Abstract
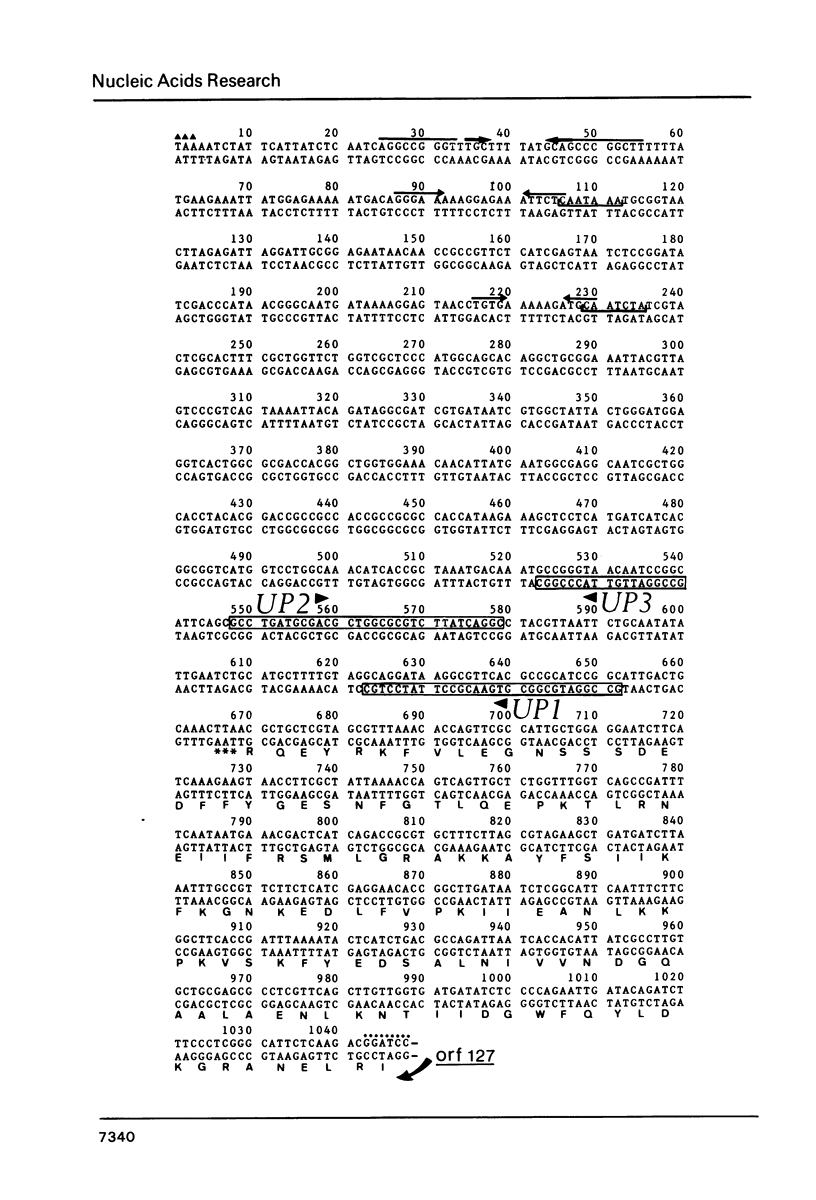
The entire threonine operon (thrABC) of Escherichia coli K12 was cloned, and the nucleotide sequence of the thrC gene and its 3' flanking region was determined. The translation initiation codon was identified by sequencing the N-terminal part of threonine synthase, the thrC gene product. Analysis of the deduced protein sequence (428 amino acid residues) revealed a region of homology, 35 amino acids long, between the three enzymes encoded by the threonine operon. During examination of the nucleotide sequence of the 1045 base pair fragments following the thrC gene, we detected some potential rho-independent and rho-dependent transcription termination signals.
Full text
PDF

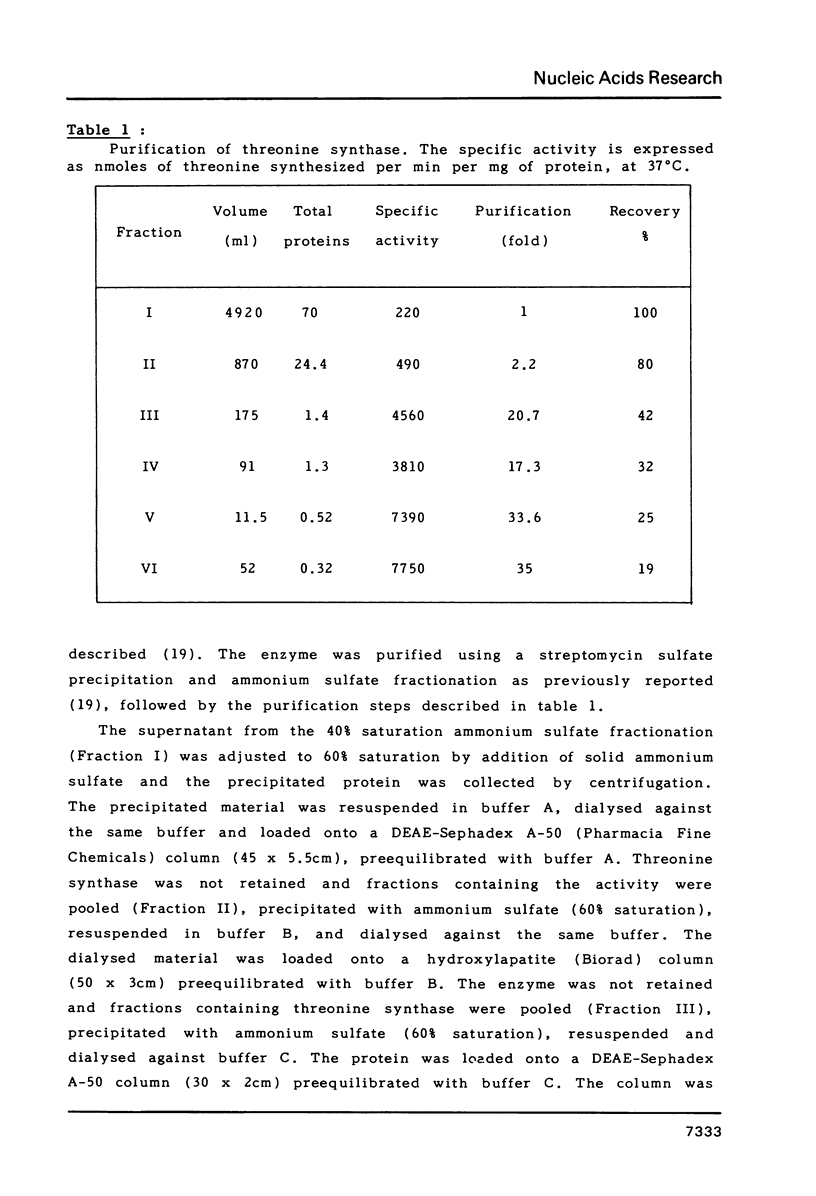


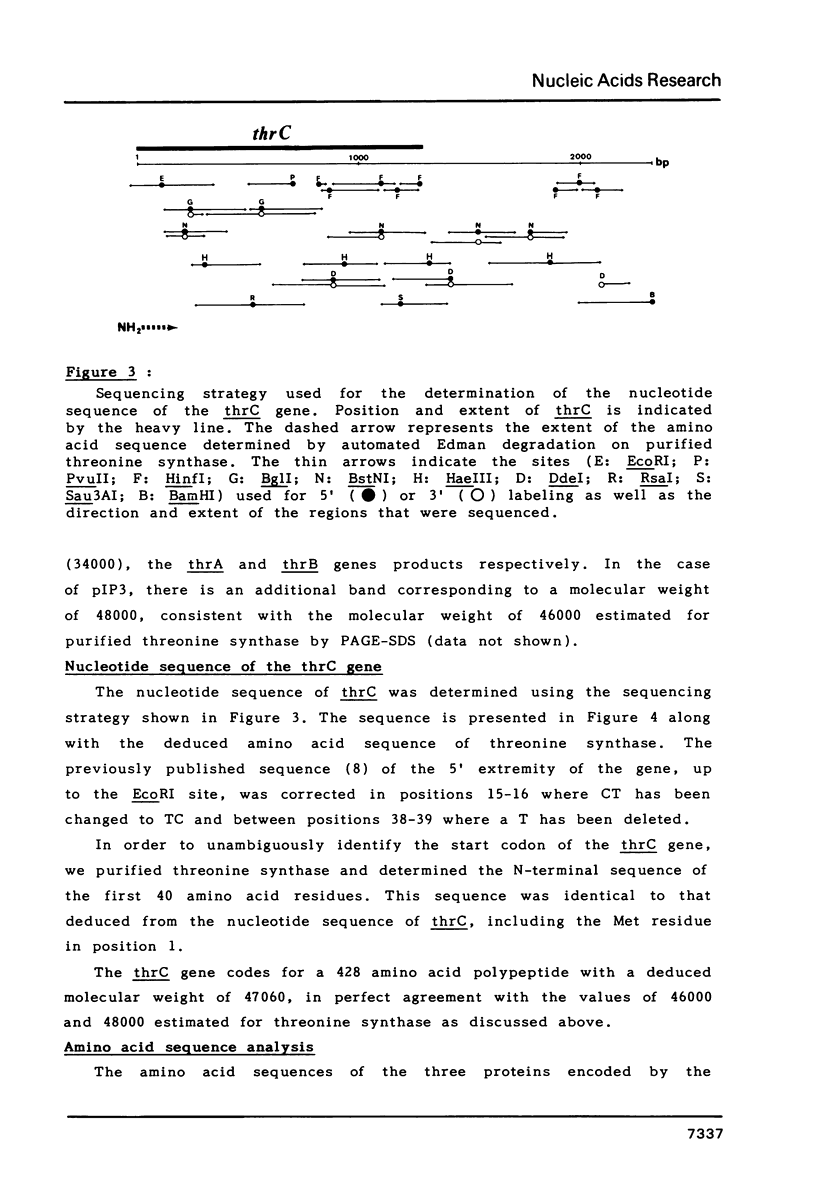







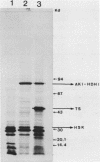
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange reaction: quantitave assay for restriction endonuclease-generated 5'-phosphoroyl termini in DNA. J Biol Chem. 1977 May 25;252(10):3176–3184. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burr B., Walker J., Truffa-Bachi P., Cohen G. N. Homoserine kinase from Escherichia coli K12. Eur J Biochem. 1976 Mar 1;62(3):519–526. doi: 10.1111/j.1432-1033.1976.tb10186.x. [DOI] [PubMed] [Google Scholar]
- Clément J. M., Hofnung M. Gene sequence of the lambda receptor, an outer membrane protein of E. coli K12. Cell. 1981 Dec;27(3 Pt 2):507–514. doi: 10.1016/0092-8674(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Cossart P., Katinka M., Yaniv M. Nucleotide sequence of the thrB gene of E. coli, and its two adjacent regions; the thrAB and thrBC junctions. Nucleic Acids Res. 1981 Jan 24;9(2):339–347. doi: 10.1093/nar/9.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P., Katinka M., Yaniv M., Saint Girons I., Cohen G. N. Construction and expression of a hybrid plasmid containing the Escherichia coli thrA and thrB genes. Mol Gen Genet. 1979 Aug;175(1):39–44. doi: 10.1007/BF00267853. [DOI] [PubMed] [Google Scholar]
- Daniel J. Azide-dependent mutants in E. coli K12. Nature. 1976 Nov 4;264(5581):90–92. doi: 10.1038/264090a0. [DOI] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975 Jul;123(1):102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer S. B., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XVI. Pattern of multivalent repression in strain K-12. J Bacteriol. 1968 May;95(5):1680–1684. doi: 10.1128/jb.95.5.1680-1684.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M. Multivalent repression in the biosynthesis of threonine in Salmonella typhimurium and Escherichia coli. Biochem Biophys Res Commun. 1963 Feb 6;10:277–282. doi: 10.1016/0006-291x(63)90430-3. [DOI] [PubMed] [Google Scholar]
- Falcoz-Kelly F., Janin J., Saari J. C., Véron M., Truffa-Bachi P., Cohen G. N. Revised structure of aspartokinase I-homoserine dehydrogenase I of Escherichia coli K12. Evidence for four identical subunits. Eur J Biochem. 1972 Aug 4;28(4):507–519. doi: 10.1111/j.1432-1033.1972.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Gardner J. F. Initiation, pausing, and termination of transcription in the threonine operon regulatory region of Escherichia coli. J Biol Chem. 1982 Apr 10;257(7):3896–3904. [PubMed] [Google Scholar]
- Gardner J. F. Regulation of the threonine operon: tandem threonine and isoleucine codons in the control region and translational control of transcription termination. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1706–1710. doi: 10.1073/pnas.76.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Yanofsky C. Nucleotide sequence and expression of Escherichia coli trpR, the structural gene for the trp aporepressor. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7117–7121. doi: 10.1073/pnas.77.12.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Ames G. F., Barnes W. M., Clement J. M., Hofnung M. A novel intercistronic regulatory element of prokaryotic operons. Nature. 1982 Aug 19;298(5876):760–762. doi: 10.1038/298760a0. [DOI] [PubMed] [Google Scholar]
- Horowitz N. H. On the Evolution of Biochemical Syntheses. Proc Natl Acad Sci U S A. 1945 Jun;31(6):153–157. doi: 10.1073/pnas.31.6.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenow H., Overgaard-Hansen K., Patkar S. A. Proteolytic cleavage fo native DNA polymerase into two different catalytic fragments. Influence of assay condtions on the change of exonuclease activity and polymerase activity accompanying cleavage. Eur J Biochem. 1971 Oct 14;22(3):371–381. doi: 10.1111/j.1432-1033.1971.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Yeh W. K. Origins of metabolic diversity: evolutionary divergence by sequence repetition. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3996–4000. doi: 10.1073/pnas.76.8.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsot C., Saint-Girons I., Cossart P. DNA sequence change of a deletion mutation abolishing attenuation control of the threonine operon of E. coli K12. Mol Gen Genet. 1982;188(3):455–458. doi: 10.1007/BF00330048. [DOI] [PubMed] [Google Scholar]
- Plamann M. D., Stauffer L. T., Urbanowski M. L., Stauffer G. V. Complete nucleotide sequence of the E. coli glyA gene. Nucleic Acids Res. 1983 Apr 11;11(7):2065–2075. doi: 10.1093/nar/11.7.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambach A., Hogness D. S. Translation of Drosophila melanogaster sequences in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5041–5045. doi: 10.1073/pnas.74.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rossi J., Egan J., Hudson L., Landy A. The tyrT locus: termination and processing of a complex transcript. Cell. 1981 Nov;26(3 Pt 1):305–314. doi: 10.1016/0092-8674(81)90199-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Schrenk W. J., Weisberg R. A. A simple method for making new transducing lines of coliphage lambda. Mol Gen Genet. 1975;137(2):101–107. doi: 10.1007/BF00341676. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Roeder W. D., Bogosian G., Somerville R. L., Weith H. L. DNA sequence of the E. coli trpR gene and prediction of the amino acid sequence of Trp repressor. Nucleic Acids Res. 1980 Apr 11;8(7):1551–1560. doi: 10.1093/nar/8.7.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstedt M. T., Greer S. B. Threonine synthetase of Bacillus subtilis. The nature of an associated dehydratase activity. J Biol Chem. 1973 Feb 10;248(3):1032–1044. [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thèze J., Saint-Girons I. Threonine locus of Escherichia coli K-12: genetic structure and evidence for an operon. J Bacteriol. 1974 Jun;118(3):990–998. doi: 10.1128/jb.118.3.990-998.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théze J., Margarita D. Characterization of a defective lambda bacteriophage transducing the threonine operon of Escherichia coli K12. Mol Gen Genet. 1974;132(1):41–48. doi: 10.1007/BF00268229. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Cohen S. N. 3'-end labeling of DNA with [alpha-32P]cordycepin-5'-triphosphate. Gene. 1980 Jul;10(2):177–183. doi: 10.1016/0378-1119(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Véron M., Saari J. C., Villar-Palasi C., Cohen G. N. The threonine-sensitive homoserine dehydrogenase and aspartokinase activities of Escherichia coli K 12. Intra and intersubunit interactions between the catalytic regions of the bifunctional enzyme. Eur J Biochem. 1973 Oct 5;38(2):325–335. doi: 10.1111/j.1432-1033.1973.tb03065.x. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold B., Graffunder H., Kohls H. A device coupled to a modified sequenator for the automated conversion of anilinothiazolinones into PTH amino acids. Anal Biochem. 1976 Oct;75(2):621–633. doi: 10.1016/0003-2697(76)90117-2. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Christie G. E., Platt T. Tandem termination sites in the tryptophan operon of Escherichia coli. Proc Natl Acad Sci U S A. 1981 May;78(5):2913–2917. doi: 10.1073/pnas.78.5.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamao F., Inokuchi H., Cheung A., Ozeki H., Söll D. Escherichia coli glutaminyl-tRNA synthetase. I. Isolation and DNA sequence of the glnS gene. J Biol Chem. 1982 Oct 10;257(19):11639–11643. [PubMed] [Google Scholar]