Abstract
Orchestrated excitation–contraction coupling in heart muscle requires adequate spatial arrangement of systems responsible for ion movement and metabolite turnover. Co-localization of regulatory and transporting proteins into macromolecular complexes within an environment of microanatomical cell components raises intracellular diffusion barriers that hamper the mobility of metabolites and signaling molecules. Compared to substrate diffusion in the cytosol, diffusional restrictions underneath the sarcolemma are much larger and could impede ion and nucleotide movement by a factor of 103–105. Diffusion barriers thus seclude metabolites within the submembrane space enabling rapid and vectorial effector targeting, yet hinder energy supply from the bulk cytosolic space implicating the necessity for a shunting transfer mechanism. Here, we address principles of membrane protein compartmentation, phosphotransfer enzyme-facilitated interdomain energy transfer, and nucleotide signal dynamics at the subsarcolemma–cytosol interface. This article is part of a Special Issue entitled ‘Local Signaling in Myocytes’.
Keywords: ADP, ATP, ATP-sensitive K+ channel, Energy metabolism, Ion homeostasis, Macromolecular complex, Partitioning, Phosphotransfer, Submembrane
1. Introduction
Established in Ancient Rome, the strategic principle divide et impera (divide and conquer) implies the fragmentation of a large concentration of power into pieces that individually can be more readily defeated and then managed allowing to gain and maintain control on a whole dominion. In biology, the evolutionary advantage of eukaryotes over prokaryotes is signified by intracellular partitioning of biological processes enabling more efficient and better controlled cellular and ultimately body metabolic well-being [1,2]. Localization of metabolic pathways within organelles enables eukaryotic cells to concomitantly carry out distinct metabolic activities. To this end, compartmentation ensures a targeted hierarchy that: i) establishes physical boundaries for metabolic reactions; ii) creates microenvironments for spatial/temporal regulation of biological processes; and iii) secures vectors of cell response matching environmental signaling—all through functionally specialized cellular spaces and specifically positioned enzymes and substrates within organelle compartments [3]. With compartmentation, environmental signaling and feed-back rectification of cellular metabolic balance dictate the operation of molecular effectors, such as ion channels, in a milieu of physical barriers imposed by biomembranes and structural protein complexes [4]. A case in point is the high-fidelity orchestration of cardiac muscle function, which requires a fine-tuned system of inter-compartment communication for proper operation, exemplified by movement of ions and energy substrates in excitation–contraction coupling. Recent evidence suggest that bringing signaling proteins and ion channels into spatial proximity, in a not well-mixed cytoplasm, is an essential principle of how diverse cellular targets can decipher and translate promiscuous messengers, e.g., intracellular Ca2+ or cyclic nucleotides, into selective responses. Thus assessing the principles and properties of compartmentation of ion channels, metabolites and energy pathways in a partitioned cell environment expands current understanding of heart homeostasis maintenance.
2. Ion channels in compartmentalized membrane macromolecular complexes
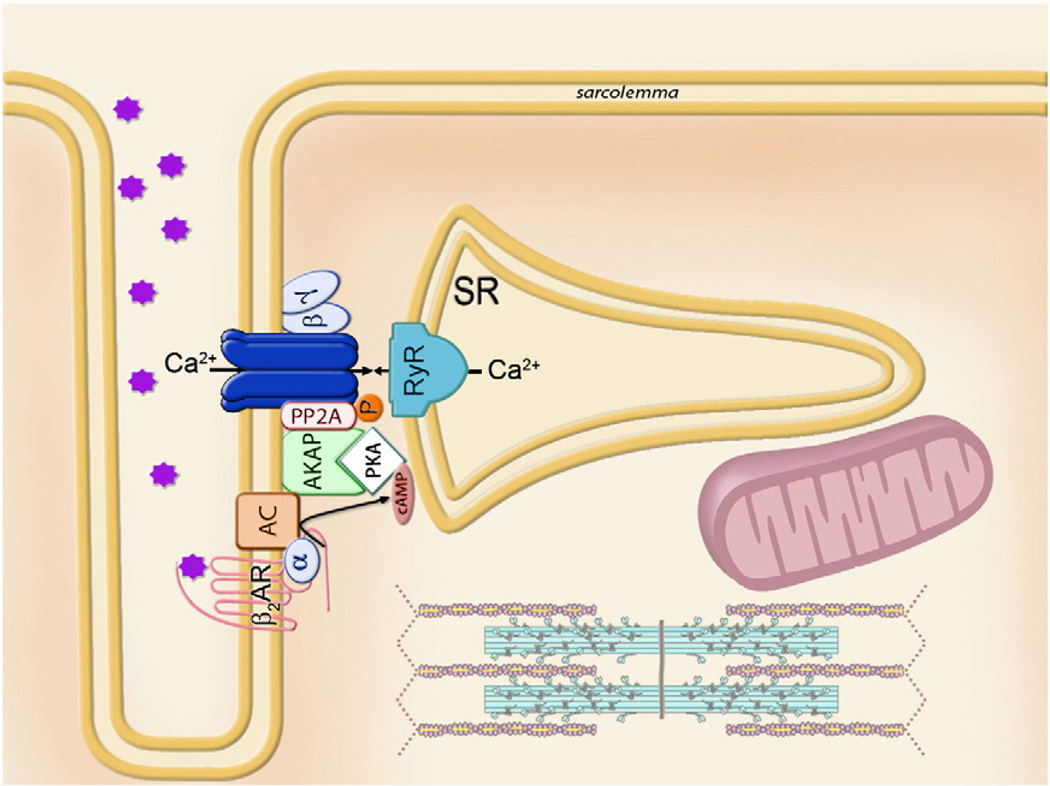
A relevant question in cell signaling, and in particular cardiac physiology, is how a limited variety of intracellular second messengers can differentially relay environmental signals associated with a large number of surface neurotransmitter or hormone receptors to effectively regulate electrical, mechanical and metabolic activity [5–8]. Indeed, many membrane receptors interact with heteromeric G-proteins in response to ligand binding [9]. Dissociation of G-proteins into Gα and Gβγ subunits initiates the production of second messengers (e.g., cAMP) and propagation of signals within the cell resulting in altered activity of different downstream effector proteins, such as enzymes and ion channels [10]. The rationale has emerged that cells ensure specificity of signaling pathways through organized macromolecular signaling complexes in the plasma membrane that may contain critical transducers, e.g., adenylate cyclase, protein kinase A (PKA) and PP2A phosphatase, as well as L-type Ca2+ channels all coupled to G-protein receptors [11,12]. Assuming that components of macromolecular complexes are physically anchored in the membrane vicinity, rapid and specific activation of the correct signaling pathway can be ensured. Such role for PKA anchoring proteins (AKAPs) is underscored by a specific binding motif to regulatory subunits (RII) serving as an adapter that maintains the holoenzyme at specific subcellular locations (Fig. 1) [13].
Fig. 1.
Model of a G-protein coupled signaling multiprotein module, which integrates β2-adrenoreceptor (β2AR) with the heteromeric G-protein (α and βγ subunits) and adenylate cyclase (AC), which by producing cAMP activates protein kinase A (PKA), anchored to the membrane through AKAP. Phosphorylation status and therefore operation of the final effector, L-type Ca2+ channel, is regulated by the ratio between local PKA and phosphatase (PP2A) activities. The Ca-channel macromolecular complex ensures rapid and vectorial activation of the specific effector and downstream pathways, exemplified here as Ca2+ release from sarcoplasmic reticulum (SR) through the ryanodine receptors (RyR).
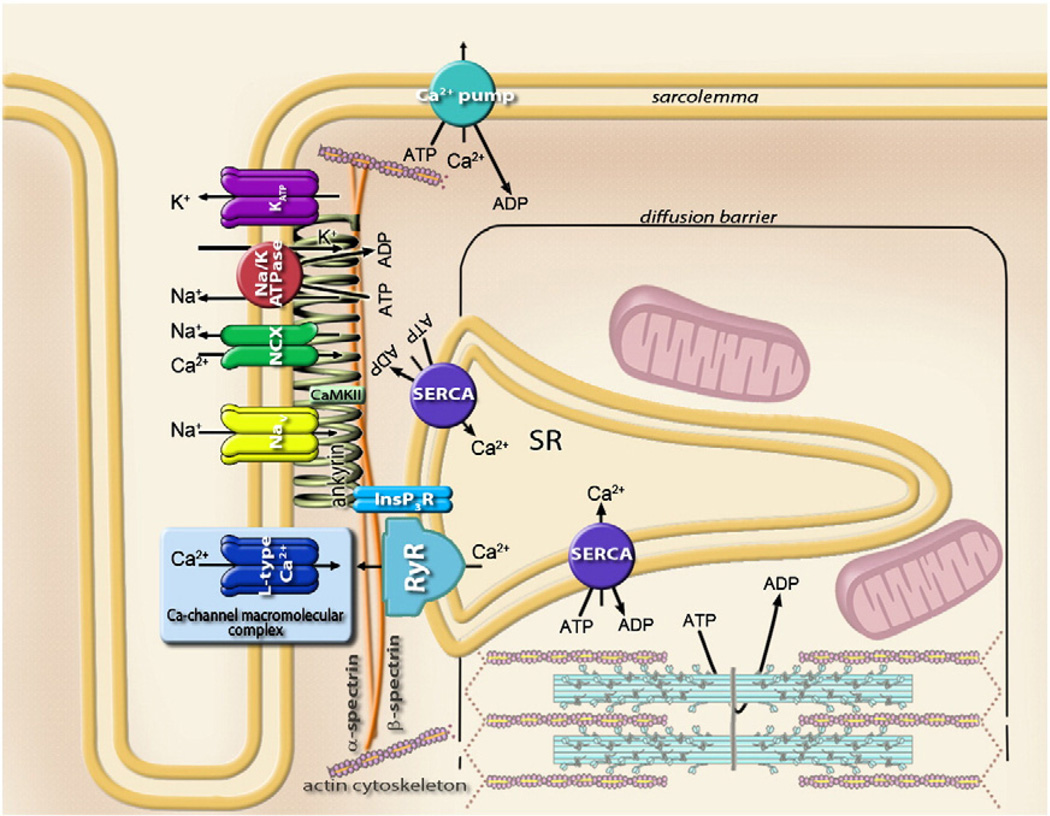
An alternative mechanism of protein clustering has been associated with the anchoring role of cytoskeletal proteins, such as spectrins and ankyrins, which line the intracellular side of the plasma membrane [14–16]. Specifically, ankyrin-G and βIV-spectrin are critical structural components required for clustering of voltage-gated Na+ channels (Nav1.5) [17–19] and calcium/calmodulin-dependent protein kinase II (CaMKII) in cardiac cells [20,21]. Disruption of βIV-spectrin-dependent co-localization of CaMKII and Nav1.5 results in lack of phosphorylated Na+ channels and aberrant cell excitability [21]. High-resolution imaging, using a glass micropipette as a probe in scanning ion conductance microscopy [22,23], has revealed the position of ATP-sensitive potassium (KATP) channels in the Z-grooves of cardiac myocytes [24], sarcolemmal regions that interact with the intracellular cytoskeleton and where transverse tubules (T-tubules) create membrane junctions with the sarcoplasmic reticulum (SR) [25]. In the same region, voltage-gated Na+ channels along with the IP3 receptor, Na+-Ca2+ exchanger and Na+/K+ ATPase were identified, and all, including KATP channels, found to be coordinated through the membrane-binding domains of ankyrins to the spectrin-actin based membrane cytoskeleton (Fig. 2) [26–29]. Such compartmentation is efficient at coordination of essential components that control excitation–contraction coupling in a manner that is rapid, yet precise. Readiness for a prompt response is supported by voltage-gated Ca2+ (CaV) channels distributed throughout T-tubules [25,30]. Conformational rearrangements of CaV proteins in response to changes in membrane potential are translated directly to ryanodine receptors (RyR) initiating Ca2+ release from the sarcoplasmic reticulum [31]. Indeed, the so-called Calcium Release Units (CRU), a group of Ca2+ permeant RyR or IP3R channels clustered in the membrane of the sarcoplasmic reticulum, are responsible for the elementary intracellular Ca2+ release events (calcium sparks) that compartmentalize to the Z-line/T-tubule regions of sarcomeres [32–34]. Thus, the local ensemble of channel proteins directly regulating membrane excitability, along with exchangers and pumps aimed at ion sequestration supporting ion homeostasis, ensure rapid and near simultaneous release of Ca2+ to bind adjacent troponin molecules and enable high-fidelity regulation of myosin–actin contraction (Figs. 1 and 2) [12].
Fig. 2.
Sarcolemmal metabolic unit comprised of the cardiac ankyrin-B membrane-associated protein complex, the Ca-channel macromolecular complex (see Fig. 1) and components of the sarcoplasmic reticulum. Ankyrin bound to β2-spectrin targets Na+/K+ ATPase, voltage-dependent Na+ (Nav) and ATP-sensitive K+ (KATP) channels, Na+–Ca2+ exchanger (NCX), IP3 receptor (IP3R), and, through interactions with obscurin (not shown), protein phosphatase 2A (PP2A). Coordination of calmodulin-dependent kinase (CaMKII) by β-spectrin in the proximity to Na+ channel is also indicated.
A remarkable mechanism of ion channel localization appears to be the dynamic compartmentation between the endoplasmic reticulum (ER) and voltage-independent Ca2+ channels responsible for store-operated Ca2+ entry (SOCE), also known as Ca2+-release-activated Ca2+ (CRAC) current identified in non-excitable cells [12,35] as well as embryonic and neonatal cardiac myocytes [36]. While Ca2+ release from ER in response to the phospholipase C (PLC) product, inositol-1,4,5-trisphosphate (IP3), triggers transient intracellular Ca2+ elevation, the mechanism of sustained subsequent Ca2+ entry is largely unknown [35,37]. The hallmark observation that slow, over seconds, activation of SOCE through plasma membrane was not initiated by a rise in cytoplasmic Ca2+ but rather started following depletion of Ca2+ in the endoplasmic reticulum stores [38], suggested an intimate interaction between unidentified components of ER and CRAC channels. Discovery of the stromal interaction molecule 1 (STIM1) as an ER Ca2+ sensor, and Orai1 as a functional component of CRAC channels, were key steps in defining the mechanism of SOCE [39,40]. Depletion of Ca2+ stores is sensed by STIM1, resulting in accumulation in ER regions adjacent to the plasma membrane. In turn, Orai1 molecules move from a dispersed distribution in plasma membrane to be assembled directly opposite to STIM1 clusters, presumably compartmentalizing both proteins in close proximity and enabling STIM1 to activate CRAC channels [41,42].
3. Diffusion restriction—new dimension in cell signaling
To ensure effective and precise control over cellular functions, spatial distribution of individual membrane components, which facilitates their recognition and interaction, has to be accompanied by an additional dimension of intracellular compartmentation. Analysis of the involvement of the Na+-Ca2+ exchanger in cardiac excitation–contraction coupling suggested a unique component of cardiomyocyte architecture, namely a diffusion barrier underneath the sarcolemma that secludes a so-called “fuzzy space” from the rest of the cell [43]. The Na+–Ca2+ exchanger may support Ca2+ influx by “pumping” internal Na+ out, or Ca2+ efflux by “pumping” external Na+ into the cell in a stoichiometry of 3 Na+ to 1 Ca2+ depending on the thermodynamic driving force and electrochemical gradient for Na+ ions [44]. In a ventricular myocyte with dimensions 10 by 10 by 100 µm, volume 10−11 l and capacitance 50 pF, depolarization from −80 mV to +20 mV would require a charge translocation of ~5 × 10−12 Coulomb (30 × 106 ions) that can increase Na+ concentration in the whole cell volume by only 5 µM above normal intracellular Na+ levels of ~8 mM. This average cytosolic Na+ concentration is insufficient to activate Na+–Ca2+ exchange due to the much higher value of the apparent dissociation constant for Na+ estimated at ~20 mM [45]. Therefore the volume into which entering Na+ ions accumulate must be significantly restricted. If such diffusion-restricted space is limited to 100–200 nm below the inner surface of the sarcolemma (0.02–0.04% of total cell volume), a 2500–5000-fold elevation of Na+ concentration would be then sufficient to rise Ca2+ levels in this hypothetical volume through the Na+–Ca2+ exchanger between 1 and 10 µM/ms contributing, thereby, to excitation–contraction coupling [43,45]. In subsequent studies the apparent diffusion coefficient for Na+ ions in the “fuzzy space” was estimated to be 103 to 104-fold below that expected in bulk cytoplasm [46].
An analogous approach that implemented ion channels as prototypic membrane sensors allowed understanding of the spatial and temporal nature of cAMP as well as ATP/ADP signaling [47–51]. Having established nucleotide-dependent regulation of cyclic nucleotide-gated (CNG) and ATP-sensitive K+ (KATP) channels, the local concentration of nucleotides in the plasma membrane proximity, where channels reside, could also be determined [52–56].
Evidence obtained using cyclic nucleotide-sensing CNG channels indicate the presence of significant diffusional limitations for cAMP near the inner surface of the plasma membrane [57]. Specifically, the operation of CNG channels indicated that submembrane elevation of cAMP in response to activation of adenylyl cyclase (AC) was approximately 12-fold higher than throughout the cell [52]. Co-localization of CNG channels and AC would be insufficient to explain this difference since in the absence of a diffusional barrier each newly synthesized cAMP molecule would diffuse away from the sensor faster than the next one can be produced, provided that the diffusion coefficient for cAMP is 3 × 10−6 cm2/s (the coefficient for free diffusion of cAMP in cytosol) [57]. Analysis of cAMP flux from the submembrane to the cytosolic space revealed that the local concentration of cAMP measured by CNG channels required that diffusion within the 200 nm near membrane “fuzzy space” must be limited by an apparent diffusion coefficient of ~10−13 cm2/s [52,57], indicating that the submembrane compartment is an unstirred zone with extremely restricted nucleotide diffusion.
Notably, an alternative and independent analysis of nucleotide fluxes in the submembrane space, performed with KATP channels, provides close estimates for diffusion restrictions [49,53,55]. In contrast to CNG channels that are activated by a rise in cAMP, KATP channels, by virtue of tight coupling with cellular energetics, open in response to a drop of intracellular ATP and a concomitant increase of ADP levels [50,58–60]. As cytosolic ATP levels are well maintained by intracellular energetics and KATP channel activity is poorly correlated with changes in total ATP/ADP [61–63], it has been proposed that sarcolemma-associated ATPases depress local ATP concentration setting a nucleotide ratio at the channel site, within the diffusion-restricted submembrane space, distinct from cytosolic levels [49,55,58,59,61,64,65]. This notion is in accord with the finding that KATP channels complement membrane macromolecular complexes that also include Na+ channels, Na+/K+ ATPase and Na+–Ca2+ exchangers, and are co-localized with Ca2+ channels and Ca2+-scavenging ATPases [24,26,27,66,67]. Thus, compartmentalized KATP channels appear to function in a microenvironment where local nucleotide content is distinct from average cytosolic levels [4,50]. Indeed, application of 20–40 mM of Na+ to permeabilized cardiac myocytes [49] or to giant excised patches [53] produced ouabain-sensitive KATP channel openings, as a result of elevated Na+/K+ ATPase activity, despite the inhibitory bulk ATP concentration. Even in excised giant patches, where plasma membrane itself and structures adjacent to the sarcolemma could be significantly disturbed, the Na+/K+ ATPase-driven depletion of ATP, detected by KATP channels within the 200 nm submembrane space, can be explained by an apparent diffusion coefficient of 7 × 10−10 cm2/s [53], much lower than ~10−6 cm2/s, the coefficient of free diffusion for nucleotides in the cytosol [68].
In cardiac cells, myoplasmic ATP under normal (aerobic) conditions is estimated at ~6–10 mM, and under severe metabolic poisoning by cyanide total ATP levels drop to 80% of control levels [61,65,69]. This drop is insufficient to explain KATP channel-driven shortening of action potentials in response to chemical ischemia. Nucleotide-dependent KATP channel gating indicates that for MgADP to open at least 1% of KATP channels, which has been suggested to be sufficient to elicit detectable action potential shortening [58], ATP at the channel site needs to be reduced to <3 mM [55]. Assuming an average bulk intracellular ATP concentration of 7 mM, a sarcolemmal ATPase flux (JATPase) of 4.7 × 10−6 µmol/cm2/s, estimated in working hearts based on 18O-asssited 31P NMR [55,70], can induce a 4 mM drop of ATP levels only at an apparent diffusion coefficient of 2.3 × 10−11 cm2/s within a 200 nm submembrane space [55]. Adding to consideration a local adenylate kinase activity (2·ADP ↔ ATP + AMP), which can support submembrane ATP from ADP produced by local ATPase activity, the diffusion coefficient must be further reduced to 1.6 × 10−11 cm2/s [49]. Although the value of restricted nucleotide diffusion has been obtained with certain assumptions, a five orders of magnitude difference from the diffusion coefficient in the cytosol cannot be compensated through realistic variations of submembrane space thickness, ATPase flux, or bulk ATP.
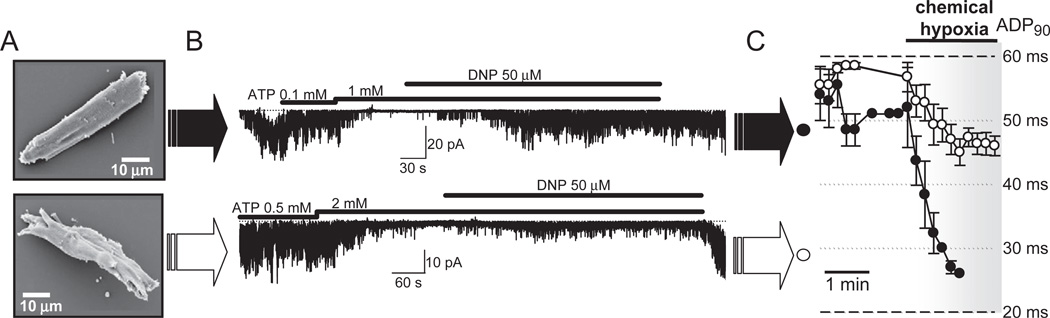
A number of observations do indicate the existence of such a surprising barrier secluding the unstirred layer near the plasma membrane. The physical nature of the reduced metabolite mobility near the sarcolemma may result from protein aggregates [26,71], membrane surface positive charges [72], reticular membranes and other organelles [4], and the underlying barrier should be considered as a significant contributor to intracellular signaling. The diffusion barrier differentiates a transient reaction of submembrane effectors to local accumulation of cAMP from a sustained response of cytosolic effectors to cAMP signaling [47,51,52]. Further evidence for the role of submembrane compartmentation in cardiac physiology was obtained in a heart failure model induced by transgenic expression of tumor necrosis factor [73,74]. Cardiac myocytes from transgenic failing hearts underwent significant distortion of cellular architecture (Fig. 3A) [75]. Cell remodeling disturbed energy communication between the cytosol and the submembrane space revealed by sarcolemmal KATP channel recordings. While in excised membrane patches KATP channels displayed normal biophysical and regulatory properties, at both cellular and organ levels KATP channels demonstrated a blunted response to chemical hypoxia due to disrupted energy communication between cell compartments (Figs. 3B and C) [75]. Intracellular structural rearrangements that prevented prompt KATP channel openings following onset of DNP-induced metabolic inhibition could have also set an environment that impeded efflux of local glycolysis-produced ATP from submembrane space, restraining KATP channel activity [76–78]. Thus, in the setting of cardiomyopathy, spatial remodeling including disturbances in submembrane compartments is associated with compromised feed-back regulation of energy expenditure by KATP channels, secluded in the sarcolemma, in response to development of bulk energy deficit [49,50,75,79–82].
Fig. 3.
A: Remodeled ventricular myocyte from the heart of a TNFα-TG transgenic mouse manifests distorted cellular architecture (bottom) compared to the rod-shaped cell from control wildtype counterparts (top), visualized by scanning electron microscopy. B: In the open cell-attached mode of the patch-clamp technique, obtained by cell permeabilization with digitonin (Abraham et al. [55]), uncoupling of bulk mitochondria with 2,4-dinitrophenol (DNP), in the constant presence of inhibitory concentration of ATP, activated sarcolemmal KATP channel activity in cardiac myocytes from control hearts (top). Impeded by cellular remodeling, the communication between the cytosol and the sarcolemma resulted in a blunted KATP channel response to DNP-induced metabolic inhibition (bottom), despite intact intrinsic KATP channel gating properties (data not shown). C: Under chemical hypoxia, induced by DNP, APD90 (action potential duration measured at 90% of its amplitude) was markedly shortened in controlled (closed circlers), but not in TNFα-remodeled hearts (open circles) due to disturbed energy signals communication to sarcolemmal KATP channels (see Hodgson et al. [75] for further information).
4. Energy communication in a compartmentalized cellular environment
The modern concept that failing hearts are engines out of fuel has emerged with recognition that altered energetics precipitate organ failure [81,83–85]. In cellular compartmentalized environments, where metabolite mobility between microdomains is limited, unregulated local ATP utilization, in the absence of mechanisms capable of shunting diffusional barriers, may significantly deplete local energy stores generating energy deficient phenotypes [50,86]. The earlier concept implying that ATP diffuses freely in an isotropic cell milieu considered as a “well-mixed bag of enzymes” [87], no longer explains ATP compartmentation in myofibrils, which could result in a paradoxical energy deficit in cardiac cells under conditions of high and well-preserved average ATP levels [4,79,88]. Diffusion restrictions in the submembrane space would hamper communication between bulk and submembrane domains, restricting membrane processes to function within local fluctuations of metabolites. While adenine nucleotides (ATP, ADP) are the recognized cellular energetic currency, the molecular mechanisms of energy signaling between compartments remain partially conceptualized.
Energy consumption is no longer viewed as a process whereby freely diffusing high-energy phosphates are consumed where needed throughout the cell. Several observations support the notion that energy consumption is also compartmentalized [4,71,79,89,90]. In the heart, selective inhibition of anaerobic glycolysis versus mitochondrial oxidative phosphorylation has revealed a differential efficacy of these systems in supporting specific membrane versus contractile functions, which cannot be explained by changes in total transcellular high-energy phosphate levels [76,77,91,92]. Anomalously slow ATP diffusion, as a result of macromolecular obstacles, was identified within sarcomeres, indicating that even under a physiological increase in cardiac workload, with an elevation of the rate of myosin ATPase from 3.4 to 5.2 mM/s, ATP concentration in the center of the sarcomeric A-zone rapidly disappears reaching 0.1 mM, which without effective energy transfer, may result in energy deficit, contractile dysfunction and cell damage [79]. Phosphotransfer enzymes creatine and adenylate kinases (CK and AK) have been recognized as systems that can facilitate energy transfer between sites of ATP production and utilization [84,93–98]. Widely distributed in cellular compartments, CK and AK are co-localized with energy sensing KATP channels [55,64,86,98–102] and thereby with cytoskeleton-bound sarcolemmal complexes [26,27], integrating into membrane metabolic units (Fig. 4A). As nucleotide-sensing KATP channels are co-localized within the metabolic unit with ion transporting ATPases, channel openings induced by a local drop of ATP and an increase of ADP levels can accelerate repolarization of action potentials by driving membrane potential towards the K+ equilibrium restraining, thereby, associated energy expenses. Thus KATP channels, when necessary, can provide a feed-back response to limit processes occurring during action potential propagation, including the operational time of L-type Ca2+ channels and associated Ca2+ release from sarcoplasmic reticulum as well as the concomitant activity of the Na+–Ca2+ exchanger, myosin-, Ca2+- and Na+/K+-ATPases [50,58,103,104].
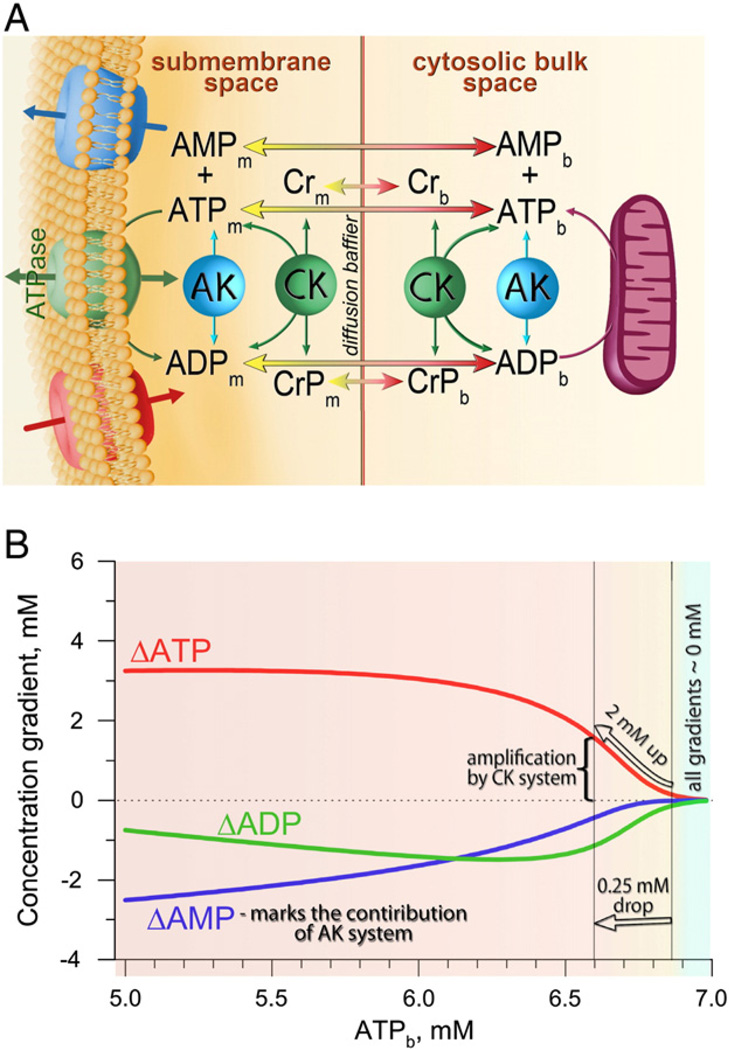
Fig. 4.
A: Diffusion of adenine nucleotides between intracellular compartments facilitated by creatine (CK) and adenylate (AK) kinase systems, implying that delivery of ATP from one cellular compartment to another, in addition to passive diffusion flux, is accompanied by influx into submembrane space of high-energy phosphate equivalents (creatine phosphate, CrP) or efflux of AMP, which then are locally involved in CK- or AK-catalyzed ATP synthesis. Indexes ‘m’ and ‘b’ denote the metabolite concentrations in the submembrane and cytosolic bulk spaces, respectively. Influx and efflux of ions through sarcolemmal channels and concomitant ATPase flux are also indicated. B: Differences in ATP, ADP and AMP concentrations (ΔATP, ΔADP and ΔAMP) between the bulk and submembrane space at different bulk ATP concentrations (ATPb). Positive gradient values correspond to the drop of nucleotide concentrations directed from the diffusion barrier towards the sarcolemma. Nucleotide gradients were constructed by resolving equation 6 from Selivanov et al. [49], at JATPase = 4.7 × 10−6 µmol/cm2/s, D = 1.6 × 10−11 cm2/s, 7 mM total nucleotide pool, 200 nm thickness of subsarcolemmal space and variable bulk ATP.
The reversible CK reaction, ADP + CrP↔Cr + ATP, phosphorylates ADP by transferring phosphate from creatine phosphate (CrP) producing creatine (Cr) catalyzing CrP-ATP phosphotransfer. The high value of the reaction equilibrium constant KCK=160 [105] indicates that an augmentation of ATP hydrolysis depletes the intracellular pool of CrP to a greater extent than the pool of ATP. Such buffering could be interpreted as a result of facilitated delivery of ATP from one cellular compartment to another accompanied by diffusion of high-energy phosphate equivalents (CrP), which are then locally involved in CK-catalyzed ATP synthesis [49,50]. Indeed, changes in CK reaction flux tightly follow cellular energetic dynamics due to significant partaking of the CK system in energy communication between cell compartments [55,70,93].
Adenylate kinase (AK) can also facilitate nucleotide intercompartmental exchange by AMP diffusion flux (JAMP) with an equilibrium constant KAK = 1 [70,100,105,106]. Located in the mitochondria, cytosol, and membrane-bound [107,108] adenylate kinase has a distinct role in setting the cell response to stress through activation of AMP-dependent processes [109,110]. Gene deletion of creatine kinase or adenylate kinase isoforms generates phenotypes with increased electrical vulnerability, disturbed muscle energetic economy and decreased tolerance to metabolic stress [92,107].
Integration of the CK system with the energy network, assuming conditions of equilibrium, implies that membrane ATPase flux, in addition to ATP diffusion, is equalized by CK activity manifested by creatine phosphate influx (JCrP) to submembrane or creatine efflux (−JCr) to bulk space (Fig. 4A). As AMP is a co-product of AK catalysis, the local AK reaction is marked by efflux of AMP from submembrane to bulk space (−JAMP). Thus ATP consumption in the submembrane “fuzzy space” (JATPase) can be described through ATP, CrP and AMP diffusion fluxes as JATPase=JATP + JCrP−JAMP (Fig. 4A) [49]. Resolving this equation using Fick's 1st law (assuming equilibrium conditions) with a diffusion coefficient value of 1.6 × 10−11 cm2/s (defined above), ATPase flux of 4.7 × 10−6 µmol/cm2/s, 7 mM total nucleotide bulk pool and a thickness of the submembrane compartment 200 nm [49,50] allowed the following prediction regarding phosphotransfer reactions setting nucleotide gradients between compartments (Fig. 4B): i) within a wide range of membrane ATPase activity, the cooperative action of CK and AK virtually nullifies differences between bulk and submembrane nucleotide concentrations (ΔATP = [ATP]b − [ATP]m = 0) at a high bulk ATP; ii) CK phosphotransfer is able to amplify cytosolic signals at a diffusion barrier, e.g., reduction by only 0.25 mM (from 6.85 to 6.6 mM) in cytosolic ATP generates a 2 mM ATP gradient between the cytosol and sarcolemma through the diffusion-restricted submembrane space (ΔATP); iii) at minor changes of bulk ATP, AK does not significantly contribute in setting gradients, but with further reductions in bulk ATP, flux through the AK system becomes significant in maintaining ΔATP constant (Fig. 4B). Thus, CK/AK systems, in a compartmentalized environment, can amplify and attenuate nucleotide signals at diffusion barriers [49,50].
Such modulation of nucleotide gradients at a diffusion barrier can be derived from properties of the phosphotransfer systems. The high value of the equilibrium constant for the CK reaction (KCK = 160) implies that even a minor drop of bulk ATP under conditions of increased energy expenses would result in significant reduction of bulk CrP through the CK reaction and, thereby, a constrained diffusion flux of CrP (JCrP) over intracellular diffusion barriers. This would induce a significant drop of CrP in the submembrane space, reduction of local submembrane CK activity and would result in an amplified fall of submembrane ATP (d[ATP]m) compared to only small changes in bulk ATP (d[ATP]b). Modulation of nucleotide signals can be expressed as a ratio between changes in submembrane over bulk ATP, i.e., d[ATP]m/d[ATP]b. In the absence of active signal modulators, a passive signal response is presented by a ratio value of 1 (Fig. 5A).
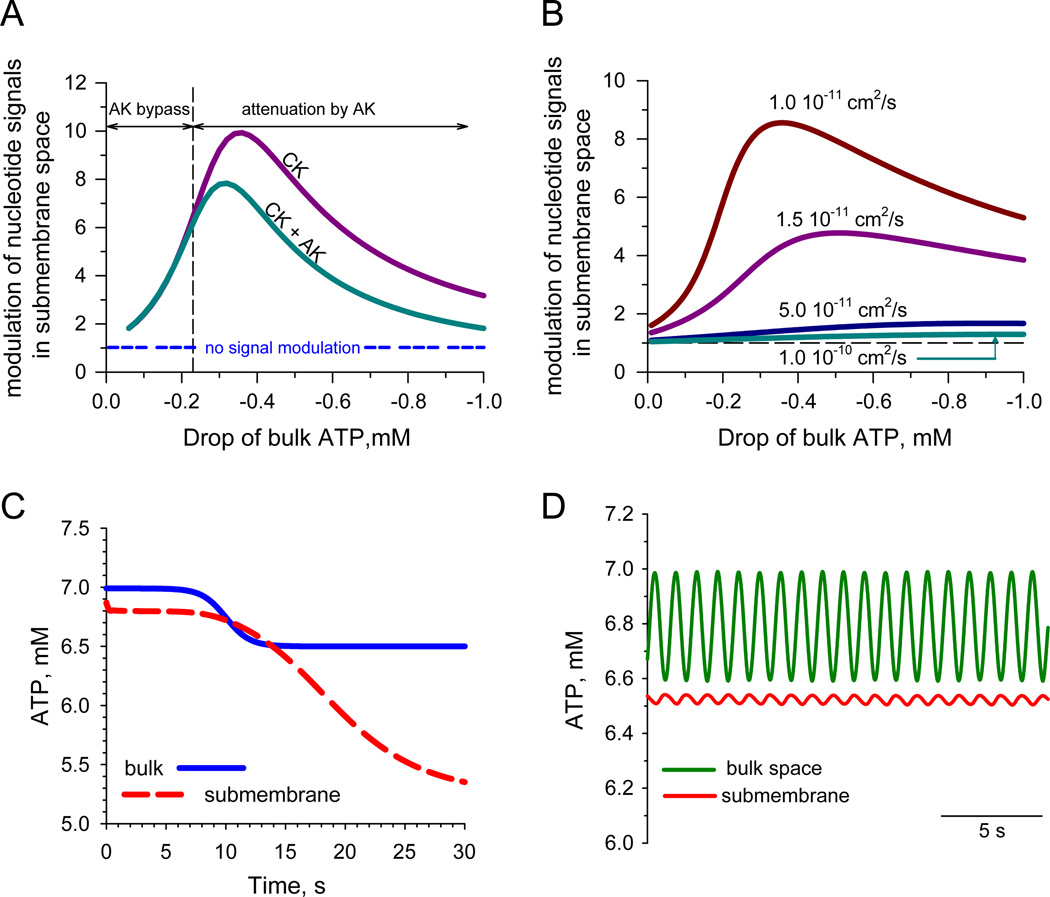
Fig. 5.
A: Effectiveness of modulation of nucleotide signals, i.e. changes of ATP in submembrane space (Δ[ATP]m) relative to a drop of ATP in bulk cytosolic levels (Δ[ATP]b), was calculated as d[ATP]m/d[ATP]b for creatine kinase (CK) alone and co-active CK and AK systems. The horizontal dotted line corresponds to a passive signal response (i.e. no signal modulation) in the absence of systems catalyzing phosphotransfer reactions. Note that higher changes in ATPb undergo a lower amplification, an effect enhanced by AK when a drop of ATPb exceeds the “AK bypass” threshold. B: Modulation of nucleotide signals under co-active CK and AK systems at different values of the apparent diffusion coefficient for nucleotides in submembrane space. C: Kinetic simulation of a nucleotide signal in submembrane space in response to a sustained drop of bulk ATP. Calculations were performed by resolving the system of differential equations (in the text) using JATPase = 4.7 × 10−6 µmol/cm2/s, D = 1.6 − 10−11 cm2/s, 200 nm thickness of subsarcolemmal space, 7 mM total nucleotide ([ANP]) and 40 mM Cr/CrP ([CrT]) pools. The nucleotide signal, generated in cytosol as 0.5 mM drop of ATP (blue solid line) is amplified in the membrane vicinity and reaches steady-state within >30 s delay (red dotted line). D: Same approach and values of parameters were used to simulate cytosolic ATP oscillations during contraction at 1 Hz frequency that were effectively filtered out in the vicinity of the sarcolemma. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Computations with parameters of ATPase activity and diffusion coefficient performed for CK alone and cooperatively active CK and AK systems [49,84,92] revealed that the effectiveness of signal transmission driven by CK is insensitive to AK modulation at smaller variations in bulk ATP (Fig. 5A, zone of the AK bypass, where the signal response curves constructed for CK alone and CK + AK are superimposed). Downward deviation of the signal response curve relative to the curve constructed for CK alone indicates the contribution of the AK system into signal attenuation following a further drop in bulk ATP (Fig. 5A, zone of signal attenuation by AK). Such profile of interplay between phosphotransfer systems quantitatively depends on diffusion restriction values (Fig. 5B), and is in agreement with experimental observations that reduction of CK flux is accompanied by up-regulation of AK phosphotransfer [108,111].
Signal transmission over diffusion barriers is not instantaneous; it depends on diffusion coefficient rates and phosphotransfer reaction properties catalyzing signal conversion. A diffusion barrier may determine not only amplification and attenuation (tuning) of nucleotide signals, but also “filtering” of sustained trends in cell energetics, protecting submembrane reactions from non-sustained nucleotide fluctuations and securing an uncluttered nucleotide sensor response [112,113]. The time course of nucleotide and creatine kinase substrate levels in any point between bulk and sarcolemma can be resolved using Fick's 2nd law in combination with phosphotransfer reaction fluxes and total adenine nucleotide [ANP] and creatine derivatives [CrT] pools:
Resolving this system of equations, based on the equilibrium for ATPase, CK and AK reactions, using apparent diffusion coefficients for ATP: D = 1.6 × 10−11 cm2/s and creatine: DCr = 2.4 × 10−11 cm2/s along with parameters defined above, revealed a significant delay of cytosolic signal transmission into the subsarcolemmal compartment making evident that short-living nucleotide changes that occurred in the cytosol will be essentially cutoff or low-pass filtered at a strong diffusion barrier (Fig. 5C). Brief changes in ATP levels during the cardiac contractile cycle do not communicate into changes of membrane excitability (Fig. 5D) [114]. Only changes in cell energetics approaching ~1 min in duration would be communicated through diffusion barriers into the submembrane compartment. Low-pass nucleotide signal filtering at the diffusion barrier provides a paradigm for conversion of sustained trends but not momentary fluctuations in cell energetics, a fundament of cell compartmentation defining cardiac tissue physiology.
5. Concluding remarks
In cardiac myocytes, co-localization of metabolic transducers and downstream effectors is achieved through specialized anchoring components, allowing vectorial matching of environmental signals. Sole co-localization of signal transducers and effectors may not by itself secure rapid and efficient targeting as demonstrated for voltage-dependent Na+ channels and the Na+–Ca2+ exchanger, or for adenylate cyclase and the cAMP-gated channel. To this end, an additional cellular factor, namely diffusional restriction in the proximity of macromolecular complexes, needs to be considered to comprehensively assess intracellular signaling. While the morphological basis for diffusion restrictions remains intriguing, the concept of energy transfer over diffusion barriers emerges as critical in understanding mechanisms of metabolic balance maintenance. In accord with the “divide and conquer” concept, cellular diffusion restrictions force phosphotransfer systems to play multiple roles in energy communication to compartmentalized membrane metabolic units. Under normal metabolic state they facilitate energy delivery to sarcolemmal energy consuming systems that are secluded from the cytosolic bulk space. Notably, AK is in reserve until a substantial drop in local ATP and concomitant increase in ADP recruit the AK system for local ATP support and initiation of AMP-dependent signaling cascades [86,109,110]. KATP channels anchored within sarcolemmal metabolic units spare local energy resources during excitation–contraction coupling [50,61,82]. Under stress, in response to CK and AK signals indicating that bulk cellular energetics experience a sustained energy blackout, but not in response to transient fluctuations of bulk nucleotide levels, KATP channel-driven shortening of action potentials would limit cellular energy demand [55,58,63,64,104]. Thus, spatial integration of macromolecular sarcolemmal or sarcomeric complexes, operating in diffusion-restricted intracellular compartments, with metabolic circuits responsible for transmission, sensing and processing of energy signals into cellular responses, ensures cardiac energy homeostasis.
Footnotes
Disclosure statement
None.
Contributor Information
Alexey E. Alekseev, Email: alekseev.alexey@mayo.edu.
Santiago Reyes, Email: reyesramirez.santiago@mayo.edu.
Vitaly A. Selivanov, Email: selivanov@ub.edu.
Petras P. Dzeja, Email: dzeja.petras@mayo.edu.
Andre Terzic, Email: terzic.andre@mayo.edu.
References
- 1.Alberts B, Johnson A, Lewis J, Raff R, Roberts K, Walter P. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. The compartmentalization of cells; pp. 659–669. [Google Scholar]
- 2.Saks V, Monge C, Guzun R. Philosophical basis and some historical aspects of system biology: from Hegel to Noble—applications for bioenergetic research. Int J Mol Sci. 2009;10:1161–1192. doi: 10.3390/ijms10031161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin W. Evolutionary origins of metabolic compartmentation in eukaryotes. Philos Trans R Soc Lond B Biol Sci. 2010;365:847–855. doi: 10.1098/rstb.2009.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saks V, Beraud N, Wallimann T. Metabolic compartmentation—a system level property of muscle cells: real problems of diffusion in living cells. Int J Mol Sci. 2008;9:751–767. doi: 10.3390/ijms9050751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 6.Tamargo J, Caballero R, Gómez R, Valenzuela C, Delpón E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Parekh AB. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J Physiol. 2008;586:3043–3054. doi: 10.1113/jphysiol.2008.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaccolo M. cAMP signal transduction in the heart: understanding spatial control for the development of novel therapeutic strategies. Br J Pharmacol. 2009;158:50–60. doi: 10.1111/j.1476-5381.2009.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 10.Clapham DE, Neer EJ. G protein β γ subunits. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 11.Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, et al. A β2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- 12.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Gray PC, Scott JD, Catterall WA. Regulation of ion channels by cAMP-dependent protein kinase and A-kinase anchoring proteins. Curr Opin Neurobiol. 1998;8:330–334. doi: 10.1016/s0959-4388(98)80057-3. [DOI] [PubMed] [Google Scholar]
- 14.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 15.Dubreuil RR. Functional links between membrane transport and the spectrin cytoskeleton. J Membr Biol. 2006;211:151–161. doi: 10.1007/s00232-006-0863-y. [DOI] [PubMed] [Google Scholar]
- 16.Kostin S, Hein S, Arnon E, Scholz D, Schaper J. The cytoskeleton and related proteins in the human failing heart. Heart Fail Rev. 2000;5:271–280. doi: 10.1023/A:1009813621103. [DOI] [PubMed] [Google Scholar]
- 17.Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci U S A. 2004;101:17533–17538. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe JS, Palygin O, Bhasin N, Hund TJ, Boyden PA, Shibata E, et al. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J Cell Biol. 2008;180:173–186. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baines AJ, Bennett PM, Carter EW, Terracciano C. Protein 4.1 and the control of ion channels. Blood Cells Mol Dis. 2009;42:211–215. doi: 10.1016/j.bcmd.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, et al. A β(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansma PK, Drake B, Marti O, Gould SA, Prater CB. The scanning ion-conductance microscope. Science. 1989;243:641–643. doi: 10.1126/science.2464851. [DOI] [PubMed] [Google Scholar]
- 23.Korchev YE, Raval M, Lab MJ, Gorelik J, Edwards CR, Rayment T, et al. Hybrid scanning ion conductance and scanning near-field optical microscopy for the study of living cells. Biophys J. 2000;78:2675–2679. doi: 10.1016/S0006-3495(00)76811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korchev YE, Negulyaev YA, Edwards CR, Vodyanoy I, Lab MJ. Functional localization of single active ion channels on the surface of a living cell. Nat Cell Biol. 2000;2:616–619. doi: 10.1038/35023563. [DOI] [PubMed] [Google Scholar]
- 25.Flucher BE, Franzini-Armstrong C. Formation of junctions involved in excitation -contraction coupling in skeletal and cardiac muscle. Proc Natl Acad Sci U S A. 1996;93:8101–8106. doi: 10.1073/pnas.93.15.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hashemi SM, Hund TJ, Mohler PJ. Cardiac ankyrins in health and disease. J Mol Cell Cardiol. 2009;47:203–209. doi: 10.1016/j.yjmcc.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kline CF, Kurata HT, Hund TJ, Cunha SR, Koval OM, Wright PJ, et al. Dual role of KATP channel C-terminal motif in membrane targeting and metabolic regulation. Proc Natl Acad Sci U S A. 2009;106:16669–16674. doi: 10.1073/pnas.0907138106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terzic A, Kurachi Y. Actin microfilament disrupters enhance KATP channel opening in patches from guinea-pig cardiomyocytes. J Physiol. 1996;492:395–404. doi: 10.1113/jphysiol.1996.sp021316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cunha SR, Mohler PJ. Ankyrin-based cellular pathways for cardiac ion channel and transporter targeting and regulation. Semin Cell Dev Biol. 2011;22:166–170. doi: 10.1016/j.semcdb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong TT, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, et al. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010;8:e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinch R, Greenstein JL, Winslow RL. Multi-scale models of local control of calcium induced calcium release. Prog Biophys Mol Biol. 2006;90:136–150. doi: 10.1016/j.pbiomolbio.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Wang SQ, Wei C, Zhao G, Brochet DX, Shen J, Song LS, et al. Imaging microdomain Ca2+ in muscle cells. Circ Res. 2004;94:1011–1022. doi: 10.1161/01.RES.0000125883.68447.A1. [DOI] [PubMed] [Google Scholar]
- 33.Maltsev AV, Maltsev VA, Mikheev M, Maltseva LA, Sirenko SG, Lakatta EG, et al. Synchronization of stochastic Ca2+ release units creates a rhythmic Ca2+ clock in cardiac pacemaker cells. Biophys J. 2011;100:271–283. doi: 10.1016/j.bpj.2010.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rovetti R, Cui X, Garfinkel A, Weiss JN, Qu Z. Spark-induced sparks as a mechanism of intracellular calcium alternans in cardiac myocytes. Circ Res. 2010;106:1582–1591. doi: 10.1161/CIRCRESAHA.109.213975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 36.Uehara A, Yasukochi M, Imanaga I, Nishi M, Takeshima H. Store-operated Ca2+ entry uncoupled with ryanodine receptor and junctional membrane complex in heart muscle cells. Cell Calcium. 2002;31:89–96. doi: 10.1054/ceca.2001.0257. [DOI] [PubMed] [Google Scholar]
- 37.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 38.Parekh AB, Penner R. Store-operated calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 39.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER–plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- 43.Lederer WJ, Niggli E, Hadley RW. Sodium-calcium exchange in excitable cells: fuzzy space. Science. 1990;248:283. doi: 10.1126/science.2326638. [DOI] [PubMed] [Google Scholar]
- 44.Levesque PC, Leblanc N, Hume JR. Role of reverse-mode Na+-Ca2+ exchange in excitation–contraction coupling in the heart. Ann N Y Acad Sci. 1991;639:386–397. doi: 10.1111/j.1749-6632.1991.tb17327.x. [DOI] [PubMed] [Google Scholar]
- 45.Miura Y, Kimura J. Sodium-calcium exchange current. Dependence on internal Ca and Na and competitive binding of external Na and Ca. J Gen Physiol. 1989;93:1129–1145. doi: 10.1085/jgp.93.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Despa S, Bers DM. Na/K pump current and [Na]i in rabbit ventricular myocytes: local [Na]i depletion and Na buffering. Biophys J. 2003;84:4157–4166. doi: 10.1016/S0006-3495(03)75140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci U S A. 2001;98:13049–13054. doi: 10.1073/pnas.221381398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iancu RV, Ramamurthy G, Harvey RD. Spatial and temporal aspects of cAMP signalling in cardiac myocytes. Clin Exp Pharmacol Physiol. 2008;35:1343–1348. doi: 10.1111/j.1440-1681.2008.05020.x. [DOI] [PubMed] [Google Scholar]
- 49.Selivanov VA, Alekseev AE, Hodgson DM, Dzeja PP, Terzic A. Nucleotide-gated KATP channels integrated with creatine and adenylate kinases: amplification, tuning and sensing of energetic signals in the compartmentalized cellular environment. Mol Cell Biochem. 2004;256–257:243–256. doi: 10.1023/b:mcbi.0000009872.35940.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reyes S, Park S, Terzic A, Alekseev AE. KATP channels process nucleotide signals in muscle thermogenic response. Crit Rev Biochem Mol Biol. 2010;45:506–519. doi: 10.3109/10409238.2010.513374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rich TC, Tse TE, Rohan JG, Schaack J, Karpen JW. In vivo assessment of local phosphodiesterase activity using tailored cyclic nucleotide-gated channels as cAMP sensors. J Gen Physiol. 2001;118:63–78. doi: 10.1085/jgp.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karpen JW, Rich TC. Resolution of cAMP signals in three-dimensional microdomains using novel, real-time sensors. Proc West Pharmacol Soc. 2004;47:1–5. [PubMed] [Google Scholar]
- 53.Kabakov AY. Activation of KATP channels by Na/K pump in isolated cardiac myocytes and giant membrane patches. Biophys J. 1998;75:2858–2867. doi: 10.1016/S0006-3495(98)77728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gribble FM, Loussouarn G, Tucker SJ, Zhao C, Nichols CG, Ashcroft FM. A novel method for measurement of submembrane ATP concentration. Biol Chem. 2000;275:30046–30049. doi: 10.1074/jbc.M001010200. [DOI] [PubMed] [Google Scholar]
- 55.Abraham MR, Selivanov VA, Hodgson D, Pucar D, Zingman LV, Wieringa B, et al. Coupling of cell energetics with membrane metabolic sensing: Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knockout. J Biol Chem. 2002;277:24427–24434. doi: 10.1074/jbc.M201777200. [DOI] [PubMed] [Google Scholar]
- 56.Zingman LV, Alekseev AE, Bienengraeber M, Hodgson D, Karger AB, Dzeja PP, et al. Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron. 2001;31:233–245. doi: 10.1016/s0896-6273(01)00356-7. [DOI] [PubMed] [Google Scholar]
- 57.Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol. 2000;116:147–161. doi: 10.1085/jgp.116.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261:H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- 59.Alekseev AE, Hodgson DM, Karger AB, Park S, Zingman LV, Terzic A. ATP-sensitive K+ channel channel/enzyme multimer: metabolic gating in the heart. J Mol Cell Cardiol. 2005;38:895–905. doi: 10.1016/j.yjmcc.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 61.Elliott AC, Smith GL, Allen DG. Simultaneous measurements of action potential duration and intracellular ATP in isolated ferret hearts exposed to cyanide. Circ Res. 1989;64:583–591. doi: 10.1161/01.res.64.3.583. [DOI] [PubMed] [Google Scholar]
- 62.Decking UK, Reffelmann T, Schrader J, Kammermeier H. Hypoxia-induced activation of KATP channels limits energy depletion in the guinea pig heart. Am J Physiol. 1995;269:H734–H742. doi: 10.1152/ajpheart.1995.269.2.H734. [DOI] [PubMed] [Google Scholar]
- 63.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, et al. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002;99:3278–3283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nichols CG, Lederer WJ. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J Physiol. 1990;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiss JN, Venkatesh N, Lamp ST. ATP-sensitive K+ channels and cellular K+ loss in hypoxic and ischaemic mammalian ventricle. J Physiol. 1992;447:649–673. doi: 10.1113/jphysiol.1992.sp019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swift F, Birkeland JAK, Tovsrud N, Enger UH, Aronsen JM, Louch WE, et al. Altered Na+/Ca2+-exchanger activity due to downregulation of Na+/K+-ATPase α2-isoform in heart failure. Cardiovasc Res. 2008;78:71–78. doi: 10.1093/cvr/cvn013. [DOI] [PubMed] [Google Scholar]
- 67.Chase A, Orchard CH. Ca efflux via the sarcolemmal Ca ATPase occurs only in the t-tubules of rat ventricular myocytes. Mol Cell Cardiol. 2011;50:187–193. doi: 10.1016/j.yjmcc.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 68.Kinsey ST, Moerland TS. Metabolite diffusion in giant muscle fibers of the spiny lobster Panulirus argus. J Exp Biol. 2002;205:3377–3386. doi: 10.1242/jeb.205.21.3377. [DOI] [PubMed] [Google Scholar]
- 69.Bittl JA, DeLayre J, Ingwall JS. Rate equation for creatine kinase predicts the in vivo reaction velocity: 31P NMR surface coil studies in brain, heart, and skeletal muscle of the living rat. Biochemistry. 1987;26:6083–6090. doi: 10.1021/bi00393a021. [DOI] [PubMed] [Google Scholar]
- 70.Pucar D, Dzeja PP, Bast P, Juranic N, Macura S, Terzic A. Cellular energetics in the preconditioned state: protective role for phosphotransfer reactions captured by 18O-assisted 31P NMR. J Biol Chem. 2001;276:44812–44819. doi: 10.1074/jbc.M104425200. [DOI] [PubMed] [Google Scholar]
- 71.Weiss JN, Korge P. The cytoplasm: no longer a well-mixed bag. Circ Res. 2001;89:108–110. [PubMed] [Google Scholar]
- 72.Deutsch N, Matsuoka S, Weiss JN. Surface charge and properties of cardiac ATP-sensitive K+ channels. J Gen Physiol. 1994;104:773–800. doi: 10.1085/jgp.104.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McTiernan CF, Feldman AM. The role of tumor necrosis factor α in the pathophysiology of congestive heart failure. Curr Cardiol Rep. 2000;2:189–197. doi: 10.1007/s11886-000-0068-4. [DOI] [PubMed] [Google Scholar]
- 74.Bradham WS, Bozkurt B, Gunasinghe H, Mann DL, Spinale FG. Tumor necrosis factor-α and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc Res. 2002;53:822–830. doi: 10.1016/s0008-6363(01)00503-x. [DOI] [PubMed] [Google Scholar]
- 75.Hodgson DM, Zingman LV, Kane GC, Perez-Terzic C, Bienengraeber M, Ozcan C, et al. Cellular remodeling in heart failure disrupts KATP channel-dependent stress tolerance. EMBO J. 2003;22:1732–1742. doi: 10.1093/emboj/cdg192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weiss JN, Hiltbrand B. Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit heart. J Clin Invest. 1985;75:436–447. doi: 10.1172/JCI111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weiss JN, Lamp ST. Glycolysis preferentially inhibits ATP-sensitive K+ channels in isolated guinea pig cardiac myocytes. Science. 1987;238:67–69. doi: 10.1126/science.2443972. [DOI] [PubMed] [Google Scholar]
- 78.Hong M, Kefaloyianni E, Bao L, Malester B, Delaroche D, Neubert TA, et al. Cardiac ATP-sensitive K+ channel associates with the glycolytic enzyme complex. FASEB J. 2011 doi: 10.1096/fj.10-176669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Selivanov VA, Krause S, Roca J, Cascante M. Modeling of spatial metabolite distributions in the cardiac sarcomere. Biophys J. 2007;92:3492–3500. doi: 10.1529/biophysj.106.101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O'Cochlain F, Gao F, et al. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 82.Alekseev AE, Reyes S, Yamada S, Hodgson-Zingman DM, Sattiraju S, Zhu Z, et al. Sarcolemmal ATP-sensitive K+ channels control energy expenditure determining body weight. Cell Metab. 2010;11:58–69. doi: 10.1016/j.cmet.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Towbin JA, Bowles NE. The failing heart. Nature. 2002;415:227–233. doi: 10.1038/415227a. [DOI] [PubMed] [Google Scholar]
- 84.Dzeja PP, Redfield MM, Burnett JC, Terzic A. Failing energetics in failing hearts. Curr Cardiol Rep. 2000;2:212–217. doi: 10.1007/s11886-000-0071-9. [DOI] [PubMed] [Google Scholar]
- 85.Ingwall JS, Shen W. On energy circuits in the failing myocardium. Eur J Heart Fail. 2010;12:1268–1270. doi: 10.1093/eurjhf/hfq193. [DOI] [PubMed] [Google Scholar]
- 86.Dzeja P, Terzic A. Adenylate kinase and AMP signaling networks: metabolic monitoring, signal communication and body energy sensing. Int J Mol Sci. 2009;10:1729–1772. doi: 10.3390/ijms10041729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barros LF, Martínez C. An enquiry into metabolite domains. Biophys J. 2007;92:3878–3884. doi: 10.1529/biophysj.106.100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McLellan G, Weisberg A, Winegrad S. Energy transport from mitochondria to myofibril by a creatine phosphate shuttle in cardiac cells. Am J Physiol. 1983;254:C423–C427. doi: 10.1152/ajpcell.1983.245.5.C423. [DOI] [PubMed] [Google Scholar]
- 89.Entman ML, Kaniike K, Goldstein MA, Nelson TE, Bornet EP, Futch TW, et al. Association of glycogenolysis with cardiac sarcoplasmic reticulum. J Biol Chem. 1976;251:3140–3146. [PubMed] [Google Scholar]
- 90.Bizeau ME, Willis WT, Hazel JR. Differential responses to endurance training in subsarcolemmal and intermyofibrillar mitochondria. J Appl Physiol. 1998;85:1279–1284. doi: 10.1152/jappl.1998.85.4.1279. [DOI] [PubMed] [Google Scholar]
- 91.Doorey AJ, Barry WH. The effects of inhibition of oxidative phosphorylation and glycolysis on contractility and high-energy phosphate content in cultured chick heart cells. Circ Res. 1983;53:192–201. doi: 10.1161/01.res.53.2.192. [DOI] [PubMed] [Google Scholar]
- 92.Kaasik A, Veksler V, Boehm E, Novotova M, Minajeva A, Ventura-Clapier R. Energetic crosstalk between organelles: architectural integration of energy production and utilization. Circ Res. 2001;89:153–159. doi: 10.1161/hh1401.093440. [DOI] [PubMed] [Google Scholar]
- 93.Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the "phosphocreatine shuttle". Am J Physiol. 1984;246:C365–C377. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- 94.Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wallimann T, Dolder M, Schlattner U, Eder M, Hornemann T, O'Gorman E, et al. Some new aspects of creatine kinase (CK): compartmentation, structure, function and regulation for cellular and mitochondrial bioenergetics and physiology. Biofactors. 1998;8:229–234. doi: 10.1002/biof.5520080310. [DOI] [PubMed] [Google Scholar]
- 96.Noma T. Dynamics of nucleotide metabolism as a supporter of life phenomena. J Med Invest. 2005;52:127–136. doi: 10.2152/jmi.52.127. [DOI] [PubMed] [Google Scholar]
- 97.Saks V, Monge C, Anmann T, Dzeja P. Integrated and organized cellular energetic systems: theories of cell energetics, compartmentation and metabolic channeling. In: Saks V, editor. Molecular System Bioenergetics: Energy for Life. Weinheim, Germany: Wiley-VCH; 2007. pp. 59–109. [Google Scholar]
- 98.Janssen E, Kuiper J, Hodgson D, Zingman LV, Alekseev AE, Terzic A, et al. Two structurally distinct and spatially compartmentalized adenylate kinases are expressed from the AK1 gene in mouse brain. Mol Cell Biochem. 2004;256–257:59–72. doi: 10.1023/b:mcbi.0000009859.15267.db. [DOI] [PubMed] [Google Scholar]
- 99.Elvir-Mairena JR, Jovanovic A, Gomez LA, Alekseev AE, Terzic A. Reversal of the ATP-liganded state of ATP-sensitive K+ channels by adenylate kinase activity. J Biol Chem. 1996;271:31903–31908. doi: 10.1074/jbc.271.50.31903. [DOI] [PubMed] [Google Scholar]
- 100.Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, et al. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci U S A. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crawford RM, Ranki JH, Botting CH, Budas GR, Jovanovic A. Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB J. 2002;16:102–104. doi: 10.1096/fj.01-0466fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jovanović S, Jovanović A, Crawford RM. M-LDH serves as a regulatory subunit of the cytosolic substrate-channelling complex in vivo. J Mol Biol. 2007;371:349–361. doi: 10.1016/j.jmb.2007.05.081. [DOI] [PubMed] [Google Scholar]
- 103.Gong B, Miki T, Seino S, Renaud J-M. A KATP channel deficiency affects resting tension, not contractile force, during fatigue in skeletal muscle. Am J Physiol. 2000;279:C1351–C1358. doi: 10.1152/ajpcell.2000.279.5.C1351. [DOI] [PubMed] [Google Scholar]
- 104.Cifelli C, Boudreault L, Gong B, Bercier JP, Renaud J-M. Contractile dysfunctions in ATP-dependent K+ channel-deficient mouse muscle during fatigue involve excessive depolarization and Ca2+ influx through L-type Ca2+ channels. Exp Physiol. 2008;93:1126–1138. doi: 10.1113/expphysiol.2008.042572. [DOI] [PubMed] [Google Scholar]
- 105.Lawson JW, Veech RL. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979;254:6528–6537. [PubMed] [Google Scholar]
- 106.Golding EM, Teague WE, Jr, Dobson GP. Adjustment of K′ to varying pH and pMg for the creatine kinase, adenylate kinase and ATP hydrolysis equilibria permitting quantitative bioenergetic assessment. J Exp Biol. 1995;198:1775–1782. doi: 10.1242/jeb.198.8.1775. [DOI] [PubMed] [Google Scholar]
- 107.Pucar D, Janssen E, Dzeja PP, Juranic N, Macura S, Wieringa B, et al. Compromised energetics in the adenylate kinase AK1 gene knockout heart under metabolic stress. J Biol Chem. 2000;275:41424–41429. doi: 10.1074/jbc.M007903200. [DOI] [PubMed] [Google Scholar]
- 108.Janssen E, Dzeja PP, Oerlemans F, Simonetti A, Heerschap A, de Haan A, et al. Adenylate kinase 1 gene deletion disrupts muscle energetic economy despite metabolic rearrangement. EMBO J. 2000;19:6371–6381. doi: 10.1093/emboj/19.23.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hardie DG. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 110.Kim AS, Miller EJ, Young LH. AMP-activated protein kinase: a core signalling pathway in the heart. Acta Physiol. 2009;196:37–53. doi: 10.1111/j.1748-1716.2009.01978.x. [DOI] [PubMed] [Google Scholar]
- 111.Dzeja PP, Terzic A. Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. FASEB J. 1998;12:523–529. doi: 10.1096/fasebj.12.7.523. [DOI] [PubMed] [Google Scholar]
- 112.Veuthey AL, Stucki J. The adenylate kinase reaction acts as a frequency filter towards fluctuations of ATP utilization in the cell. Biophys Chem. 1987;26:19–28. doi: 10.1016/0301-4622(87)80003-0. [DOI] [PubMed] [Google Scholar]
- 113.Weiss JN, Yang L, Qu Z. Systems biology approaches to metabolic and cardiovascular disorders: network perspectives of cardiovascular metabolism. J Lipid Res. 2006;47:2355–2366. doi: 10.1194/jlr.R600023-JLR200. [DOI] [PubMed] [Google Scholar]
- 114.Kusuoka H, Inoue M, Tsuneoka Y, Watari H, Hori M, Abe H. Augmented energy consumption during early systole as a mechanism of cyclical changes in high-energy phosphates in myocardium assessed by phosphorus nuclear magnetic resonance. Jpn Circ J. 1985;49:1099–1107. doi: 10.1253/jcj.49.1099. [DOI] [PubMed] [Google Scholar]