Abstract
The bacteriophage lambda O protein participates in the initiation of lambda DNA replication. The lambda O gene was cloned into plasmid pKC30 such that its expression was controlled by the lambda PL promoter. A lambda prophage-coded thermosensitive cI repressor was used to regulate transcription of the cloned O gene. Thermal inactivation of the lambda cI repressor resulted in overproduction of the O protein until it constituted approximately 20% of the total cellular protein of Escherichia coli. A simple three-step purification protocol was developed that yields several milligrams of homogeneous O protein per gram of cell paste. The precise position of the O gene in the known lambda DNA sequence was identified from the amino-terminal sequence of the isolated O protein. Purified O protein stimulated the replication of plasmid lambda dv DNA in vitro and specifically bound to duplex DNA fragments carrying the lambda replication origin.
Full text
PDF





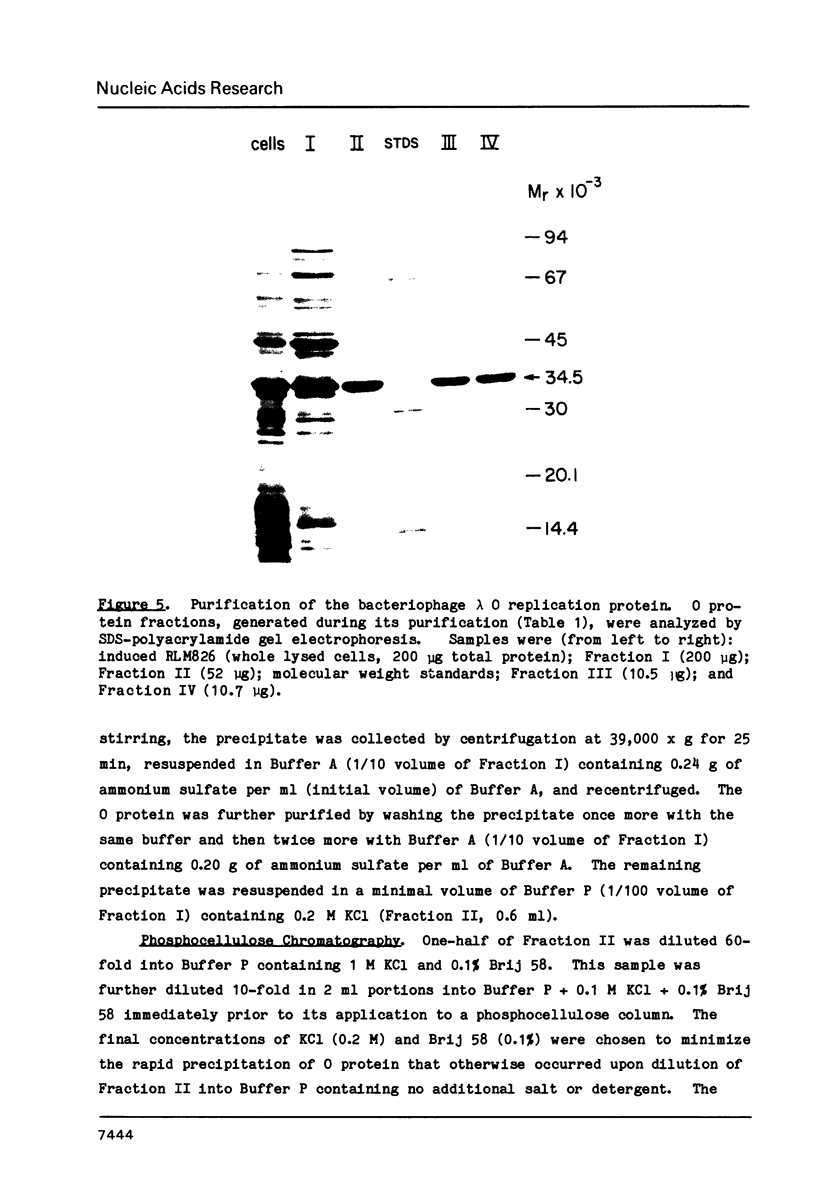
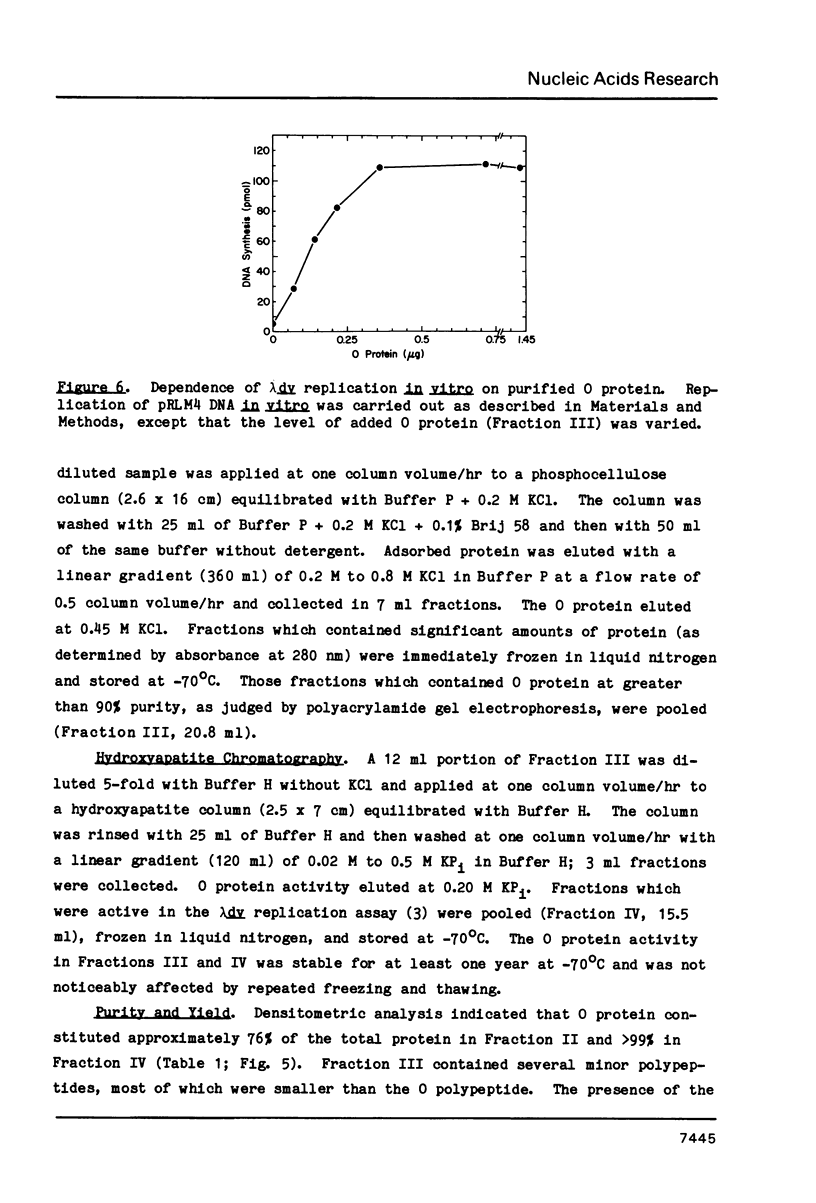


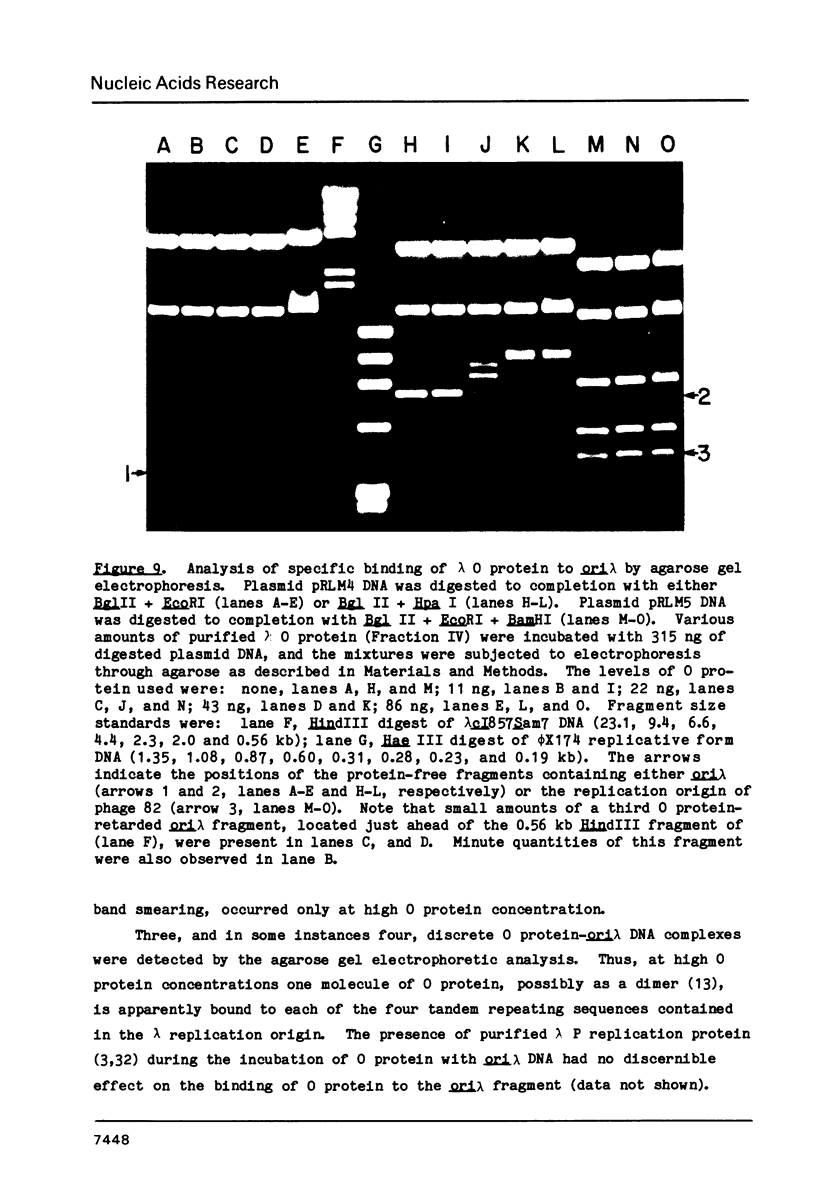




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Promoter occlusion: transcription through a promoter may inhibit its activity. Cell. 1982 Jul;29(3):939–944. doi: 10.1016/0092-8674(82)90456-1. [DOI] [PubMed] [Google Scholar]
- Anderl A., Klein A. Replication of lambda dv DNA in vitro. Nucleic Acids Res. 1982 Mar 11;10(5):1733–1740. doi: 10.1093/nar/10.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin C. C., Anderson P. M., Wold F. The amino acid sequence of Escherichia coli cyanase. J Biol Chem. 1983 Jan 10;258(1):276–282. [PubMed] [Google Scholar]
- Danner D. B., Deich R. A., Sisco K. L., Smith H. O. An eleven-base-pair sequence determines the specificity of DNA uptake in Haemophilus transformation. Gene. 1980 Nov;11(3-4):311–318. doi: 10.1016/0378-1119(80)90071-2. [DOI] [PubMed] [Google Scholar]
- Denniston-Thompson K., Moore D. D., Kruger K. E., Furth M. E., Blattner F. R. Physical structure of the replication origin of bacteriophage lambda. Science. 1977 Dec 9;198(4321):1051–1056. doi: 10.1126/science.929187. [DOI] [PubMed] [Google Scholar]
- Fried M. G., Crothers D. M. CAP and RNA polymerase interactions with the lac promoter: binding stoichiometry and long range effects. Nucleic Acids Res. 1983 Jan 11;11(1):141–158. doi: 10.1093/nar/11.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furano A. V. Content of elongation factor Tu in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4780–4784. doi: 10.1073/pnas.72.12.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth M. E., McLeester C., Dove W. F. Specificity determinants for bacteriophage lambda DNA replication. I. A chain of interactions that controls the initiation of replication. J Mol Biol. 1978 Dec 5;126(2):195–225. doi: 10.1016/0022-2836(78)90359-5. [DOI] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Gottesman M., Shaw J. E., Pearson M. L. Protein degradation in E. coli: the lon mutation and bacteriophage lambda N and cII protein stability. Cell. 1981 Apr;24(1):225–233. doi: 10.1016/0092-8674(81)90518-3. [DOI] [PubMed] [Google Scholar]
- Hines J. L., Chauncey T. R., Agarwal K. L. Preparation and properties of the HpaI and HpaII endonucleases. Methods Enzymol. 1980;65(1):153–163. doi: 10.1016/s0076-6879(80)65021-6. [DOI] [PubMed] [Google Scholar]
- Klein A., Lanka E., Schuster H. Isolation of a complex between the P protein of phage lambda and the dnaB protein of Escherichia coli. Eur J Biochem. 1980 Mar;105(1):1–6. doi: 10.1111/j.1432-1033.1980.tb04467.x. [DOI] [PubMed] [Google Scholar]
- Klinkert J., Klein A. Cloning of the replication gene P of bacteriophage lambda: effects of increased P-protein synthesis on cellular and phage DNA replication. Mol Gen Genet. 1979 Mar 20;171(2):219–227. doi: 10.1007/BF00270008. [DOI] [PubMed] [Google Scholar]
- Kuypers B., Reiser W., Klein A. Cloning of the replication gene O of E. coli bacteriophage lambda and its expression under the control of the lac promoter. Gene. 1980 Aug;10(3):195–203. doi: 10.1016/0378-1119(80)90049-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moore D. D., Denniston K. J., Blattner F. R. Sequence organization of the origins of DNA replication in lambdoid coliphages. Gene. 1981 Jun-Jul;14(1-2):91–101. doi: 10.1016/0378-1119(81)90151-7. [DOI] [PubMed] [Google Scholar]
- Norgard M. V. Rapid and simple removal of contaminating RNA from plasmid DNA without the use of RNase. Anal Biochem. 1981 May 1;113(1):34–42. doi: 10.1016/0003-2697(81)90040-3. [DOI] [PubMed] [Google Scholar]
- Scherer G. Nucleotide sequence of the O gene and of the origin of replication in bacteriophage lambda DNA. Nucleic Acids Res. 1978 Sep;5(9):3141–3156. doi: 10.1093/nar/5.9.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Skalka A. M. DNA replication--bacteriophage lambda. Curr Top Microbiol Immunol. 1977;78:201–237. [PubMed] [Google Scholar]
- Tsurimoto T., Hase T., Matsubara H., Matsubara K. Bacteriophage lambda initiators: preparation from a strain that overproduces the O and P proteins. Mol Gen Genet. 1982;187(1):79–86. doi: 10.1007/BF00384387. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Purification of bacteriophage lambda O protein that specifically binds to the origin of replication. Mol Gen Genet. 1981;181(3):325–331. doi: 10.1007/BF00425606. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Purified bacteriophage lambda O protein binds to four repeating sequences at the lambda replication origin. Nucleic Acids Res. 1981 Apr 24;9(8):1789–1799. doi: 10.1093/nar/9.8.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Replication of lambda dv plasmid in vitro promoted by purified lambda O and P proteins. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7639–7643. doi: 10.1073/pnas.79.24.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., McMacken R., Kornberg A. dnaB protein of Escherichia coli. Purification and role in the replication of phiX174 DNA. J Biol Chem. 1978 Jan 10;253(1):261–269. [PubMed] [Google Scholar]
- Wickner S. H. DNA replication proteins of Escherichia coli and phage lambda. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):303–310. doi: 10.1101/sqb.1979.043.01.037. [DOI] [PubMed] [Google Scholar]
- Wold M. S., Mallory J. B., Roberts J. D., LeBowitz J. H., McMacken R. Initiation of bacteriophage lambda DNA replication in vitro with purified lambda replication proteins. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6176–6180. doi: 10.1073/pnas.79.20.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., McMacken R. Regulation of expression of the Escherichia coli dnaG gene and amplification of the dnaG primase. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4907–4911. doi: 10.1073/pnas.79.16.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem J., Uchida H., Sunshine M., Saito H., Georgopoulos C. P., Feiss M. Genetic analysis of two genes, dnaJ and dnaK, necessary for Escherichia coli and bacteriophage lambda DNA replication. Mol Gen Genet. 1978 Aug 4;164(1):9–14. doi: 10.1007/BF00267593. [DOI] [PubMed] [Google Scholar]