Abstract
Loss of mobility influences the quality of life for patients with neuromuscular diseases. Common measures of mobility and chronic muscle damage are the six-minute walk test and serum creatine kinase. Despite extensive pre-clinical studies of therapeutic approaches, characterization of these measures is incomplete. To address this, a six-minute ambulation assay, serum creatine kinase, and myoglobinuria were investigated for the mdx mouse, a dystrophinopathy mouse model commonly used in pre-clinical studies. Mdx mice ambulated shorter distances than normal controls, a disparity accentuated after mild exercise. An asymmetric pathophysiology in mdx mice was unmasked with exercise, and peak measurements of serum creatine kinase and myoglobinuria were identified. Our data highlights the necessity to consider asymmetric pathology and timing of biomarkers when testing potential therapies for muscular dystrophy.
Keywords: mdx, Duchenne muscular dystrophy, Biomarkers, pre-clinical studies, ambulation
1. INTRODUCTION
Duchenne muscular dystrophy (DMD) is caused by mutations in the X-linked dystrophin gene that lead to a complete loss of dystrophin expression in muscle. Loss of dystrophin expression leads to a decrease in mobility and progressive muscle necrosis in DMD. Initial clinical diagnosis is from the identification of muscle weakness, elevated serum creatine kinase (sCK) or the appearance of myoglobinuria, and a muscle biopsy. A patient endpoint measure commonly used to assess mobility and efficacy of a therapeutic intervention is the six-minute walk test. In pre-clinical studies, common endpoint measures to assess the efficacy of a potential therapy are sCK and histological analysis of muscle pathology. Current treatments that improve endpoint measures in patients are limited. Part of the problem may be the design and interpretation of pre-clinical studies using the mdx mouse. This aspect is highlighted in three recent publications, demonstrating publication bias in basic research [1], over-optimistic or biased conclusions from animal models [2], and the need for quality pre-clinical data [3].
The mdx mouse, the animal model commonly used in pre-clinical studies for Duchenne muscular dystrophy, lacks dystrophin due to a point mutation in the dystrophin gene [4]. The mdx muscle pathology is similar to young DMD boys, but the clinical course is less severe than older patients. Investigations using the mdx mouse have provided insights into the complex mechanisms leading to muscle degeneration, progression of muscle weakness, and clinical presentation of the disease. Our goal for this study was to refine three pre-clinical endpoint measures for the mdx mouse: the six-minute ambulation assay, sCK, and myoglobinuria. More accurate pre-clinical endpoint measure for the mdx mice, used in conjunction with other refined endpoint measures, will enhance the translatability of pre-clinical data for DMD and possibly other loss of mobility conditions, to the clinic.
2. MATERIALS AND METHODS
2.1. Mice
The Jackson Laboratory mouse strains used in this study: C57BL/6J, B6.129P2-Nos3tm1Unc/J, B6.129S4-Nos1tm1Plh/J, B6.129P2-Nos2tm1Lau/J, and C57BL/10ScSn-Dmdmdx/J (abbreviated as C57BL/6, eNOS-null, nNOS-null, iNOS-null, and mdx, respectively). Other mice used were: B6.129Sv-MbtmDjg and B6.129Sv-UtrntmJrs (abbreviated as Mb-null [5] and Utrn-null [6], respectively). Mb-null;mdx, mdx;iNOS-null, mdx;nNOS-null, and mdx;Utrn-null mice were generated by intercrossing mice to produce mice on a mixed C57BL/6J;C57BL/10ScSnJ background. Phosphodiesterase 5A inhibitor treatment for mdx mice was previously described [7]. To limit mild strain differences that could be out bred [8], mice were bred at the University of Iowa to produce a large enough number of mice from the same breeding pair for each cohort of experiments. To minimize gender variation [8], mice were males at 10 weeks old unless otherwise stated, and had food and water ad libitum with exposure to the same care handler. Mice were synchronized, group housed, and tested in rooms on the same shifted 12:12-hour light:dark cycle. Testing time for all mice was at Zeitgeber time 14-17, when the mice were physiologically awake and active [9]. Mouse housing and activity rooms were under specific pathogen-free conditions. All mouse experiments were performed in accordance with animal usage guidelines and regulations set forth by NIH and the University of Iowa Institutional Animal Care and Use Committee. Mice were maintained within a centralized barrier animal facility at the University of Iowa directed by the Office of Animal Resources.
2.2. Six-minute ambulation distance test
Mouse activity was monitored using the VersaMax Animal Activity Monitoring System as previously described [7]. Mice were tested in individual chambers, for 6 × 1 minute intervals before and immediately after exercise. Data were transferred to a Microsoft Excel worksheet and calculations were done within the Excel program. For each minute of ambulation, the average total distance was determined and standard errors were calculated. The cumulative distance per minute up to six-minutes and the standard error for each minute were charted.
2.3. Magnetic resonance imaging (MRI)
Mice were anesthetized using a mixture of ketamine and xylazine (87.5 and 12.5 mg/kg, I.P.), and the plane of anesthesia was confirmed by absence of the pedal reflex. For scans done after exercise, imaging started less than 10 minutes after exercise to allow for mice to be anesthetized. Images were captured in the axial and coronal planes with a Varian Unity/Inova 4.7 T small-bore MRI system (Varian, Palo Alto, CA), using an acquisition consisting of T2-weighted fast spin-echo sequence (TR/TE = 5000/48ms) with in-plane resolution of 0.11 × 0.22 mm and slice thickness of 0.6 mm.
2.4. Treadmill exercise
Mice were mildly exercised with an adjustable variable-speed belt treadmill from AccuPacer as previously described [7].
2.5. Contractile properties of isolated muscle
Contractile properties were measured for isolated male mdx extensor digitorum longus muscles from 5 unexercised or 5 post-exercised mice ages 16-19 weeks. Measurements post-exercise were done within 15-30 minutes after exercise. Mice were anesthetized with an intra-peritoneal injection of 1.3% avertin (0.015 ml/g body weight) and muscles removed. The distal tendon was clamped to a post and the proximal tendon was tied to a dual mode servomotor (Aurora Scientific) with 6-0 suture. Contractile properties, optimal fiber length and maximal isometric tetanic force, were determined as previously described [10]. The lengthening contraction protocol (LCP) was previously described [11]. The force deficit was defined as the difference between pre- and post-LCP force expressed as a percentage of pre-LCP force [11]. P-value calculations were made using a Student t test.
2.6. Serum creatine kinase assay
Serum CK levels before and after exercise were measured as previously described [7]. For this study, blood collection and assays were done at designated time points. To reduce variability in data sets [8], all mice used were male, from the same breeding colony, and of the same age. The groups of mice tested for Figure 3A, 3F, and 3G were from different breeding colonies. Normal sCK levels were 100-500 U/L. Elevated sCK levels were greater than 500 U/L and hyperCKemia values were considered greater than 20,000 U/L. For blood collection, mice were placed in a restrainer, which they freely walked out of after blood collection. The initial drop of blood after the tail vein nick was wiped away and the subsequent 25 μl of blood was collected without tail manipulation. Serum was diluted 1:9 and 1:49 (v:v), to ensure activity levels within the limits of the assay, and assayed in triplicate. There was a minimum of 24 hours between blood collections from the same mouse. For sCK at different intervals, sCK from eight sets of mdx mice were measured 24 hours prior to exercise. On test day (0d), all eight sets of mice were exercised and sCK was assayed 2 hours post-exercise; 24 h following the initial exercise, the 1d set of mdx mice were run again and sCK was assayed; the same procedure was followed for all other groups for subsequent interval days after the initial run. For sCK at different intervals within a 120-minute time-frame after exercise, nine sets of mice were used with each set of mice representing a time point.
Fig. 3.
Post-exercise ambulation, serum creatine kinase (sCK), and myoglobinuria. (A) Six-minute ambulation distance post-exercise and after a repeated exercise 24 h later (n=4). (B)Serum CK from C57BL/6 and mdx mice before (pre) and at different time points after exercise (n=4 for each). (C) The presence of heme in the urine was assessed using urine dipsticks. Key for heme amounts is displayed (n=3 for each). (D) Urine collected pre- and post-exercise from littermate mdx and Mb-null;mdx mice were tested on dipstick strips (n=3 per analysis). (E) Serum CK before and 2 hours after exercise from Mb-null;mdx mice (n=4). (F) After an initial bout of exercise, eight sets of mdx mice, at different intervals (1d, 3d, 5d, 7d, 9d, 11d, 13d, and 14d), were re-exercised and sCK was measured (0d n=40, 1d n=5, 3d n=5, 5d n=6, 7d n=4, 9d n=4, 11d n=3, 13d n=6, and 14d n=7), and myoglobinuria was tested (inset). (G) Nine sets of mdx mice were exercised and then 24 h later were exercised again. Each set of mice represented a time point. Serum CK was assayed at T=0, 15, 30, 45, 60, 75, 90, 105, and 120 min after the repeated exercise. (T=0 n=11, T=15 n=6, T=30, 45, and 60 n=6, T=75, 90, 105, and 120 n=4), and myoglobinuria was tested with representative results in the inset. Error bars are SEM.
2.7. Urine collection and dip stick assay
Urine was collected from each mouse by allowing mice to urinate over a fresh piece of aluminum foil. Voided urine was transferred to a fresh microcentrifuge tube, the color noted, and tested immediately. Urine was applied to the reagent strip (Siemens Multistix® 10SG) in which the development of a blue color indicated high levels of a heme protein. None of the mice had hematuria.
2.8. Evans Blue Dye uptake imaging and quantification
Evans Blue Dye (EBD) injection was described previously [12]. After 3 h, mice were run on a treadmill. Mice were sacrificed and quadriceps and gastrocnemius muscles were collected. Axial 7 μm cryo-sections of skeletal muscle were visualized [12]. For quantification, each muscle was placed in 1 ml of N,N-dimethyl formamide for 48 h to extract the EBD. Absorbance of EBD in each solution was measured at 630 nm. Values were normalized by dividing by the weight of the tissue.
2.9. Statistical analysis
Unless otherwise stated, the data were calculated according to an analysis of variance. P-value calculations were made using a Student t test. Data are expressed as mean ± SEM. See Table S1 for values, averages, and SEM for ambulation.
3. RESULTS
3.1. Ambulation distance within six minutes to measure mdx mouse performance
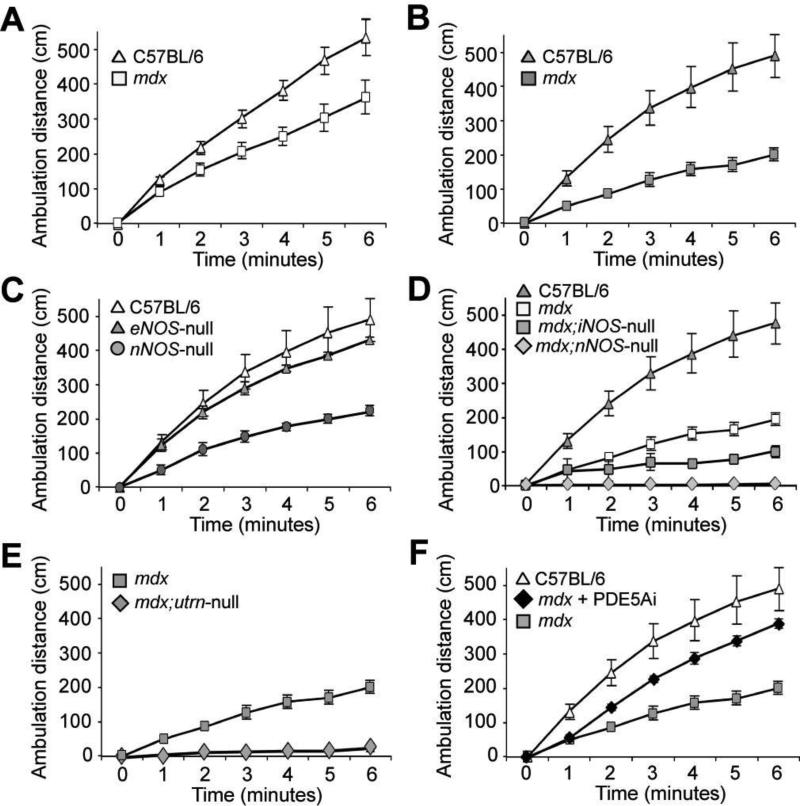
We combined and adapted the step-activity monitor and six-minute walk test in the form of a six-minute ambulation distance assay to assess mouse performance (Fig. 1). Comparing basal ambulatory performance over six minutes, we found that mdx mice show less ambulatory distance over six minutes than normal wild-type mice (Fig. 1A). This difference in ambulation performance between mdx and wild-type mice parallels that of DMD and control boys in the six-minute walk test [13]. Since ambulatory DMD boys are not sedentary like normally housed mdx mice, we ensured the clinical relevance of the ambulatory assay by physically challenging the mice before measuring their ambulatory performance. As ambulatory DMD boys do not perform hard activities to exhaustion, the mdx mice were challenged with mild and limited treadmill exercise, as previously described [7], before ambulation was assessed. We chose this mild, limited exercise as opposed to an exhaustive protocol, during their physiologically active time, to assay performance of active mice, and not that of muscle fatigued mice. C57BL/6 mice showed no significant difference in ambulation before and after exercise. However, with exercise, ambulation of mdx mice was affected and the difference between wild-type and mdx mouse ambulation distance was accentuated (Fig. 1B). These ambulation data clearly demonstrate a distinction between control and mdx mouse performance, particularly after mild exercise.
Fig. 1.
Six-minute ambulation distance as an endpoint measure in preclinical investigations for DMD. An ambulation distance assay was developed by adapting the step-activity and six-minute walk tests for mice using an open field activity chamber. (A) Basal ambulation distances versus time of C57BL/6 and mdx mice were charted. Ambulation distances were charted after mice were challenged with mild exercise (B, C, D, E, and F). (B) C57BL/6 versus mdx mice. (C) C57BL/6 versus eNOS- and nNOS-null mice. (D) C57BL/6 versus mdx, mdx;iNOS-null and mdx;nNOS-null mice (E) mdx versus mdx;Utrn-null mice. (F) C57BL/6 versus mdx treated with phosphodiesterase5A inhibitor and untreated mdx mice (n=6 for mdx, C57BL/6, nNOS-null, eNOS-null mice, and mdx+PDE5Ai; n=4 for mdx;nNOS-null, mdx;Utrn-null, and mdx;iNOS-null mice. Error bars indicate SEM).
To validate the ambulation assay, we tested post-exercise performance of other mouse models used to examine pathogenic mechanisms of dystrophin deficiency. Constitutively active endothelial and neuronal nitric oxide synthase (eNOS and nNOS, respectively) enzymes play important roles in vasomodulation, but only genetic deletion of nNOS in mice leads to mdx-like debility after mild exercise [7]. Basal ambulation distances of eNOS-null were similar to that of wild-type mice (fig. S1A). This similarity is consistent with the indistinguishable pre-exercise vertical activity between wild-type and eNOS- and nNOS-null mouse strains[7]. Post-exercise, however, only nNOS-null mice displayed reduced ambulation (Fig. 1C). Previous reports show that genetic ablation of nNOS in mdx mice does not alter mdx muscle pathology [14, 15] and that the induction of iNOS expression in mdx mice contributes to muscle fiber damage [16, 17]. Comparative ambulation distances from wild-type, mdx, mdx;iNOS-null, and mdx;nNOS-null mice demonstrated that genetic ablation of iNOS or nNOS decreased mdx performance after exercise (Fig. 1D), but had little effect on the basal ambulation of mdx mice (fig. S1B). Our results from comparing mdx and mdx;nNOS-null ambulation is consistent with our data comparing pre- and post-exercise activity of the same mice, which demonstrated that nNOS perturbation affects performance [7]. Despite reports that mdx;iNOS-null muscle have less muscle damage [17], their reduced ambulation compared to mdx mice was consistent with increased muscle pathology in 6- and 10- week old mdx;iNOS-null mice (fig. S2). The mdx;Utrn-null mice showed significant loss of ambulation after mild exercise (Fig. 1E), consistent with their more severe muscle pathology [18]. Lastly, since we previously found that phosphodiesterase 5A inhibition was able to improve post-exercise vertical activity [7], we compared post-exercise ambulation distance of control and mdx mice to that of mdx mice pre-treated with phosphodiesterase 5A inhibitor (Fig. 1F). Phosphodiesterase 5A inhibitor treatment improved mdx mouse ambulation. These data demonstrate that the six-minute ambulation assay is a highly responsive test of disease severity and functional response with mdx mice.
3.2. Bilateral analysis of mdx hind leg skeletal muscles and asymmetric pathology
When mdx mice were treadmill exercised, each mdx mouse, unlike wild-type controls, consistently leaned to one side of the treadmill. Notably, mdx mice subjected to multiple rounds of treadmill exercise leaned to the same side for each run suggesting that dystrophin deficiency causes asymmetric skeletal muscle pathology or weakness. To test this, we performed T2-weighted MRI of wild-type and mdx mouse hind-quarters, without exercise and after mild exercise (Fig. 2A). Post-exercise changes in T2-weighted MRI show up as lighter areas on scans and indicate changes in water content, which represent muscle edema or inflammation. Coronal MRI scans of wild-type hind leg muscles showed no T2-weighted changes (Fig. 2A, Wild-type panels). Coronal MRI scans of mdx mice without exercise showed no T2-weighted changes, except some mice showed small areas of fibrosis (Fig. 2A, mdx no-ex, small arrowheads). Coronal images of the same mdx mice all showed a large increase in T2-weighted changes after exercise (Fig. 2A, mdx post-ex, large arrowheads) that were always more in one leg than the contra-lateral leg, even in older mdx mice (fig. S3).
Fig. 2.
Asymmetric pathology of mdx mice. (A) Representative coronal magnetic resonance imaging scans of wild-type and mdx mouse hind quarters. Scans shown for no-ex and post-ex are from the same mouse. Localized areas of fibrosis (small white arrows) are detectable in mdx mice. T2-weighted changes (large white arrows) from mdx mice are indicative of muscle edema or inflammation (n=5 for each). (B) Representative microscope images from bilateral analysis of quadriceps muscles (quads) and gastrocnemius muscles (gastrocs) from mdx mice post-exercise. Areas of Evans Blue Dye uptake into damaged muscle fibers (white arrows) and side the mouse leaned on are noted (n=6). (C) Four mdx mice were I.V. injected with Evans Blue Dye then subjected to mild exercise. Quadriceps (quad) and gastrocnemius (gastroc) muscles were isolated separately. Total Evans Blue Dye was quantified for each muscle isolated. Measurements were done in triplicate, error bars are SEM. (D) Absolute differences in percent force deficits between left and right mdx extensor digitorum longus muscles. Error bars are SEM. (*) Significant difference (P=0.012) was observed between values for exercised mice compared with those of non-exercised mice.
The leaning and sometimes unilateral appearance of T2-weighted changes in mdx hind leg muscles suggested asymmetric pathology and weakness. We examined mdx muscle damage by EBD. Damaged or necrotic muscle fibers stain positive for endogenous extracellular proteins like albumin or IgG/M [4]. EBD binds albumin. In unexercised mdx mice, muscle fibers that stain positive for EBD are also the ones that are positive for albumin or mouse IgG/M [12, 19]. Thus, detection of EBD uptake into skeletal muscle is an accurate index of muscle pathology. To test asymmetric muscle pathology with mdx mice, we performed bilateral examination of EBD staining in mdx quadriceps and gastrocnemius muscles after exercise (Fig. 2B). We found that EBD detection in the quadriceps and gastrocnemii muscles was asymmetric in every mouse tested; in some cases, only one side featured detectable EBD/albumin uptake into muscle (fig. S3). Quantitatively, total EBD measurements confirmed variable EBD uptake from each mdx mouse and asymmetrical EBD uptake in mdx quadriceps and gastrocnemius muscles after exercise (Fig. 2C). To test if this asymmetrical pathology translated to asymmetrical muscle contractile properties, we compared the susceptibility to contraction-induced injury between the right and left muscles of mdx mice (Fig. 2D). For mice without exercise and mice after exercise, isolated extensor digitorum longus muscles were exposed to a lengthening contraction protocol and force deficit was determined. Exercise heightened the disparity between right and left muscles for susceptibility to injury. With exercise, the more injury-prone muscles of each mouse incurred force deficits of 50 ± 4% which were typically two-fold greater than the force deficits of the contra-lateral muscles, which averaged 31 ± 3%. Thus, post-stretch force deficits were also asymmetric in mdx mice.
3.3. Timing of indices of muscle pathology in mdx mice
Each time mdx exercised, they leaned to the same side. However, repeating the exercise did not significantly affect the post-exercise ambulation performance (Fig. 3A). This lack of alteration in ambulation after a repeated exercise suggested that there was no further muscle damage occurring. To test this, we first investigated indices of muscle damage and necrosis by determining the peak analysis time for sCK in wild-type and mdx mice after exercise (Fig. 3B). We found that sCK levels from mdx mice were generally elevated above control mouse levels without exercise (Fig. 3B, 4903.09 +/- 443.76 U/L). After exercise, sCK consistently peaked to hyperCKemia levels 2 hours after exercise (Fig. 3B, 55,850.77 +/- 5523.04 U/L) and gradually dissipated to below pre-exercise sCK levels (Fig. 3B, 565.45 +/- 200.66 U/L) by 24 hours. With wild-type mice, sCK readings were consistently below 500 U/L with a slight peak 1 hour post-exercise. Wild-type sCK activity readings went below pre-exercise levels by 6 hours.
We assayed the urine of mdx mice pre- and post-exercise using urine dipsticks (Fig. 3C). Similar to the pattern of sCK post-exercise, heme detection in mdx urine peaked 2 hours post-exercise and dissipated by 6 hours, with no evidence of heme in the urine by 24 hours post-exercise. To determine which heme protein the dipstick test was detecting, we crossed myoglobin-null (Mb-null) [5] and mdx mice, then tested Mb-null;mdx mouse sCK and urine after mild exercise (Fig. 3D, E). Mb-null;mdx were negative for the presence of heme in their urine post-exercise, but had elevated sCK before exercise and hyperCKemia post-exercise. Thus, a positive result on urine dipsticks from mdx mice indicates that mdx mice have exercise-induced myoglobinuria, which corresponded with hyperCKemia levels.
Consistent with no significant change in mdx ambulation performance, mdx sCK levels did not increase as it did during the initial exercise. We repeated the mild exercise with different sets of mdx mice at 1, 3, 5, 7, 9, 11, 13 and 14 days after the initial exercise, and measured sCK at the 2 hour time point (Fig. 3F). After the rise in sCK to hyperCK levels following the first bout of exercise on day 0 (0d), sCK from the mdx mice rose to that same level only when the interval between exercise was 14 days. Myoglobinuria followed the same pattern (Fig. 3F, inset).
To test for early peaking of sCK, we exercised mdx mice twice in a 24 hour interval, and collected sCK at several time points up to 2 hours following the second bout of exercise (Fig. 3G). Twenty-four hours since an initial mild exercise, different sets of mdx mice were mildly exercised again and sCK was measured at time intervals post-exercise (0, 15, 30, 45, 60, 75, 90, 105, and 120-minutes). Different sets of mice were used for each time point. Readings for sCK remained relatively close to elevated pre-exercise levels within each time point, consistent with ongoing regeneration. At the 2 hour mark, sCK readings were the lowest. Myoglobinuria was undetectable during this 2 hour analysis (Fig. 3G, inset).
4. DISCUSSION
The mdx mouse is extensively used as the pre-clinical animal model for dystrophin-deficiency to investigate therapeutic approaches for DMD. There are promising therapeutic interventions emerging for DMD that are in Phase I/II clinical trials like exon skipping, myostatins, and compounds that up-regulate utrophin [20]. Despite these recent advances, there are a multitude of potential therapies that have not succeeded in clinical trials, currently leaving patients with no curative treatment. This discrepancy has caused researchers to question the mdx mouse as a model for DMD as well as the validity of translating promising results from mdx preclinical studies into clinical trials. Our data demonstrate that when properly assayed, the mdx mouse is an ideal model for preclinical studies for DMD.
A six-minute ambulation test was designed for mice as an endpoint measure for DMD preclinical studies. Timed sub-maximal exercise performance is used as a standard outcome measure in clinical trials to test the efficacy of a treatment on improving mobility. This mild exercise is used for a variety of diseases in which patients experience physical limitations. The step-activity monitor and six-minute walk test are two forms of this kind of testing that are used with normal and ambulatory DMD boys [13, 21]. We combined these two assays into the six-minute ambulation distance test for mice. Like with DMD boys, mdx mice showed reduced ambulation. The reduced ambulation of the mdx;iNOS-null mouse was a surprise, but not so when we found that the muscles were consistently more fibrotic than mdx muscles. Our mdx;iNOS-null data suggest that some iNOS activity is necessary during the degeneration-regeneration of dystrophic muscle to prevent extensive muscle fibrosis. The reduced ambulation of the mdx;nNOS-null mice after mild exercise is in line with our previous findings of lower post-exercise vertical activity with these mice [7], and is also consistent with a report that shows myopathic functional deficits with reduced bulk and impaired contractile function in nNOS-deficient muscle [22]. The increased performance in the six-minute ambulation assay of mdx mice treated with phosphodiesterase 5A inhibitor was consistent with our previous data that this acute pre-treatment increases mdx post-exercise vertical activity [7]. This pharmacological treatment along with the other mdx mouse models used in this study validated the six-minute ambulation assay as a performance outcome measure for DMD pre-clinical studies using the mdx mouse. In addition, we demonstrated that after mild exercise with the mdx mouse, this assay is responsive to varying degrees of muscle pathology in that ambulation increased or decreased with known changes in muscle function among the different mdx mouse models and with a pharmacological treatment.
The combination of the exercise and ambulation assay provided several informative pieces of data. The reduced ambulation with mdx mice is in agreement with previous studies that show mdx mice have reduced locomotor activity based on a SHIRPA test [23] and reduced voluntary running compared to their wild-type counterparts [24-27]. Even the leaning of mdx mice during exercise on the treadmill was not too surprising since it is similar to what ambulatory DMD boys do to maintain balance and gait while walking [28]. What was important to this observation of leaning to one side of the treadmill was that we did bilateral analysis of muscle pathology and found mdx mice have asymmetric muscle pathology in their quadriceps and gastrocnemius skeletal muscles and have asymmetric muscle force. These findings are significant, as several therapeutic studies for DMD use muscle pathology as an endpoint measure, and many therapeutic preclinical studies test one leg of an mdx mouse and use the contra-lateral leg as a control.
The asymmetric mdx muscle pathology may be similar to the variability in dystrophinopathy patients, with some muscles being affected while neighboring, or other muscles being spared from pathology. Like with humans, mice have a lateral preference [29], i.e. display handedness, but in generally, most mouse strains show an equal distribution of right- and left-handedness [30, 31]. Whether this laterality influences mdx physiopathology and susceptibility to contraction-induced injury or muscle pathology influences the laterality of mdx mice, remains unknown. Innate laterality could influence mdx pathology, whereby the dominant side gets more use than the other and eventually becomes more severely affected. Conversely, asymmetric mdx muscle pathology could influence laterality. Clinically, muscle weakness in DMD patients is often described as symmetric, in that both left and right sides will show muscle weakness. However, DMD patients do have asymmetric muscle weakness. The weakness is symmetrical in that both sides show muscle weakness, but the degree of left to right weakness may be variable. The variability could be due to handedness or to a learned adaptive response. Whether there is asymmetric muscle pathology with DMD patients is unknown as systematic bilateral analysis of skeletal muscle would require multiple muscle biopsies that would be difficult to ethically justify. Nevertheless, our findings demonstrate that asymmetric muscle pathology as well as asymmetric weakness should be considered when taking biopsies for testing therapies in animal models, particularly the mdx mouse.
The ongoing necrosis of skeletal muscle and the asymmetric pathology and weakness could contribute to the reduced ambulation and leaning during exercise of mdx mice. If an mdx mouse leaned to one side during the treadmill exercise, the same mouse would lean to the same side when the treadmill exercise was repeated. In addition, repeating the exercise with the same mouse the next day did not significantly affect the post-exercise ambulation performance. Consistent with no change in ambulation performance, mdx sCK levels did not increase as it did during the initial exercise. Despite each skeletal muscle fiber being susceptible to contraction-induced injury due to the lack of dystrophin, and chronic inflammation in the mdx mouse [32], this lack of increase in sCK after subsequent exercise suggested that repeat exercise does not result in further muscle damage. The release of CK and myoglobin from normal muscle, however, is thought to activate an inflammatory response that enhances clearance of these proteins, such that subsequent muscle damage fails to give rise to elevated muscle-cell proteins in the blood [33-35]. We found no evidence of sCK or myoglobinuria peaking sooner. It is not clear why biomarkers of muscle damage follow this pattern. Nevertheless, our data demonstrate the importance of mouse activity and analysis time for these muscle damage biomarkers. If the normally sedentary mdx mouse is active one to fourteen days before a sCK analysis, sCK levels will likely be lower than expected when analyzed. Thus, a low sCK reading in an mdx mouse preclinical study may not be a response to the therapeutic agent, but simply a reflection of mouse activity during the trial period.
In summary, we present the use of a six-minute ambulation test, in conjunction with refined analyses of muscle pathology and biomarkers of muscle damage, as pre-clinical endpoint measures relevant for muscular dystrophy pre-clinical trials using mdx mice. Future pre-clinical study designs for muscular dystrophy should take into account possible baseline asymmetry of muscle pathology. Our data is consistent with other reports that describe physiological function being altered after performance testing with mdx mice [23], thus sCK levels can also be affected by pre-sCK assessment exercise and/or activity. Therefore, future study designs should consider the circadian rhythm and mouse activity in mouse physiological performance analyses before muscle damage biomarkers are assayed. Positive findings using these endpoint measures may enhance the translatability of future preclinical studies for Duchenne and other muscular dystrophies.
Supplementary Material
SUPPLEMENTARY MATERIAL
Fig. S1A-D. Pre-exercise six-minute ambulation. A. Comparing C57BL/6 with eNOS-null mice. B. Comparing C57BL/6 with mdx, mdx;iNOS-null, and mdx;nNOS-null mice. C. Comparing mdx with mdx;Utrn-null mice. D. Comparing C57BL/6 with mdx +/- phosphodiesterase 5A inhibitor.
Table S1. Pre-exercise six-minute ambulation data.
Table S2. Post-exercise six-minute ambulation data.
Fig. S1E. Pre- and post-exercise six-minute ambulation assay for nNOS-null mice
Fig. S2. Muscle pathology of mdx;iNOS-null mice.
Fig. S3. Asymmetric muscle pathology in mdx mice.
References for supplemental material.
Acknowledgments
We thank M.G. Anderson, S.A. Moore and R.K. Shields for comments, and K. Garringer, M.J. Schneider, and S.J. Prouty for technical assistance. We thank D.J. Garry (University of Minnesota) for the Mb-null mice and J.R. Sanes (Harvard Medical School) for the Utrn-null mice. This work was supported in part by a Paul D. Wellstone Muscular Dystrophy Cooperative Research Center Grant (NINDS/U54-NS053672). Y.M.K. was supported by an NIH/National Research Service Award (NRSA) Training Fellowship from the University of Iowa Cardiovascular Interdisciplinary Research (NHLBI/T32 HL07121), an individual NRSA Fellowship (NIAMS/F32-AR48742), an NIAMS/R01-AR051199, a Senator Paul D. Wellstone Fellowship (NINDS/U54-NS053672-S), an American Recovery and Reinvestment Act grant (NIAMS/R01-AR051199-S) and an Institute for Clinical and Translation Science Pilot Grant Award (NCRR/UL1-RR0024979). E.P.R. was supported by a Muscular Dystrophy Association Development Grant (67814). K.P.C. is an Investigator of the Howard Hughes Medical Institute. All authors revised the manuscript and gave final approval for the version to be published.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All the authors concur with the submission of this manuscript. This manuscript is approved by the University Of Iowa Office Of Research. If accepted, this manuscript shall not be published elsewhere in the same form in either the same or any other language, without consent of the Editor or Publisher.
The authors declare no competing financial interests
REFERENCES
- 1.Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8:e1000344. doi: 10.1371/journal.pbio.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O'Collins V, et al. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.London AJ, Kimmelman J, Emborg ME. Research ethics. Beyond access vs. protection in trials of innovative therapies. Science. 2010;328:829–30. doi: 10.1126/science.1189369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 5.Garry DJ, Ordway GA, Lorenz JN, Radford NB, Chin ER, Grange RW, et al. Mice without myoglobin. Nature. 1998;395:905–8. doi: 10.1038/27681. [DOI] [PubMed] [Google Scholar]
- 6.Grady RM, Merlie JP, Sanes JR. Subtle neuromuscular defects in utrophin-deficient mice. J Cell Biol. 1997;136:871–82. doi: 10.1083/jcb.136.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–5. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spurney CF, Gordish-Dressman H, Guerron AD, Sali A, Pandey GS, Rawat R, et al. Preclinical drug trials in the mdx mouse: assessment of reliable and sensitive outcome measures. Muscle Nerve. 2009;39:591–602. doi: 10.1002/mus.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson S, Lupi D, Hankins MW, Peirson SN, Foster RG. The effects of rod and cone loss on the photic regulation of locomotor activity and heart rate. Eur J Neurosci. 2008;28:724–9. doi: 10.1111/j.1460-9568.2008.06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang R, Urso ML, Zambraski EJ, Rader EP, Campbell KP, Liang BT. Adenosine A(3) receptor stimulation induces protection of skeletal muscle from eccentric exercise-mediated injury. Am J Physiol Regul Integr Comp Physiol. 2010;299:R259–67. doi: 10.1152/ajpregu.00060.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol. 1997;139:375–85. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Elfring GL, et al. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve. 2009 doi: 10.1002/mus.21544. [DOI] [PubMed] [Google Scholar]
- 14.Chao DS, Silvagno F, Bredt DS. Muscular dystrophy in mdx mice despite lack of neuronal nitric oxide synthase. J Neurochem. 1998;71:784–9. doi: 10.1046/j.1471-4159.1998.71020784.x. [DOI] [PubMed] [Google Scholar]
- 15.Crosbie RH, Straub V, Yun HY, Lee JC, Rafael JA, Chamberlain JS, et al. mdx muscle pathology is independent of nNOS perturbation. Hum Mol Genet. 1998;7:823–9. doi: 10.1093/hmg/7.5.823. [DOI] [PubMed] [Google Scholar]
- 16.Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–30. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet. 2009;18:482–96. doi: 10.1093/hmg/ddn376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90:729–38. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 19.Wooddell CI, Zhang G, Griffin JB, Hegge JO, Huss T, Wolff JA. Use of Evans blue dye to compare limb muscles in exercised young and old mdx mice. Muscle Nerve. 2010;41:487–99. doi: 10.1002/mus.21527. [DOI] [PubMed] [Google Scholar]
- 20.Pichavant C, Aartsma-Rus A, Clemens PR, Davies KE, Dickson G, Takeda S, et al. Current Status of Pharmaceutical and Genetic Therapetuic Approaches to Treat DMD. Mol Ther. 2011;19:830–40. doi: 10.1038/mt.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald CM, Widman LM, Walsh DD, Walsh SA, Abresch RT. Use of step activity monitoring for continuous physical activity assessment in boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 2005;86:802–8. doi: 10.1016/j.apmr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Percival JM, Anderson KN, Gregorevic P, Chamberlain JS, Froehner SC. Functional deficits in nNOSmu-deficient skeletal muscle: myopathy in nNOS knockout mice. PLoS One. 2008;3:e3387. doi: 10.1371/journal.pone.0003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafael JA, Nitta Y, Peters J, Davies KE. Testing of SHIRPA, a mouse phenotypic assessment protocol, on Dmd(mdx) and Dmd(mdx3cv) dystrophin-deficient mice. Mamm Genome. 2000;11:725–8. doi: 10.1007/s003350010149. [DOI] [PubMed] [Google Scholar]
- 24.Buyse GM, Van der Mieren G, Erb M, D'Hooge J, Herijgers P, Verbeken E, et al. Long-term blinded placebo-controlled study of SNT-MC17/idebenone in the dystrophin deficient mdx mouse: cardiac protection and improved exercise performance. Eur Heart J. 2009;30:116–24. doi: 10.1093/eurheartj/ehn406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter GT, Wineinger MA, Walsh SA, Horasek SJ, Abresch RT, Fowler WM., Jr. Effect of voluntary wheel-running exercise on muscles of the mdx mouse. Neuromuscular disorders : NMD. 1995;5:323–32. doi: 10.1016/0960-8966(94)00063-f. [DOI] [PubMed] [Google Scholar]
- 26.Dupont-Versteegden EE, McCarter RJ, Katz MS. Voluntary exercise decreases progression of muscular dystrophy in diaphragm of mdx mice. Journal of applied physiology. 1994;77:1736–41. doi: 10.1152/jappl.1994.77.4.1736. [DOI] [PubMed] [Google Scholar]
- 27.Wineinger MA, Abresch RT, Walsh SA, Carter GT. Effects of aging and voluntary exercise on the function of dystrophic muscle from mdx mice. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 1998;77:20–7. doi: 10.1097/00002060-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Gaudreault N, Gravel D, Nadeau S, Houde S, Gagnon D. Gait patterns comparison of children with Duchenne muscular dystrophy to those of control subjects considering the effect of gait velocity. Gait Posture. 2010 doi: 10.1016/j.gaitpost.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Collins RL. On the inheritance of handedness. I. Laterality in inbred mice. J Hered. 1968;59:9–12. doi: 10.1093/oxfordjournals.jhered.a107656. [DOI] [PubMed] [Google Scholar]
- 30.Signore P, Chaoui M, Nosten-Bertrand M, Perez-Diaz F, Marchaland C. Handedness in mice: comparison across eleven inbred strains. Behav Genet. 1991;21:421–9. doi: 10.1007/BF01065977. [DOI] [PubMed] [Google Scholar]
- 31.Signore P, Nosten-Bertrand M, Chaoui M, Roubertoux PL, Marchaland C, Perez-Diaz F. An assessment of handedness in mice. Physiology & behavior. 1991;49:701–4. doi: 10.1016/0031-9384(91)90305-8. [DOI] [PubMed] [Google Scholar]
- 32.Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black CD, McCully KK. Muscle injury after repeated bouts of voluntary and electrically stimulated exercise. Med Sci Sports Exerc. 2008;40:1605–15. doi: 10.1249/MSS.0b013e3181788dbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyatt JP, Clarkson PM. Creatine kinase release and clearance using MM variants following repeated bouts of eccentric exercise. Med Sci Sports Exerc. 1998;30:1059–65. doi: 10.1097/00005768-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Marqueste T, Giannesini B, Fur YL, Cozzone PJ, Bendahan D. Comparative MRI analysis of T2 changes associated with single and repeated bouts of downhill running leading to eccentric-induced muscle damage. J Appl Physiol. 2008;105:299–307. doi: 10.1152/japplphysiol.00738.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY MATERIAL
Fig. S1A-D. Pre-exercise six-minute ambulation. A. Comparing C57BL/6 with eNOS-null mice. B. Comparing C57BL/6 with mdx, mdx;iNOS-null, and mdx;nNOS-null mice. C. Comparing mdx with mdx;Utrn-null mice. D. Comparing C57BL/6 with mdx +/- phosphodiesterase 5A inhibitor.
Table S1. Pre-exercise six-minute ambulation data.
Table S2. Post-exercise six-minute ambulation data.
Fig. S1E. Pre- and post-exercise six-minute ambulation assay for nNOS-null mice
Fig. S2. Muscle pathology of mdx;iNOS-null mice.
Fig. S3. Asymmetric muscle pathology in mdx mice.
References for supplemental material.