaHCO3 (14.9 mCi/mmol), and was incubated for 30 min at 30°C with constant mixing at 500 RPM. A 50 μl sample of acid-precipitated [14C]malonyl-CoA product was collected on Whatman #1 filters, air-dried, and quantified by scintillation counting.
2.4. Growth experiments
For growth curves, WT cells were diluted into fresh media containing inhibitors or DMSO solvent control and cell density was monitored every 48 h for up to 10 days using a FACScan flow cytometer (Becton Dickinson). Inhibitors were prepared in filter-sterilized DMSO and used as 100X stocks, resulting in final DMSO concentration of 1% v/v. Following each cell count, cultures were diluted to maintain logarithmic phase growth, and inhibitors or DMSO was added to maintain experimental concentrations.
2.5. Metabolic labeling and lipid analysis
Metabolic labeling was performed essentially as described (Paul et al., 2004, Vigueira and Paul, 2011). Briefly, after 4 days of haloxyfop treatment, ~1 × 108 PF cells were labeled with 25 μCi of [11,12-3H]laurate (C12:0; 60 mCi/mmol) for 2 h in a 28°C CO2 incubator. Total lipids were extracted in chloroform/methanol/water (10:10:3 v/v/v) and equal CPMs/lane were analyzed by normal phase TLC using chloroform/methanol/water (10:10:3 v/v/v) as the mobile phase. Labeled lipid species were identified based on known migration patterns in this TLC system (Doering et al., 1993). To analyze the fatty acids by chain length, total lipid extracts were converted to fatty acid methyl esters (FAMEs), extracted in hexane, and equal CPMs/lane were analyzed by C18 reverse-phase TLC using chloroform/methanol/water (5:15:3 v/v/v) as the mobile phase. TLCs were sprayed with En3Hance (Perkin-Elmer) and exposed to x-ray film at −80°C. For chain length markers, FAMEs were prepared in parallel from 30 μCi of [3H]fatty acids: [11,12-3H]laurate (C12; 60 mCi/mmol), [9,10-3H]myristate (C14; 60 mCi/mmol), [9,10-3H(N)]palmitate (C16; 60 mCi/mmol), and [9,10-3H]stearate (C18; 60 mCi/mmol).
2.6. Streptavidin blotting
Streptavidin blotting can detect the biotin prosthetic group on ACC and was performed essentially as described (Vigueira and Paul, 2011). Briefly, PF parasites were treated for 4 days with haloxyfop. 20 μg of whole cell lysate were fractionated on 8% SDS-PAGE gels and transferred to nitrocellulose. The blot was cut, and the top half was probed for ACC with SA-HRP (1:400 in 0.2% dry milk, 1X Tris-buffered saline (TBS), 0.05% Tween-20). The bottom was probed with a mouse anti-tubulin (clone B-5-1-2), diluted 1:50,000 in Wash Buffer (5% dry milk, 1X TBS, 0.05% Tween-20) followed by HRP-conjugated goat anti-mouse IgG secondary antibody diluted 1:10,000 in Wash Buffer. Semi-quantitative analysis of blots was performed using densitometry (NIH Image J software) of appropriately exposed films (unsaturated signal within the linear range of the film).
2.7. Statistics
One-tailed Student’s t-test analyses between control and treatments were performed using Microsoft Excel. We judged statistical significance to be p < 0.01. Error bars represent standard deviation from the mean.
2.8. Genetic sequence acquisition
ACC protein sequences were acquired from the genetic sequence database at the National Center for Biotechnical Information. The accession numbers for each sequence are listed: Trypanosoma brucei (GenBank ID: Tb927.8.7100), Saccharomyces cerevisiae (GenBank ID: NM_001183193.1), Homo sapiens ACC1 (GenBank ID: U19822), Homo sapiens ACC2 (GenBank ID: U89344), Rattus norvegicus (GenBank ID: J03808), Toxoplasma gondii (GenBank ID: AF157612), Lolium multiflorum (GenBank ID: AY710293.1), Zea mays (GenBank ID: U19183), and Alopecurus myosuroides (GenBank ID: AJ310767.1).
3. Results
3.1. Effect of FOPs and sethoxydim on TbACC activity
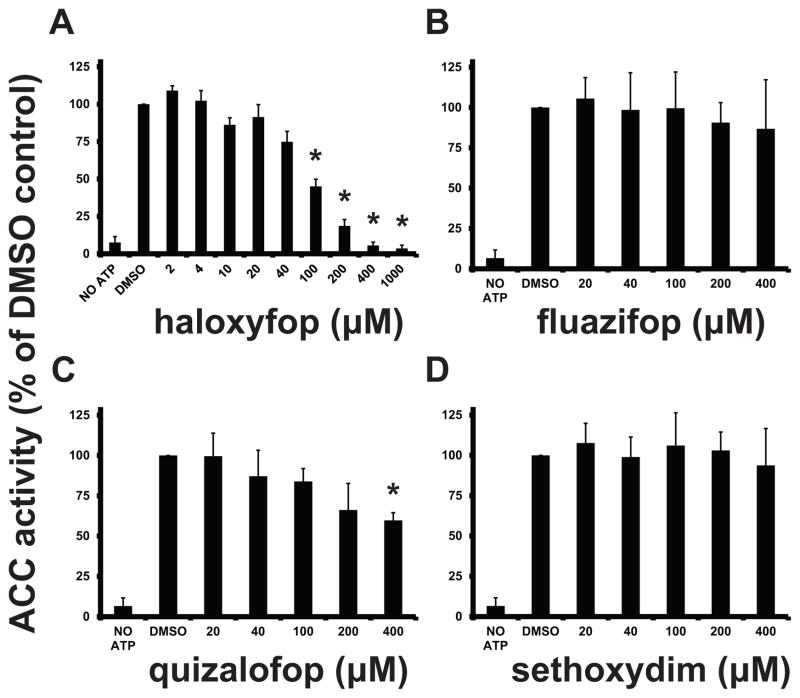
We tested three compounds from the FOP family and one compound from the DIM family of herbicides for their effect on TbACC enzymatic activity in PF lysate. TbACC activity is assayed in desalted cell lysate by measuring the incorporation of the [14C]CO2 from [14C]NaHCO3 into the acid-resistant [14C]malonyl-CoA product (Vigueira and Paul, 2011). Haloxyfop was the most potent inhibitor of the assay with an EC50 of 67 μM, and EC90 of 400 μM (Fig. 1A). The other tested FOPs (fluazifop and quizalofop) and the DIM compound (sethoxydim) had either no inhibitory activity or had EC50 values >400 μM (Fig. 1B–D). As haloxyfop showed promising activity, we used this compound in our subsequent studies.
Fig. 1.
Effect of FOP and DIM herbicides on TbACC activity in PF lysate. ACC activity in PF cell lysates was measured after a 30 min incubation with 2 μM-1 mM haloxyfop (A), or 20–400 μM fluazifop (B), quizalofop (C), sethoxydim (D). Values are expressed as a percentage of the DMSO control. DMSO concentrations were maintained at 1% v/v for all conditions. The mean of 3 experiments is shown. Error bars indicate the SD. The * indicates p < 0.01 for the difference between DMSO control and herbicide-treated conditions, Student’s t-test.
3.2. TbACC contains residues that confer resistance in other ACCs
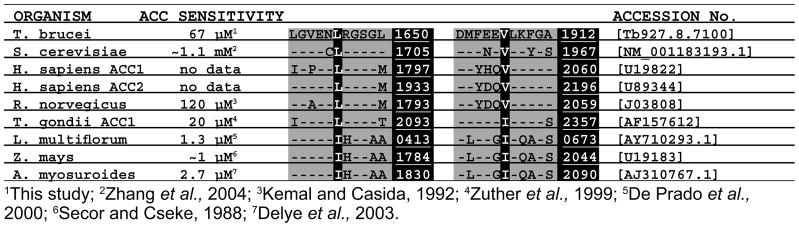
Previous work on FOPS has revealed that enzyme resistance can be traced to two key amino acid residues in the haloxyfop binding pocket of the ACC CT domain, corresponding to L1705 and V1967 in Saccharomyces cerevisiae (Zhang et al., 2004). In ACCs that have been experimentally determined to be resistant to inhibition by haloxyfop, the proteins possess the L/V motif at equivalent positions (Fig. 2). However, in sensitive ACCs, there are typically deviations from this pattern at either or both positions: L-1705-I or V-1967-I variants. TbACC has an L/V pair (L1650 and V1912) identical to the yeast L/V pair and would therefore be predicted to be resistant to haloxyfop.
Fig. 2.
Alignment of multidomain ACC amino acid sequences surrounding the resistance-conferring residues in the CT domain. The organism names are listed in the first column and the EC50 or IC50 values for each are listed in the second column. The resistance-conferring residues, equivalent to S. cerevisiae L1705 and V1967, are highlighted and the highlighted numbers represent the equivalent amino acid positions in each sequence. Dashes indicate residues identical to those in T. brucei. Accession numbers are provided in the far right column. The L. multiflorum sequence is a partial cDNA sequence, thus the highlighted numbers refer to the amino acid positions in the partial gene product. No EC50 or IC50 data is available for the human ACCs.
3.3. Effect of haloxyfop on growth in culture
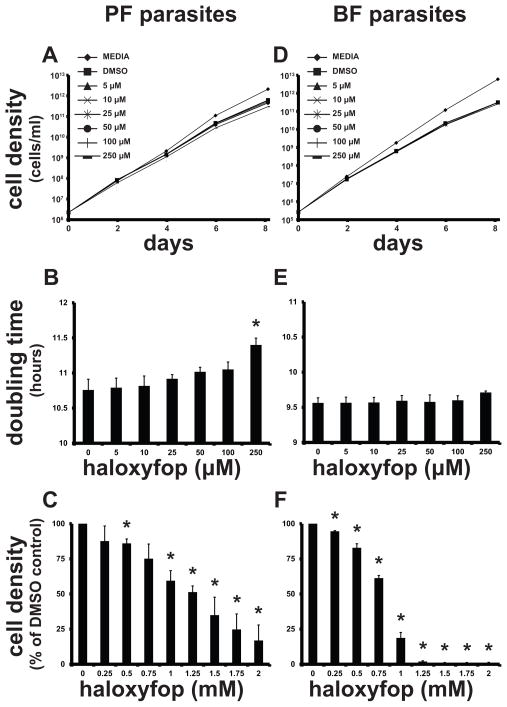
We have previously demonstrated that RNAi of ACC causes little reduction in parasite growth rate when cells are cultured in normal media (Vigueira and Paul, 2011). Therefore, we sought to determine the maximum concentration of haloxyfop that could be tolerated by the parasites without having a major impact on growth rate. For growth of PF cells, haloxyfop concentrations up to 100 μM had no significant effect on doubling time, though 250 μM haloxyfop caused a slight, statistically significant increase (Fig. 3B). For BF cells, growth remained unchanged in the presence of up to 250 μM haloxyfop (Fig. 3E). The overall effect on growth rate over ten days was minimal in both PF and BF parasites (Fig. 3A, D), suggesting that potentially lethal, off-target effects are kept to a minimum at haloxyfop concentrations ≤250 μM. At higher haloxyfop concentrations (250 μM to 2 mM), we observed a statically significant reduction in cell growth over 48 h (Fig 3C, F) with an EC50 of 1.2 mM for PF parasites and an EC50 of 0.8 mM for BF parasites.
Fig. 3.
Effect of haloxyfop on in vitro growth of T. brucei. (A,B,D,E) WT PF (A,B) and BF (D,E) cells were diluted into media containing 5–250 μM haloxyfop or DMSO control, and the cell densities of the cultures were recorded every other day for 8 days. Cumulative culture density is shown in A and D, and culture doubling times are shown in B and E. (C,F) WT PF (C) and BF cells (F) were diluted into media containing 250 μM–2 mM haloxyfop or DMSO control, and the cell densities of the culture were determined after 48 h. Values are expressed as a percentage of DMSO control. For all panels, the mean of three replicates is shown. Error bars show SD. The * indicates p < 0.01 for the difference between DMSO and haloxyfop-treated conditions, Student’s t-test.
We detected a slight effect of the DMSO solvent on growth, with a 9% and 17% reduction in PF and BF parasites, respectively. The effect of 1% v/v DMSO on BF parasite viability has been quantified previously and is consistent with our observations (Sharlow et al., 2010).
3.4. Effect of haloxyfop on fatty acid elongation
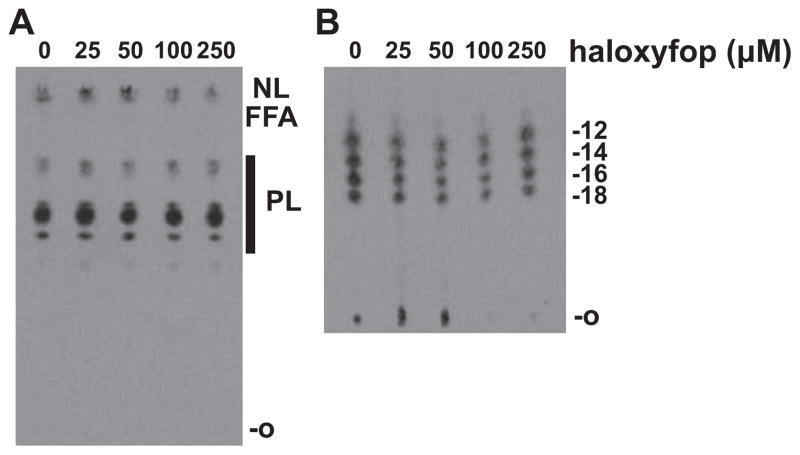
To determine whether haloxyfop treatment targets TbACC in intact cells, we assessed FA elongation in vivo as T. brucei will readily take up, elongate and incorporate exogenous fatty acids into more complex lipids. We used haloxyfop concentrations that did not exhibit a major growth defect in PF parasites, as our previous work showed that ACC RNAi inhibited in vivo fatty acid elongation while showing no growth defect in normal media (Vigueira and Paul, 2011). After a 4 day treatment with 10–250 μM haloxyfop, we incubated PF cells with [3H]laurate (C12:0) and assessed its elongation by the ELO pathway. A chain length analysis of FAMEs by reverse-phase TLC demonstrated no reduction in the ability of the parasite to elongate [3H]laurate (C12:0) to longer fatty acids (C14:0, C16:0, C18:0) upon haloxyfop treatment (Fig. 4B). Additionally, normal phase TLC of bulk lipids revealed no gross differences between haloxyfop-treated and untreated parasites in the level of incorporation of [3H]laurate into phospholipids, free fatty acids, or neutral lipids (Fig. 4A).
Fig. 4.
Fatty acid incorporation and elongation in the presence of haloxyfop. WT PF cells were grown for 4 days in the presence of haloxyfop or DMSO. Cells were then incubated with 25 μCi of [3H]laurate (C12) for 2 h. (A) Total lipids were extracted and equal CPMs per lane were resolved by TLC. The origin (O) and relative migration of neutral lipids (NL), free fatty acids (FFA), and phospholipids (PL) are indicated on the right. Treatment conditions are indicated at the top. (B) Fatty acid chain length analysis of FAMEs prepared from the total lipid extracts in (A). Equal CPMs per lane were resolved by C18 reverse-phase high-performance TLC. The origin (O) and markers for C12, C14, C16 and C18 FAMEs are indicated at the right. Treatment conditions are indicated at the top. A representative of two independent experiments is shown.
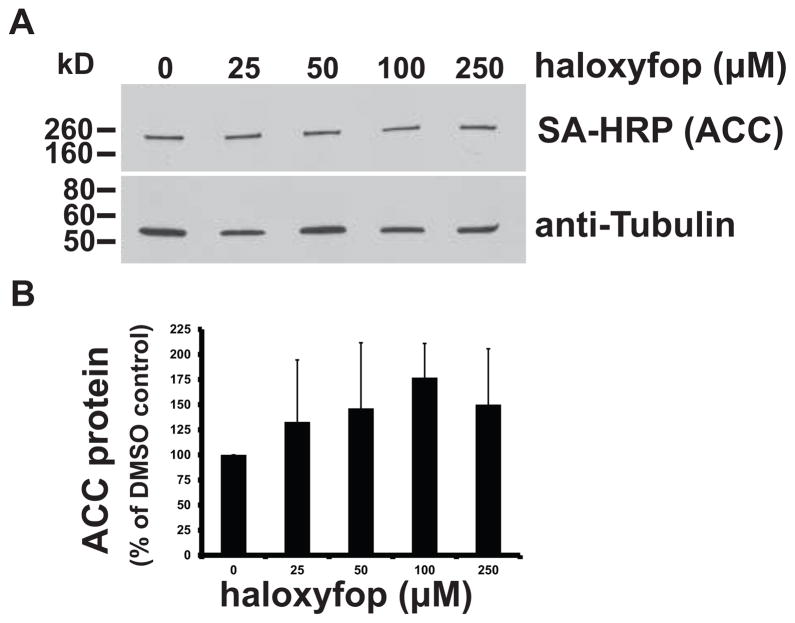
3.5. Effect of haloxyfop on ACC protein levels
We next examined the possibility that the parasite compensated for haloxyfop inhibition by increasing ACC protein expression. ACC protein can be detected by western blotting with SA-HRP, which recognizes the biotin prosthetic group of ACC (Nikolau et al., 1985, Haneji and Koide, 1989, Vigueira and Paul, 2011). After 4 days of haloxyfop treatment (10–250 μM), we observed no statistically significant changes (p >0.01) in ACC protein levels when normalized to β-tubulin protein levels (Fig. 5).
Fig. 5.
Effect of haloxyfop treatment on ACC protein levels. WT PF cells were grown for 4 days in the presence of 25–250 μM haloxyfop or DMSO. (A) Total hypotonic lysates (20 μg protein) were probed for ACC by SA-HRP blotting, which recognizes the ACC biotin prosthetic group. The lower half of the blot was probed for tubulin as a loading control. Treatment conditions are indicated at the top. One representative of three independent blots is shown. (B) Densitometric quantitation of ACC protein levels normalized to the α-tubulin loading control. Values are expressed as a percentage of the DMSO control. The mean of 3 independent replicates is shown. Error bars indicate the SD. No significant difference was observed between DMSO and haloxyfop-treated conditions (p > 0.01, Student’s t-test).
4. Discussion
Of the FOP and DIM compounds we tested, haloxyfop had the greatest inhibitory effect on TbACC activity in lysate (EC50 of 67 μM) (Fig 1). The EC50 for haloxyfop on TbACC was determined with lysate rather than with purified protein, thus it is difficult to directly compare to other IC50s reported for purified ACCs. Direct comparison of TbACC to other ACCs is also problematic because of the lab-to-lab variation in experimental procedure reported in the literature. With these limitations in mind, the sensitivity of TbACC in cell lysate is most similar to the moderate sensitivity described for that of the protozoan parasite T. gondii (IC50 20 μM) (Zuther et al., 1999) and the Norway rat, R. norvegicus (IC50 120 μM), (Kemal and Casida, 1992). Our data also suggests TbACC is less sensitive to haloxyfop than the plastid ACCs of ryegrass, corn, and blackgrass (IC50 1–3 μM) (Secor and Cseke, 1988, De Prado et al., 2000, Delye et al., 2003) and more sensitive than the ACC CT domain of the yeast, S. cerevisiae (IC50 ~1.1 mM) (Zhang et al., 2004).
The moderate sensitivity of TbACC to haloxyfop is somewhat surprising based on the presence of the L/V variant previously determined to confer enzyme resistance to this compound. Amino acid changes at these two positions cause sensitive plant ACCs to become insensitive to enzymatic inhibition by FOPS and DIMS (Zagnitko et al., 2001, Brown et al., 2002, Christoffers et al., 2002, Delye et al., 2003, White et al., 2005, Zhang and Powles, 2006b). With the exception of the Norway rat, ACCs that contain L/V variants are comparatively resistant to haloxyfop (Fig. 2).
The finding that TbACC possess the L/V variant and exhibits moderate sensitivity supports previous work indicating that although these residues appear important for conferring enzyme resistance, they alone are not necessarily predictive of sensitivity. Other residues in and around the haloxyfop binding pocket are likely to affect enzyme sensitivity and may moderate the effects of any single residue (Zhang et al., 2004, Zhang and Powles, 2006b, Liu et al., 2007). Evidently, the sensitivity of ACCs to haloxyfop lies on a continuum, making it difficult to classify the ACC of any one organism as either “sensitive” or “resistant”.
Treatment of PF and BF parasites with haloxyfop concentrations up to 250 μM had a minimal effect on parasite growth and doubling time (Fig. 3A–B, D–E). However, this effect was minor compared to the dramatic effect of higher haloxyfop concentrations on PF and BF parasite growth over 48 h (Fig. 3C, F). BF parasites were slightly more sensitive to haloxyfop treatment than PF parasites. However, both PF and BF T. brucei were remarkably less sensitive to haloxyfop than T. gondii (EC50 ~100 μM) (Zuther et al., 1999). We have previously demonstrated through RNAi experiments that TbACC is largely expendable in BF parasites in vitro and is only required when PF parasites are cultured in low-lipid media (Vigueira and Paul, 2011). Thus, we contend that inhibition of TbACC by haloxyfop should have little to no consequence on the growth rate of the parasite in vitro. Consequently, the reduction in growth we observed with haloxyfop concentrations >250 μM can be attributed to off-target effects rather than inhibition of TbACC.
Our previous work demonstrated that reduction of TbACC by RNAi causes a robust reduction in elongation of fatty acids in PF parasites (Vigueira and Paul, 2011). Given that haloxyfop treatment inhibited TbACC activity in lysate, the inability of haloxyfop to affect FAS in vivo was unexpected (Fig. 4). Assessing the effect of haloxyfop concentrations >250 μM on fatty elongation in vivo was not feasible, because any observed effects could not be separated from those resulting from the profound effect of the compound on parasite growth due to likely off-target effects (Fig. 3C, F).
One possible explanation for this disparity between haloxyfop’s effect in lysate and intact cells could be a compensatory increase in TbACC protein expression, a possibility that we ruled out (Fig 5). It is also possible that incomplete inhibition of TbACC allowed available malonyl-CoA pools to remain high enough that ELO activity appeared unaffected. Alternatively, the apparent insensitivity of TbACC to haloxyfop in intact cells may be due to the fact that the compound does not efficiently enter the cell or is partitioned into a cellular compartment not accessible to TbACC. Haloxyfop’s effect on growth at higher concentrations (Fig 3C, F) does not negate poor membrane permeability as a possible explanation, because haloxyfop could be acting at the cellular surface to cause a reduction in growth. Depolarization of the cellular membrane has been described in plants and is considered a secondary mechanism for the graminicide activity of FOPs (Häusler et al., 1991, Shimabukuro and Hoffer, 1992, Ditomaso, 1994, Holtum et al., 1994, Wright, 1994).
Another possible explanation is that haloxyfop is modified in intact T. brucei, rendering it unable to bind and inhibit TbACC. Because the haloxyfop binding pocket lies in a tight space on the face of the ACC protein dimer, any small modification of the compound could reduce the ability of haloxyfop to bind and inhibit enzymatic activity (Zhang et al., 2003). Stereochemical inversion of haloxyfop has been observed previously in rats (Bartels and Smith, 1989), however the inhibitory activity of the resulting enantiomer has not been determined.
In summary, we have demonstrated that haloxyfop inhibits TbACC in vitro, but has no detectable effect on in vivo lipid metabolism, suggesting that the toxicity of haloxyfop to T. brucei cannot be entirely attributed to TbACC inhibition. To our knowledge, this is the first report of potential off-target effects of this class of inhibitors in protozoan parasites. Furthermore, this study highlights the need for careful characterization of the mechanisms of action of small molecule inhibitors in lysates as well as in intact cells.
Acknowledgments
This work was supported in part by the National Institutes of Health 1R15AI081207 (K.P.) and Clemson University Funds (K.P.). We thank members of the Clemson University Parasite Club for their helpful suggestions. We also thank Marianne Ligon, Ben Martin, and Ciara McKnight for experimental assistance and Jim Morris for critical reading of the manuscript.
Footnotes
Abbreviations used: ACC, acetyl-CoA carboxylase; BCCP, biotin-carboxyl carrier protein; BF, bloodstream form trypanosome; CT, carboxyl-transferase; DIMs, cyclohexanediones; DMSO, dimethyl sulfoxide; ELO, microsomal elongase; FAS, fatty acid synthesis; FOPs, aryloxyphenoxypropionates; PF, procyclic form trypanosome; RNAi, RNA interference; SA-HRP, streptavidin conjugated horseradish peroxidase; TBS, tris-buffered saline; TLC, thin layer chromatography.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartels MJ, Smith FA. Stereochemical inversion of haloxyfop in the Fischer 344 rat. Drug Metabolism and Disposition. 1989;17:286–291. [PubMed] [Google Scholar]
- Brown AC, Moss SR, Wilson ZA, Field LM. An isoleucine to leucine substitution in the ACCase of Alopecurus myosuroides (black-grass) is associated with resistance to the herbicide sethoxydim. Pesticide Biochemistry and Physiology. 2002;72:160–168. [Google Scholar]
- Brun R, Schonenberger M. Cultivation and in vitro cloning of procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Tropica. 1979;36:289–292. [PubMed] [Google Scholar]
- Burri C. Chemotherapy against human African trypanosomiasis: Is there a road to success? Parasitology. 2010;137:1987–1994. doi: 10.1017/S0031182010001137. [DOI] [PubMed] [Google Scholar]
- Christoffers MJ, Berg ML, Messersmith CG. An isoleucine to leucine mutation in acetyl-CoA carboxylase confers herbicide resistance in wild oat. Genome. 2002;45:1049–1056. doi: 10.1139/g02-080. [DOI] [PubMed] [Google Scholar]
- De Prado R, Gonzalez-Gutierrez J, Menendez J, Gasquez J, Gronwald JW, Gimenez-Espinosa R. Resistance to acetyl CoA carboxylase-inhibiting herbicides in Lolium multiflorum. Weed Science. 2000;48:311–318. [Google Scholar]
- Delye C, Zhang XQ, Chalopin C, Michel S, Powles SB. An isoleucine residue within the carboxyl-transferase domain of multidomain acetyl-coenzyme A carboxylase is a major determinant of sensitivity to aryloxyphenoxypropionate but not to cyclohexanedione inhibitors. Plant Physiology. 2003;132:1716–1723. doi: 10.1104/pp.103.021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditomaso JM. Evidence against a direct membrane effect in the mechanism of action of graminicides. Weed Science. 1994;42:302–309. [Google Scholar]
- Doering TL, Pessin MS, Hoff EF, Hart GW, Raben DM, Englund PT. Trypanosome metabolism of myristate, the fatty acid required for the variant surface glycoprotein membrane anchor. The Journal of Biological Chemistry. 1993;268:9215–9222. [PubMed] [Google Scholar]
- FAO. [accessed 6/1/2011];Ethiopian fly factory guns for “poverty insect”. 2007 http://www.fao.org/newsroom/en/focus/2007/1000511/article_1000514en.html.
- Haneji T, Koide SS. Transblot identification of biotin-containing proteins in rat liver. Analytical Biochemistry. 1989;177:57–61. doi: 10.1016/0003-2697(89)90013-4. [DOI] [PubMed] [Google Scholar]
- Häusler RE, Holtum JA, Powles SB. Cross-resistance to herbicides in annual ryegrass (Lolium rigidum): IV. Correlation between membrane effects and resistance to graminicides. Plant Physiology. 1991;97:1035–1043. doi: 10.1104/pp.97.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. The Journal of Parasitology. 1989;75:985–989. [PubMed] [Google Scholar]
- Holtum JAM, Häusler RE, Devine MD, Powles SB. Recovery of transmembrane potentials in plants resistant to aryloxyphenoxypropanoate herbicides - a phenomenon awaiting explanation. Weed Science. 1994;42:293–301. [Google Scholar]
- Horn D, McCulloch R. Molecular mechanisms underlying the control of antigenic variation in African trypanosomes. Current Opinions in Microbiology. 2010;13:700–705. doi: 10.1016/j.mib.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemal C, Casida JE. Coenzyme A esters of 2-aryloxyphenoxypropionate herbicides and 2-arylpropionate anti-inflammatory drugs are potent and stereoselective inhibitors of rat liver acetyl-CoA carboxylase. Life Sciences. 1992;50:533–540. doi: 10.1016/0024-3205(92)90393-4. [DOI] [PubMed] [Google Scholar]
- Lee SH, Stephens JL, Englund PT. A fatty-acid synthesis mechanism specialized for parasitism. Nature Reviews Microbiology. 2007;5:287–297. doi: 10.1038/nrmicro1617. [DOI] [PubMed] [Google Scholar]
- Lee SH, Stephens JL, Paul KS, Englund PT. Fatty acid synthesis by elongases in trypanosomes. Cell. 2006;126:691–699. doi: 10.1016/j.cell.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Liu W, Harrison DK, Chalupska D, Gornicki P, O’Donnell CC, Adkins SW, Haselkorn R, Williams RR. Single-site mutations in the carboxyltransferase domain of plastid acetyl-CoA carboxylase confer resistance to grass-specific herbicides. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3627–3632. doi: 10.1073/pnas.0611572104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolau BJ, Wurtele ES, Stumpf PK. Use of streptavidin to detect biotin-containing proteins in plants. Analytical Biochemistry. 1985;149:448–453. doi: 10.1016/0003-2697(85)90596-2. [DOI] [PubMed] [Google Scholar]
- Paul KS, Bacchi CJ, Englund PT. Multiple triclosan targets in Trypanosoma brucei. Eukaryotic Cell. 2004;3:855–861. doi: 10.1128/EC.3.4.855-861.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CW, McLeod R, Rice DW, Ginger M, Chance ML, Goad LJ. Fatty acid and sterol metabolism: potential antimicrobial targets in Apicomplexan and trypanosomatid parasitic protozoa. Molecular and Biochemical Parasitology. 2003;126:129–142. doi: 10.1016/s0166-6851(02)00280-3. [DOI] [PubMed] [Google Scholar]
- Secor J, Cseke C. Inhibition of acetyl-CoA carboxylase activity by haloxyfop and tralkoxydim. Plant Physiology. 1988;86:10–12. doi: 10.1104/pp.86.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharlow ER, Lyda TA, Dodson HC, Mustata G, Morris MT, Leimgruber SS, Lee KH, Kashiwada Y, Close D, Lazo JS, Morris JC. A target-based high throughput screen yields Trypanosoma brucei hexokinase small molecule inhibitors with antiparasitic activity. PLoS Neglected Tropical Diseases. 2010;4:e659. doi: 10.1371/journal.pntd.0000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro RH, Hoffer BL. Effect of diclofop on the membrane potentials of herbicide-resistant and -susceptible annual ryegrass root tips. Plant Physiology. 1992;98:1415–1422. doi: 10.1104/pp.98.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AP, Surolia N, Surolia A. Triclosan inhibit the growth of the late liver-stage of Plasmodium. IUBMB Life. 2009;61:923–928. doi: 10.1002/iub.237. [DOI] [PubMed] [Google Scholar]
- Surolia N, Surolia A. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nature Medicine. 2001;7:167–173. doi: 10.1038/84612. [DOI] [PubMed] [Google Scholar]
- Tong L, Harwood HL. Acetyl-coenzyme A carboxylases: versatile targets for drug discovery. Journal of Cellular Biochemistry. 2006;99:1476–1488. doi: 10.1002/jcb.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigueira PA, Paul KS. Requirement for acetyl-CoA carboxylase in Trypanosoma brucei is dependent upon the growth environment. Molecular Microbiology. 2011;80:117–132. doi: 10.1111/j.1365-2958.2011.07563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White GM, Moss SR, Karp A. Differences in the molecular basis of resistance to the cyclohexanedione herbicide sethoxydim in Lolium multiflorum. Weed Research. 2005;45:440–448. [Google Scholar]
- WHO. [accessed on 6/1/2011];World Health Organization Fact Sheet No. 259 on African Trypanosomiasis. 2010 http://www.who.int/mediacentre/factsheets/fs259/en/
- Wright JP. Use of membrane-potential measurements to study mode of action of diclofop-methyl. Weed Science. 1994;42:285–292. [Google Scholar]
- Xiang S, Callaghan MM, Watson KG, Tong L. A different mechanism for the inhibition of the carboxyltransferase domain of acetyl-coenzyme A carboxylase by tepraloxydim. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20723–20727. doi: 10.1073/pnas.0908431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagnitko O, Jelenska J, Tevzadze G, Haselkorn R, Gornicki P. An isoleucine/leucine residue in the carboxyltransferase domain of acetyl-CoA carboxylase is critical for interaction with aryloxyphenoxypropionate and cyclohexanedione inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6617–6622. doi: 10.1073/pnas.121172798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Tweel B, Tong L. Molecular basis for the inhibition of the carboxyltransferase domain of acetyl-coenzyme-A carboxylase by haloxyfop and diclofop. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5910–5915. doi: 10.1073/pnas.0400891101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yang Z, Shen Y, Tong L. Crystal structure of the carboxyltransferase domain of acetyl-coenzyme A carboxylase. Science. 2003;299:2064–7. doi: 10.1126/science.1081366. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Powles SB. The molecular bases for resistance to acetyl co-enzyme A carboxylase (ACCase) inhibiting herbicides in two target-based resistant biotypes of annual ryegrass (Lolium rigidum) Planta. 2006a;223:550–557. doi: 10.1007/s00425-005-0095-x. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Powles SB. Six amino acid substitutions in the carboxyl-transferase domain of the plastidic acetyl-CoA carboxylase gene are linked with resistance to herbicides in a Lolium rigidum population. New Phytologist. 2006b;172:636–645. doi: 10.1111/j.1469-8137.2006.01879.x. [DOI] [PubMed] [Google Scholar]
- Zuther E, Johnson JJ, Haselkorn R, McLeod R, Gornicki P. Growth of Toxoplasma gondii is inhibited by aryloxyphenoxypropionate herbicides targeting acetyl-CoA carboxylase. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13387–92. doi: 10.1073/pnas.96.23.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]