Abstract
The Homeobox (Hox) and Paired box (Pax) gene families are key determinants of animal body plans and organ structure. In particular, they function within regulatory networks that control organogenesis. How these conserved genes elicit differences in organ form and function in response to evolutionary pressures is incompletely understood. We molecularly and functionally characterized one member of an evolutionarily dynamic gene family, plac8 onzin related protein 1 (ponzr1), in the zebrafish. ponzr1 mRNA is expressed early in the developing kidney and pharyngeal arches. Using ponzr1-targeting morpholinos, we show that ponzr1 is required for formation of the glomerulus. Loss of ponzr1 results in a nonfunctional glomerulus but retention of a functional pronephros, an arrangement similar to the aglomerular kidneys found in a subset of marine fish. ponzr1 is integrated into the pax2a pathway, with ponzr1 expression requiring pax2a gene function, and proper pax2a expression requiring normal ponzr1 expression. In addition to pronephric function, ponzr1 is required for pharyngeal arch formation. We functionally demonstrate that ponzr1 can act as a transcription factor or co-factor, providing the first molecular mode of action for this newly described gene family. Together, this work provides experimental evidence of an additional mechanism that incorporates evolutionarily dynamic, lineage-specific gene families into conserved regulatory gene networks to create functional organ diversity.
Keywords: Kidney development, Kidney evolution, Pharyngeal arch development, Zebrafish, Plac8
INTRODUCTION
The evolution of animal complexity has fascinated scientists ever since Darwin presented his ideas on embryology and ‘descent with modification’ (Darwin, 1890). Species’ adaptation to new environments required specific organ system modifications. The adaptation of fish from hyperosmotic saltwater to hypoosmotic freshwater environments is an extreme example of organ system adaptation. This movement required drastic modification of salt and water homeostasis mechanisms. Two organ systems essential for maintaining osmotic balance are the gills and kidney. In aquatic animals, the gills are gatekeepers for maintaining salt balance whereas the kidney is needed for maintaining both salt and water concentrations. Thus, maintaining homeostasis requires cooperation between these two systems. How differential adaptation of the gills and kidney to new environments is encoded in the genomes of diverse animals is not well understood.
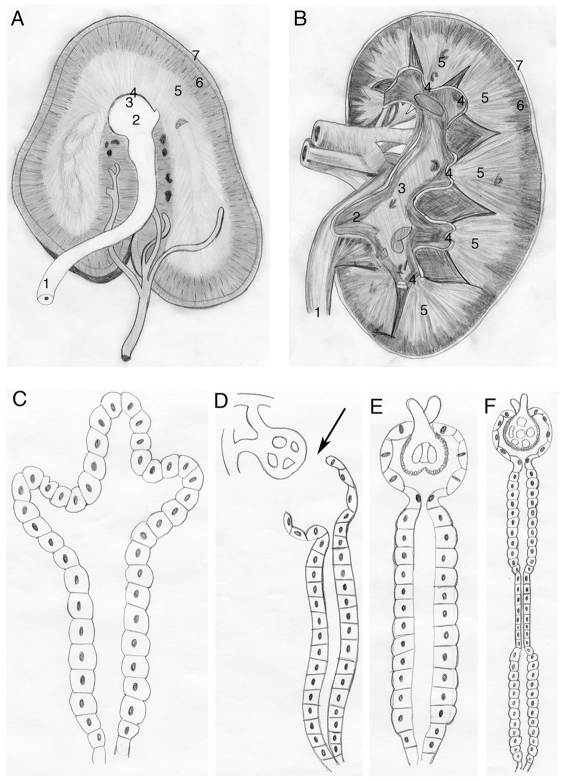
Gross anatomical observations readily demonstrate kidney diversity across species. For over a century and a half, scientists have compared adult mammalian kidneys, cataloging both similarities and differences (Wagner and Tulk, 1845). These differences are illustrated when comparing the horse (Fig. 1A) and human (Fig. 1B) kidney. Despite such outward structural diversity among mammals, the building blocks of their kidneys, the nephrons, are nearly identical. However, comparing nephrons among chordates reveals clear differences (Dantzler, 1989; Smith, 1937). As early as 1937, key variances in nephron structure were noted to track with broad evolutionary changes in vertebrates (Smith, 1937). When examining the earliest developing kidney, the pronephros, variations are observed when comparing glomerular absence (aglomerular; Fig. 1C) or presence (non-integrated or integrated glomerulus; Fig. 1D,E), and by examining the complexity of tubules and ducts extending from the glomerulus. Aglomerular kidneys (Fig. 1C), found in a subset of teleosts such as Opsanus tau and Lophius piscatorius (Marshall, 1930), remove waste by tubule lumen secretion (Beyenbach, 2004). In organisms containing a glomerulus, two examples of glomerular pronephric evolution are the non-integrated and the integrated glomerulus. The non-integrated form of the pronephros is found in Xenopus, in which the coelom separates the glomerulus from the tubules and ducts (Fig. 1D, arrow) (Dressler, 2006). By contrast, the zebrafish pronephros (Fig. 1E) represents the most common teleost kidney type – a glomerulus integrated with the pronephric tubules and duct – and is similar to the nephron found in adult mammalian kidneys (Fig. 1F). The molecular basis underlying these developmental variations of the vertebrate pronephros is unknown.
Fig. 1.
Kidney diversity. (A,B) Two examples of structural diversity seen in mammalian kidneys. Specific anatomical structures of these examples are as follows: ureter (1), pelvis (2), calyx (3), papilla (4), medulla (5), cortex (6) and fibrous capsule (7). The horse kidney (A) demonstrates a kidney type in which the papillae (4) and calyxes (3) fuse to form a single structure that projects into the pelvis (2) (Frandson et al., 2009). Rather than the familiar human (B) ‘bean’-shaped kidney, the two poles extend to form a ‘crest kidney’. The human kidney (B) demonstrates multiple papillae (4), and calyxes (3) merging into a single pelvis (2). This illustrates kidney adaptation beyond the single papilla. See Dyce et al. and Oliver (Dyce et al., 1995; Oliver, 1968). (C-F) Comparing three examples of aquatic pronephroi (C-E) with the mammalian nephron (F) to illustrate nephric structural diversity. Aglomerular nephrons (C) are seen in subsets of marine teleosts, including Opsanus tau and Lophius piscatorius. This type of nephron filters via secretion into the ductal system. (D-F) Example of non-integrated (D) versus integrated (E,F) glomerular kidneys. (D) A glomerular-filtering nephron whose tubules and ducts are separated by the coelom (arrow). An example of this type of kidney is seen in Xenopus embryos (Dressler, 2006). The zebrafish embryo nephron (E) consists of a glomerulus integrated into pronephric tubules and ducts, topological architecture similar to that seen in humans (F). When comparing E to F, however, the separate sections noted in mammalian ducts (F) cannot be visually distinguished in the zebrafish pronephros (E). See Vize and Smith (Vize and Smith, 2004).
Broadly, three related but molecularly distinct models have been proposed as the genetic basis of organ diversity. The first involves conservation of master regulatory genes (Carroll, 2005); the second outlines gene regulatory networks (GRNs) (Davidson and Erwin, 2006); and the third focuses on dynamic gene families (Demuth et al., 2006). First, the ‘master regulation’ model – whereby a handful of key conserved genes are necessary and sufficient for organ development – is the best characterized. Changes in number and regulation of key highly conserved genes is an important mechanism underlying evolutionary diversity (Carroll, 2005). For example, Hox genes specify variation in body segments, including appendage development in insects (Lewis, 1978) and leg loss in snakes (Cohn and Tickle, 1999). Two demonstrated mechanisms for Hox-driven evolutionary diversity are copy number change and expression pattern alteration via variation in regulatory sequences (Force et al., 1999). These mechanisms are responsible for at least part of the diversity seen in body structure (Gilbert, 2000).
The kidney’s master regulatory gene, paired box 2 (pax2), is conserved from flies to humans (Cagan, 2003; Lun and Brand, 1998; Sanyanusin et al., 1995; Schimmenti et al., 1997; Torres et al., 1995) and is both necessary and sufficient to specify renal tubule and ductal cell fate. However, the glomerulus has distinct evolutionary origins and does not require pax2 (Majumdar et al., 2000). Therefore, pax2 and its role in kidney diversity represent a strong model for exploring the mechanisms underlying how conserved genes are manipulated throughout evolution. Pax2 is a paired box transcription factor known to be required for pronephric development. Zebrafish pax2a mutants, no isthmus (noi) (Brand et al., 1996), show distinctive kidney phenotypes (Majumdar et al., 2000). Without pax2a, the podocyte marker wilms tumor 1a (wt1a) shows caudally expanded expression at 25 hours post fertilization (hpf) (Majumdar et al., 2000). Furthermore, cross-sections of noi mutants show improper pronephric tubule and duct formation but reveal intact glomeruli. Evidence from a detailed analysis of the structure and function of the pronephros by Howland and by Price (Howland, 1921; Price, 1910), along with the noi mutant data, suggests that the glomerulus originates and develops independently of the pronephric tubules and ducts (Drummond and Majumdar, 2003). The key question, then, is how pax2 works with other genes to code for glomerular innovation and kidney diversity.
The second model of diversity, the GRN model, also attributes evolutionary innovation to a set of conserved genes. As currently conceptualized, a GRN consists of four types of sub-circuits arranged hierarchically within a network (Davidson, 2010; Davidson and Erwin, 2006). In summary, GRNs consist of ‘kernels’ comprising a few genes and regulatory regions absolutely required to form a specific organ (Punzo et al., 2002; Silver and Rebay, 2005). Should any part of the kernel be lost, the organ does not form. Modules called ‘plug-ins’ provide subsequent layers of complexity (Cadigan and Nusse, 1997; Kingsley, 1994). Plug-ins, which interact with and regulate the kernel, are not required to be organ-specific. ‘Cis-regulatory linkages’ are considered input-output switches involved in regulation of the other sub-circuits (Bolouri and Davidson, 2002; Hersh and Carroll, 2005). ‘Differentiation gene batteries’ are the organ-specific genes that provide the final termination of the network (Gilchrist et al., 2006; Peter and Davidson, 2009). Thus, GRNs can encode diversity via changes in plug-ins and differentiation gene batteries because changes in these peripheral systems will not change the kernel.
Recently, current in-depth sequencing methods and innovative bioinformatic approaches have uncovered genes that do not follow traditional conservation models – ‘evolutionarily dynamic’ gene families (Obbard et al., 2009). In particular, vertebrate-specific gene families have been discovered (Boutet et al., 2010; Katsube et al., 2009). One such example is the vertebrate-specific Ccn family of small, reactive, cysteine-rich proteins that are crucial for signaling of many vertebrate traits, including vasculogenesis and chondrogenesis (Katsube et al., 2009).
The master regulatory gene and GRN models exhibit substantial conceptual overlap, while each offers unique insight into mechanisms that can ultimately cooperate to encode evolutionary diversity. However, how these newly described, lineage-restricted gene families are specifically utilized by core, highly conserved genes and their corresponding signaling networks through evolution is still an open question.
Here, we molecularly and functionally characterize one member of an evolutionarily dynamic gene family, plac8 onzin related protein 1 (ponzr1). Throughout development, ponzr1 expression localizes to the pronephros and pharyngeal arches. We show that ponzr1 functions downstream of pax2a and forms a feedback loop that also modifies pax2a expression. Morpholino knockdown reveals ectopic midline expression of pax2a and wt1a at 24 hpf. At 3 days post fertilization (dpf), ponzr1 knockdown results in a modified zebrafish kidney with loss of the glomerulus and disrupted podocytes. Despite the loss of the glomerulus, the resulting kidney in ponzr1 morphants unexpectedly reveals a functioning structure reminiscent of the simpler kidney found in aglomerular fish (Cagan, 2003; Vize and Smith, 2004). These data lay the foundation for a new model of kidney development in which pax2a signals for kidney differentiation in the pronephric ducts and tubules, and ponzr1 serves as a switch to signal for a more complex kidney that filters with an integrated glomerulus. Examining the second organ system involved in osmotic homeostasis, we find that the wilms tumor 1b (wt1b)-expressing pharyngeal arches, which will eventually develop into the gills, do not form in ponzr1 morphants. Finally, we show ponzr1 can function as a transcription factor or co-factor, providing the first mechanistic insight for this gene family. This protein gives us an additional mechanism of control for conserved genes using a member of a dynamic gene family to generate diversity.
MATERIALS AND METHODS
ponzr1 isolation and constructs
ponzr1 was isolated by RT-PCR from total RNA isolated from 2 dpf embryos. The reverse transcription reaction was performed with 5 μg total RNA, 100 ng of random hexamers (Invitrogen), and Superscript II (Invitrogen). PCR was with the following ponzr1 primers: 5′-CGCGGTAAAACACATTTGCTG-3′ and 5′-TATCAGCGATCACAAAGTTAACT-3′ with cDNA from 2 dpf embryos and High Fidelity Polymerase (Roche). ponzr1 PCR amplicon was gel extracted with the Gel Extraction Kit (Qiagen) and inserted into pCR4TOPO (Invitrogen) to generate pCR4TOPO-ponzr1.
To generate pT3TS-ponzr1, ponzr1 was PCR amplified with SpeI sites engineered onto each primer: 5′-GGACTAGTGCCACCATGGCAGCAATTTCTACTACG-3′, 5′-GGACTAGTCTAATATCTGATCTGCTGGGT-3′. After PCR amplification the band was gel extracted and cut with SpeI restriction enzyme (New England Biolabs) and ligated with T4 ligase (Roche) into pT3TS (Hyatt and Ekker, 1999).
pT3TSpax2a vector was generated by PCR amplifying pax2a Isoform 2 (Lun and Brand, 1998) from a pCMV-S6Pax2-Z vector (Open Biosystems) using the following primers with BglII and SpeI sites engineered onto the 5′ and 3′ primers respectively: 5′-GGAAGATCTGCCACCATGGATATTCACTGCAAAGCA, 3′-GGACTAGTCTAGTGGCGGTCATAGGCAG. ponzr1 amplicon was gel extracted (Qiagen Gel Extraction Kit) and cut with BglII and SpeI (New England Biolabs) and inserted into pT3TS (Hyatt and Ekker, 1999).
pT3TSdsRed was made by cutting the dsRed coding sequence out of pFRM2.1dsRed with BamHI and XbaI followed by ligation into BglII/SpeI sites in pT3TS to generate pT3TSdsRed.
pT3TSponzr1, pT3TSpax2a, pT3TSdsRed were linearized with XbaI and 5′ (7-methyl guanosine) capped mRNA was transcribed with T3 RNA polymerase (T3 mMESSAGE mMACHINE, Ambion).
The ponzr1 5′ untranslated region (UTR) was amplified engineering a BamH1 and EcoR1 site onto the 5′ and 3′ primers, respectively: 5′-GGATCCCAGCTTGCTCCACACAAGACAAG, 3′-GAATTCCTCACAACCGTAGTAGAAATTGCTGC. The PCR product and pCS2+-GFP was digested with BamH1 and EcoR1 and ligated. pCS2+ponzr1 5′ UTR-GFP was digested using Not1 and capped mRNA was made using SP6 RNA polymerase (SP6 mMESSAGE mMACHINE, Ambion). This mRNA was co-injected with ponzr1 morpholino oligonucleotides (MOs) to check efficacy of MO download as previously described (Chen et al., 2004).
Whole-mount in situ hybridization
pCR4TOPOponzr1 (described above) was cut with NotI (New England Biolabs) and DIG-labeled riboprobe generated by in vitro transcription with T3 RNA polymerase (Roche) according to the manufacturer’s protocol. pGEM-3Zf(+)pax2a cut with EcoRI and DIG-labeled riboprobe was transcribed with SP6 as described (Krauss et al., 1991). For double in situ hybridizations, pax2a riboprobes were made by incorporating fluorescein-labeled UTPs instead of DIG. pBluescript KS+ lim-1 (Toyama et al., 1995) was cut with SpeI and riboprobe transcribed with T7 RNA polymerase (Roche). cdh17, wt1a and podocin cDNAs were all isolated by RT-PCR from total RNA from 24 hpf embryos. cdh17 primers (5′-ACAGCTGGAGACCCTCAGAA-3′, 5′-GTCCTGAAGGCAGATGAAGC-3′), wt-1 primers (5′-TGGCTGTCACACTCCTTCTG-3′, 5′-TAGGGTTTCTCCCCTGTGTG-3′), podocin primers (5′-CAAAGCAGCCAAATCTGTGA-3′, 5′-GTCTGGAATGCTAGCGAAGG-3′). cdh17, wt1a and podocin cDNAs were all inserted into pCRIITOPO (Invitrogen). pCRIITOPOcdh17, pCRIITOPOwt1a and pCRIITOPOpodocin were all linearized with Not1 and transcribed with SP6 to generate DIG-labeled riboprobes. Whole-mount in situ hybridizations were performed as described (Thisse and Thisse, 2008).
Morpholino experiments
The following MOs were used in this study: ponzr1 MO1 5′-GAAGTCCTTGTCTTGTGTGGAGCAA-3′, ponzr1 MO2 5′-CCGTAGTAGAAATTGCTGCCATGAC-3′, Control MO 5′-CAAGACCTTGTGTTGTGTGCAGCAT-3′. Morpholino injections were performed as previously described (Nasevicius and Ekker, 2000).
Glomerular capillary experiments
Double transgenic larvae were generated by crossing the Tg(wt1b:EGFP) to Tg(gata1:dsRed) line. Embryos were imaged in fluorinated ethylene propylene tubing (Cole Parmer, USA, P/N EW-00244-ZU) as described by (Petzold et al., 2010) held in a custom-built capillary stage that allowed rotation of the capillary and rotation in the lateral plane about the focal point. Images and movies were taken using a Zeiss Examiner D1 stand, equipped with the W Plan-Aprochromat 20×/1.0 objective (Zeiss, Germany, P/N 421452-9800) and a digital videocamera (Sony, Japan, HDR-HC9) capable of HD 1080i videorecording.
Alcian Blue staining
Alcian Blue cartilage staining was performed as previously described (Walker and Kimmel, 2007) with two modifications. First, we used a 0.01% Alcian Blue concentration to stain the larvae. Second, the larvae were stained for 6 hours then washed overnight in 20% glycerol in 0.25% KOH.
Zebrafish work
All zebrafish work was conducted under full animal care and use guidelines with prior approval by the local institutional animal care and use committee. Danio rerio pax2a null noitu29–/– and pax2a hypomorphic strains noitb21–/– used in our studies were described previously (Lun and Brand, 1998). Danio rerio Tg(wt1b:EGFP)-expressing strain was previously described (Perner et al., 2007), as was the mRFP-expressing line, GBT0046, used as a filtration assay (Clark et al., 2011; Petzold et al., 2009). Danio rerio transgenic lines were described previously: Tg(atp1a1a.4:GFP) (Liu et al., 2007), Tg(enpep:GFP) (Seiler and Pack, 2011) Tg(fli1:EGFP) (Traver et al., 2003) and Tg(gata1:dsRed) (Lawson and Weinstein, 2002).
Statistics
All histograms are the mean of two or three experiments. The error bars were calculated using the mean variance. Statistics was run using a one-way ANOVA and the Newman-Keuls multiple comparison test to look for statistical significance while still accounting for multiple comparisons. All statistical analysis was run on RCF Prism v50a.
RESULTS
The evolutionarily dynamic, chordate-specific Ponzr gene family
ponzr1 was isolated as a part of a forward genetic screen for novel genes required for zebrafish organogenesis (Pickart et al., 2006). ponzr1 encodes a 124-amino acid predicted protein with a plac8 domain, a protein motif of unknown function. The human genome encodes three known proteins containing this same motif: placenta-specific 8 (PLAC8), PLAC8-like 1 (PLAC8L1), and Cornifelin (CNFN). The signature family motif contains two conserved, cysteine-rich domains (domains 1 and 2) separated by a variable region (supplementary material Fig. S1A). Using the conserved sequence obtained from the human and zebrafish Ponzr1 alignment, we mined numerous animal genomes for Ponzr family members (supplementary material Fig. S1B). Ponzr family members are found in the extended vertebrate lineage (supplementary material Fig. S1A) and do not appear in the genomes of Drosophila melanogaster or Caenorhabditis elegans (data not shown). The Ponzr gene family is evolutionarily diverse. For example, several branches of the Ponzr family tree have no human or mouse orthologs (supplementary material Fig. S1B); other branches contain two human genes that have been truncated and are thought to be no longer functional (PLAC8 pseudogenes) that align to a Ponzr member from the invertebrate chordate Ciona intestinalis (supplementary material Fig. S1B). Furthermore, even in animals with the same number of Ponzr family members, such as Ciona and Xenopus, the identified Ponzr genes are not orthologous. Zebrafish ponzr1 represents one of a dozen Ponzr genes in this teleost.
ponzr1 is expressed in the developing kidney and pharyngeal arches
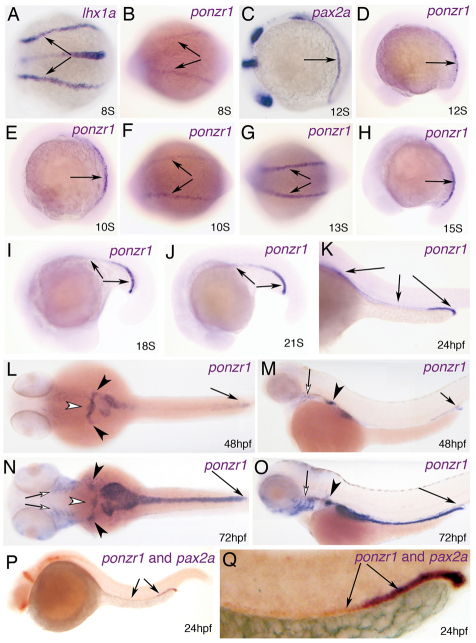
Whole-mount in situ hybridization reveals that ponzr1 is expressed in the intermediate mesoderm, which forms the developing kidney (Fig. 2B,D). This pattern resembles the expression of the pronephric progenitor marker lhx1a (previously lim-1) (Toyama and Dawid, 1997; Toyama et al., 1995) at eight somites (8S) (Fig. 2A) and pax2a at 12S (Fig. 2C). Tissue-specific expression of ponzr1 is visible throughout pronephric development (Fig. 2E-J) and can be seen in the pronephric tubules and ducts at 24 hpf (Fig. 2K). Double in situ hybridizations at 24 hpf show ponzr1 expression in the pax2a-positive tissue of the pronephric tubules and ducts (Fig. 2N,O). ponzr1 expression persists in the pronephric duct and can be detected in the pronephric tubules at 48 hpf (Fig. 2L) and 72 hpf (Fig. 2M). Unlike pax2a (Wingert et al., 2007), ponzr1 is also expressed in the developing glomerulus (Fig. 2L,M). Furthermore, ponzr1 is expressed in the pharyngeal arches at 48 hpf (Fig. 2M) and 72 hpf (Fig. 2N,O).
Fig. 2.
ponzr1 expression in the developing zebrafish kidney. (A) lhx1a mRNA expression in the intermediate mesoderm (arrows) at 8S. (B) ponzr1 mRNA expression in the intermediate mesoderm (arrows) at 8S. (C,D) pax2a mRNA expression at 12S (C) is comparable to ponzr1 (D). (E-K) ponzr1 expression is maintained throughout kidney development from intermediate mesoderm to the formation of the pronephric ducts and tubules. Lateral (E) and dorsal (F) views of ponzr1 show expression in the intermediate mesoderm (arrow) at 10S. ponzr1 expression in the pronephric ducts and tubules (arrows) at 13S (G), 15S (H), 18S (I), 21S (J) and 24 hpf (K). (L-O) At 48 hpf (L,M) and 72 hpf (N,O), ponzr1 expression is detected in the pharyngeal arches (open arrows), pronephric tubules (black arrowhead), ducts (black arrow) and glomeruli (open arrowhead). (P,Q) Double in situ hybridizations with pax2a (orange) and ponzr1 (purple) reveal overlapping expression of ponzr1 and pax2a during kidney development (arrows).
ponzr1 functions downstream of pax2a
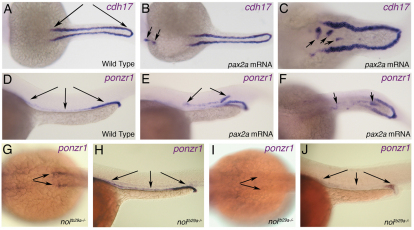
We asked whether ponzr1 is genetically downstream of the kidney master regulatory gene, pax2a, by performing gain-of-function and loss-of-function experiments. In gain-of-function experiments, overexpression of pax2a Isoform 2 mRNA results in formation of ectopic pronephric tissue that express the kidney-specific marker, cadherin 17 (cdh17) (Fig. 3A-C). This functionally demonstrates that Pax2a is a master regulatory factor sufficient to specify pronephric cell fate during embryogenesis. Overexpression of pax2a also results in commensurate ectopic ponzr1 expression in the ectopic ducts (Fig. 3D-F). To perform loss-of-function experiments, we used the pax2a mutant line, no isthmus (noitu29a–/–) (Lun and Brand, 1998). In wild-type sibling embryos at 32 hpf, ponzr1 expression was detected throughout the developing pronephric tubules and ducts (Fig. 3G,H). By contrast, ponzr1 expression in noitu29a–/– mutant embryos was not detected in the anterior pronephric tubules at 32 hpf (Fig. 3I) and was substantially diminished in the posterior pronephric ducts (Fig. 3J). Together, these data provide evidence that ponzr1 is genetically downstream of pax2a.
Fig. 3.
ponzr1 expression is regulated by pax2a. (A) Wild-type cdh17 mRNA expression in the pronephric ducts and tubules (arrows) at 24 hpf. (B-F) Injecting 25 pg of pax2a mRNA results in ectopic cdh17 expression anteriorly (B, arrows) and between the pronephric tubules (C, arrows). Endogenous ponzr1 expression is seen in the pronephric ducts and tubules at 24 hpf (D, arrows). Embryos injected with 25 pg of pax2a mRNA reveal ectopic ponzr1 expression between the pronephric tubules (E,F, arrows). (G-J) Dorsal (G,I) and lateral (H,J) views show a loss of ponzr1 expression (I,J) compared with wild-type siblings (G,H) in anterior and posterior pronephros in noitu29a–/–embryos at 32 hpf.
ponzr1 morpholino knockdown embryos exhibit altered kidney marker expression
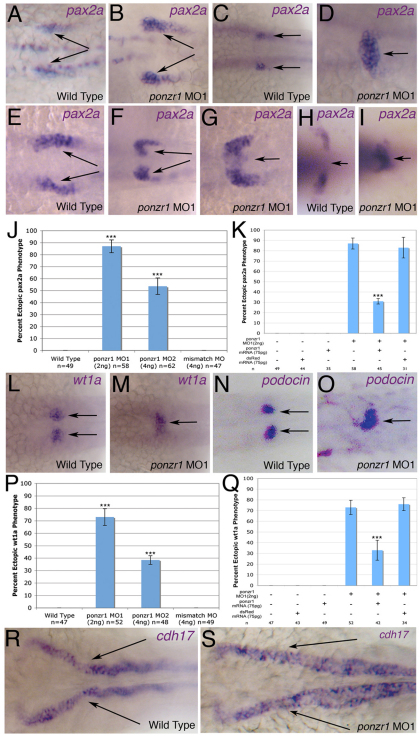
Using whole-mount in situ hybridization, we screened known markers of kidney development to ascertain whether ponzr1 knockdown affected kidney development. First, we assayed for altered expression of intermediate mesoderm markers during early pronephric development to determine the effect of ponzr1 knockdown on early kidney development. Between 4S and 12S, we noted no phenotype (supplementary material Fig. S2). At 18S, ponzr1 morphants exhibit expanded pax2a expression in the anterior pronephric tubules (Fig. 4B) compared with uninjected controls (Fig. 4A). Ectopic and expanded midline expression of pax2a was visible at 24 hpf in ponzr1 morphants (Fig. 4D) compared with controls (Fig. 4C). At the 21S intermediate developmental time point, deformation of the anterior kidney is apparent in ponzr1 morphants when assayed using pax2a expression (Fig. 4F,G, compared with 4E). The pax2a midline fusion is apparent at 3 dpf in ponzr1 morphants (Fig. 4I).
Fig. 4.
ponzr1 is required for correct kidney patterning by pax2a. (A) pax2a mRNA expression in anterior pronephric tubules (arrows) at 18S. (B) Expanded expression of pax2a at 18S (arrows) in ponzr1 morphants. (C) Wild-type expression of pax2a in the anterior pronephric tubules (arrows) at 24 hpf. (D) ponzr1 morphants show expanded/ectopic pax2a expression toward the midline at 24 hpf (arrow). (E) Wild-type pax2a at 21S (arrows). (F,G) ponzr1 morphants show a pax2a phenotype intermediate 18S and 24 hpf where the invagination (F) of pax2a-expressing cells and the start of the midline fusion (G) can be seen. (H) Wild-type pax2a at 3 dpf. (I) ponzr1 morphants show a maintenance of the ectopic midline expression of pax2a (arrow). (J) Ectopic pax2a phenotype is seen using two independent ponzr1 morpholinos but not in ponzr1 mismatch control. (K) Ectopic pax2a phenotype is rescued using ponzr1 mRNA (P<0.001) but not dsRed mRNA. (L-O) wt1a (L) and podocin (N) mark future podocytes with two distinct expression domains at 24 hpf in controls (arrows). A single midline wt1a (M) and podocin (O) expression domain is seen in ponzr1 morphants at 24 hpf. (P,Q) wt1a phenotype is seen with two ponzr1 morpholinos (P) and can be rescued using ponzr1 mRNA (Q, P<0.001). (R,S) cdh17 expression is detected in the pronephric ducts (R, arrows) and tubules at 48 hpf in controls but is expanded in the pronephric ducts (S, arrows) in ponzr1 morphants at 48 hpf. ***P<0.001
The markers of the future podocytes of the glomerulus, wt1a and podocin, are normally expressed in two distinct domains at 24 hpf (Fig. 4L,N). However, ponzr1 morphants exhibit ectopic wt1a and podocin expression in a single midline position (Fig. 4M,O). To ensure the phenotype was specific to ponzr1 loss of function, we tested for the pax2a and wt1a 24 hpf phenotypes with a second ponzr1 morpholino as well as a mismatch control (Fig. 4J,P) and found both morpholinos were significantly different from wild type and the mismatch control. Additionally, we noted a statistically significant rescue of the phenotype by co-injection of ponzr1 mRNA (Fig. 4K,Q). We tested ponzr1 MO knockdown efficiency using an artificial ponzr1 5′ UTR/green fluorescent protein (GFP) synthetic mRNA injected into zebrafish embryos [based on Chen et al. (Chen et al., 2004)]. Briefly, GFP intensity was quantified by fluorescent imaging. Each experiment was standardized with wild-type embryos as 0% (supplementary material Fig. S5A,I) and ponzr1 5′ UTR mRNA-injected embryos as 100% (supplementary material Fig. S5B,I). ponzr1 MO1-injected embryos demonstrated a 94% knockdown (supplementary material Fig. S5C,D), whereas ponzr1 MO2-injected embryos displayed a 90% knockdown (supplementary material Fig. S5E,F). By contrast, the highest dose of ponzr1 MO1 mismatch oligo showed only a 50% knockdown (supplementary material Fig. S5G-I). Therefore, in ponzr1 MO-injected animals at 24 hpf, we saw ectopic midline expression of multiple kidney markers with expanded expression of pax2a.
The three markers pax2a, wt1a and podocin label the anterior kidney, specifically the developing glomerulus. Next, we examined the pronephric tubules and ducts in ponzr1 MO knockdown embryos. cdh17 staining in 48 hpf morphants demonstrated a widening of expression in the anterior pronephric duct (Fig. 4S) compared with controls (Fig. 4R). In the Tg(atp1a1a.4:GFP) (Liu et al., 2007) (supplementary material Fig. S3) and Tg(enpep:GFP) (Seiler and Pack, 2011) (supplementary material Fig. S4) lines, no obvious phenotypes were noted at 28 hpf (A-H) and 48 hpf (I-P).
Loss of ponzr1 results in a nonfunctional glomerulus but functional pronephros
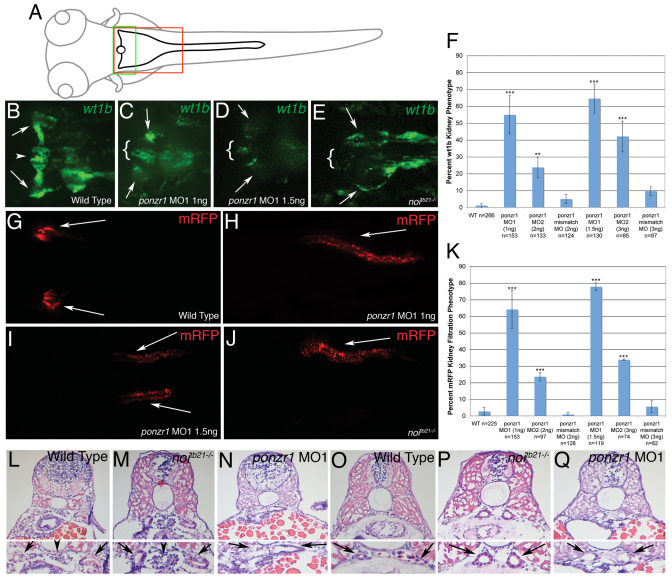
Because of the atypical 24 hpf phenotype in ponzr1 morphants, we hypothesized that at 3 dpf there would be an architectural and functional change after the pronephros was fully formed. We assessed the anterior kidney structure at 3 dpf using the Tg(wt1b:EGFP) line (Perner et al., 2007). In Tg(wt1b:EGFP) larvae, both the podocytes surrounding the glomerulus and the pronephric tubules proceeding distally and caudally from the podocytes (Fig. 5B) express EGFP. In ponzr1 MO1-injected embryos, a gap in fluorescence is noted where the podocytes should be encircling the glomerulus, and the pronephric tubule staining is largely lost (Fig. 5C,D, quantified in 5F). Furthermore, pax2a hypomorph embryos (noitb21–/–) (Lun and Brand, 1998) show an inhibition of podocyte migration from the pronephric tubules to the glomerulus with fluorescence running caudally (Fig. 5E) rather than concentrating in the midline.
Fig. 5.
ponzr1 is required for anterior kidney form and function. (A) A drawing of a larval zebrafish at 3-4 dpf. The green box is the area of the larva seen in images B-E, and the red box is the area seen in images G-J. (B) Tg(wt1b:EGFP) larvae (Perner et al., 2007) mark podocytes surrounding the glomerulus (arrowhead) and the pronephric tubules (arrows). (C,D) ponzr1 morphants show a loss of fluorescence in the area surrounding the glomerulus, except at the midline (brackets). The pronephric tubular staining is lost (arrows) in a dose-dependent manner (D: 1 ng MO1; E: 1.5 ng MO1). (E) noitb21–/– mutant embryos exhibit a loss of fluorescence around the glomerulus (brackets) and the pronephric tubule fluorescence runs posteriorly instead of laterally (arrows). (F) Two ponzr1 MOs induce a similar phenotype that is ameliorated in mismatch control MO injections. (G) mRFP appears bilaterally in the curvature of the pronephric tubules (arrows) in control embryos due to glomerular filtration. (H-J) In ponzr1 morphants, mRFP is collected in a more posterior part of the tubules – sometimes only one pronephric tubule collects fluorescence and sometimes fluorescence is seen in both (H: 1 ng MO1; I: 1.5 ng MO1). A similar phenotype is seen in noitb21–/– mutant embryos (J). (K) Percentage of embryos with the filtration phenotype. (L-Q) Larvae at 4dpf that have been sectioned and H&E stained. (L) Uninjected embryos show a glomerulus (arrowhead) with two pronephric tubules (arrows). (M) noitb21–/– mutant embryo has a glomerulus (arrowhead) but shows dilated tubules (arrow). (N) ponzr1 morphants show dilated pronephric tubules (arrows) with no glomerulus. (O) The posterior pronephric tubules in wild-type embryos (arrows). noitb21–/– mutant (P) and ponzr1 morphant embryos (Q) both show dilated tubules (arrows). **P<0.01, ***P<0.001
Because the 3 dpf kidney structure was altered, we hypothesized that the pronephros would be nonfunctional. To assay for kidney function, we used a zebrafish line that expresses an mRFP attached to a signal sequence. The mRFP is secreted into the blood and, as long as the glomerulus is functional, accumulates in the curvature of the pronephric tubules bilaterally exactly like fluorescent dextran beads (Fig. 5G) (Clark et al., 2011). Surprisingly, when examining this kidney function assay in our ponzr1 knockdown larvae we did not observe total loss of kidney function. Instead, the remaining kidney demonstrated fluorescence accumulating in the posterior pronephric tubules (Fig. 5H,I). Loss of pax2a results in a similar phenotype (Fig. 5J). The filtration phenotype is quantified in Fig. 5K.
As the wt1b fluorescence showed altered architecture of the podocytes and pronephric tubules, we examined the glomerulus using serial sections stained with haematoxylin and eosin (H&E). In wild-type embryos, the glomerulus (arrowhead, Fig. 5L) is clearly visible, as are the flanking tubules (arrows, Fig. 5L) and pronephric ducts (Fig. 5O). In noitb21–/– mutant embryos, the glomerulus is unaltered (Fig. 5M), but the pronephric ducts are overtly dilated (Fig. 5P). This phenotype is comparable to that noted in noitb29a–/– mutant embryos (Majumdar et al., 2000). By contrast, ponzr1 morphants show a loss of the central glomerulus and a dilation of the pronephric tubules (Fig. 5N) as well as the ducts (Fig. 5Q). These data confirm that ponzr1 morphants lack a centralized glomerulus but are still able to retain some kidney filtration function.
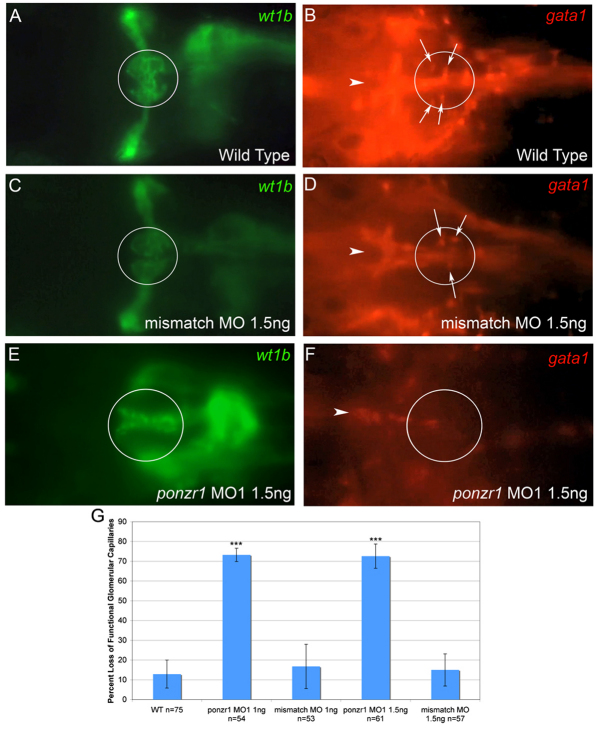
To further assess the defect in the glomerulus, in ponzr1 morphant embryos we examined the glomerular capillaries, which make up the bulk of the cells within the podocytes. Because the vasculature is so abundant in the head, we were unable to visualize the capilliaries using Tg(fli1:EGFP) (Lawson and Weinstein, 2002) (data not shown). Moreover, confidence in focal plane alignment was low in that line. Therefore, we used a double transgenic Tg(wt1b:EGFP) and Tg(gata1:dsRed) (Traver et al., 2003) to ask whether the glomerulus had functional capillaries. We used an updated Specimen in a Corrected Optical Rotational Enclosure (SCORE) protocol (Petzold et al., 2010) combined with a 20X water-emersion, long working-distance lens to visualize the samples and a high-definition camera to record the data. The Tg(wt1b:EGFP) was used to focus on the glomerulus; then we monitored RFP fluorescence to visualize blood flow through the capillaries. In both uninjected embryos (supplementary material Movie 1; Fig. 6A,B) as well as mismatch MO controls (supplementary material Movie 2; Fig. 6C,D), blood cells could be seen moving through the capillaries. However, in ponzr1 MO1-injected larvae, no blood cells could be seen flowing through the area where the glomerular capillaries should be located (supplementary material Movie 3; Fig. 6E,F). The loss of blood flow in the ponzr1 MO-injected larval glomeruli is significantly different from both wild-type and mismatch MO controls (Fig. 6G).
Fig. 6.
Loss of capillary blood flow in ponzr1 morphants. (A,C,E) Tg(wt1b:EGFP) in the podocytes was used to identify the glomerulus in a 3 dpf larvae. (B,D,F) Tg(gata1:dsRed) was used to visualize the blood cells flowing through the glomerular capillaries. (B) Wild-type larvae on the same plane as A show gata1-positive blood cells in the glomerular capillaries (arrows; see supplementary material Movie 1). The bright red line of cells running through the middle of the larvae is the dorsal aorta (arrowhead). (D) Mismatch MO-injected larvae also have blood cells flowing through the glomerular capillaries (arrows; see supplementary material Movie 2) and through the aorta (arrowhead). (F) ponzr1 morphants have no blood cells in the glomerular capillaries, but the cells flowing through the aorta can still be seen (arrowhead; see supplementary material Movie 3). (G) This phenotype is significantly different compared with both wild-type and mismatch MO-injected larvae (*** P<0.001).
Pharyngeal arch loss in ponzr1 morphants
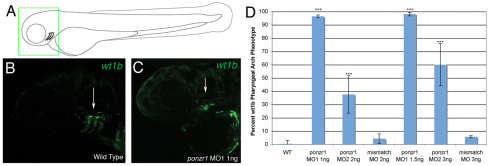
A second way in which aquatic animals manage their osmotic balance is through the gills. The gills form from the pharyngeal arches (Kimmel et al., 1995), which begin to show ponzr1 expression at 2 dpf (Fig. 2L). Additionally, wt1b is also expressed in the pharyngeal arches (Perner et al., 2007) in a pattern similar to that of ponzr1. Therefore, we examined wt1b expression in the pharyngeal arches at 3 dpf. Wild-type embryos showed three pharyngeal arches expressing wt1b (Fig. 7B). ponzr1 MO knockdown resulted in a complete loss of wt1b in the arches (Fig. 7C). This phenotype was seen with both ponzr1 morpholinos but not with control morpholinos or in control wild-type larvae (Fig. 7D).
Fig. 7.
ponzr1 is required for wt1b-expressing pharyngeal arches. (A) A drawing of a 3 dpf larval zebrafish showing where the pharyngeal arches are located. The green box is the area seen in images B and C. (B) Tg(wt1b:EGFP) larvae 3 dpf lateral view shows three pharyngeal arches express wt1b. (C) When ponzr1 MO is injected, the pharyngeal arches are lost. (D) The loss of pharyngeal arches phenotype is significantly different in the two ponzr1 MOs compared with both the wild type and mismatch controls. **P<0.01, ***P<0.001
To determine if the cartilages of the posterior pharyngeal arches are lost in ponzr1 knockdown embryos, we stained with Alcian Blue at 5 dpf. We observed complete loss of the posterior four pharyngeal arches in the ponzr1 MO1-injected larvae (supplementary material Fig. S6B) but not in wild-type or mismatch MO-injected larvae (supplementary material Fig. S6A,C).
Rescue of anterior pax2a mutant phenotype by ponzr1
Having demonstrated that ponzr1 is downstream of pax2a, we asked whether ponzr1 overexpression would be sufficient to rescue the noitb21–/– kidney phenotype at 24 hpf. We examined two known pax2a mutant phenotypes: ectopic, expanded wt1a expression in the anterior kidney (Majumdar et al., 2000); and truncated cdh17 expression in the posterior kidney. Injection of ponzr1 mRNA into pax2a mutant embryos results in a significant reduction in the frequency of embryos displaying the wt1a expression phenotype (supplementary material Fig. S7A). In contrast, addition of ponzr1 mRNA does not significantly alter the posterior cdh17 expression defect (supplementary material Fig. S7B). Therefore, ponzr1 is sufficient to rescue signaling for the anterior portion of the kidney but cannot replace pax2a under these conditions for the posterior kidney at 24 hpf.
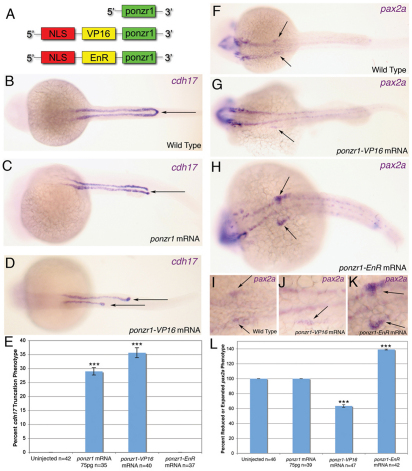
ponzr1 genetically functions as a transcription factor or co-factor
Little is known about the mechanism of action for members of the Ponzr gene family. To determine whether ponzr1 is a terminally expressed gene or a member of a gene regulatory pathway, we asked whether ponzr1 can act as a transcription factor or co-factor. We used a previously described activator/repressor genetic test (Brickman et al., 2000; Conlon et al., 1996) to probe the ability of ponzr1 ability to interact with the transcriptional regulatory system. We made DNA constructs with ponzr1 fused to either the transcriptional activator herpes simplex virus protein 16 (VP16) (Sadowski et al., 1988) or to the transcriptional repressor engrailed repressor (EnR) (Jaynes and O’Farrell, 1991) (Fig. 8A). We then injected mRNA synthesized from ponzr1-VP16 or ponzr1-EnR, respectively, into zebrafish embryos and examined cdh17 and pax2a expression patterns at 24 hpf. Expression of ponzr1-VP16 fusion caused a severe truncation of cdh17 in the pronephric ducts (Fig. 8D) similar to, but more extreme than, the overexpression of ponzr1 mRNA (Fig. 8C). The truncation phenotype is significantly increased compared with wild-type embryos (Fig. 8E). Injections of ponzr1-VP16 mRNA also resulted in a reduction in pax2a expression in the anterior pronephric tubules (Fig. 8G,J) compared with controls (Fig. 8F,I). By contrast, expression of the ponzr1-EnR mRNA resulted in enhanced pax2a expression (Fig. 8H,K), resembling the change of pax2a noted in ponzr1 morphant embryos (Fig. 4B). However, we did not observe a cdh17 phenotype in ponzr1-EnR injected embryos (Fig. 8E). The pax2a phenotypes seen in the ponzr1-VP16 and ponzr1-EnR injections are significantly different from wild type (Fig. 8L). Together, these data strongly suggest that ponzr1 normally functions as a transcription factor or co-factor during kidney development.
Fig. 8.
ponzr1 can function as a transcription factor or co-factor. (A) Diagrams showing the transcriptional activator and repressor mRNAs injected into zebrafish. The NLS was included to facilitate nuclear access of the fusion proteins. (B) Dorsal view of wild-type cdh17 expression in the pronephric ducts at 24 hpf. (C) ponzr1 mRNA-injected embryos reveal cdh17 expression loss at the cloaca (arrow). (D) ponzr1-VP16 mRNA-injected embryos show a severely truncated cdh17 expression in the pronephric ducts (arrows). (E) cdh17 phenotype in ponzr1- and ponzr1-VP16-injected embryos are significantly different from control, whereas ponzr1-EnR-injected embryos are not. (F) Wild-type pax2a expression in the anterior pronephric ducts at 24 hpf (magnified I). (G-K) ponzr1-VP16 mRNA-injected embryos (G) show reduced pax2a expression (magnified J) whereas ponzr1-EnR mRNA-injected embryos (H) demonstrate expanded pax2a expression in the anterior pronephric ducts (arrows) (magnified K) at 24 hpf. (L) Quantification of pax2a phenotypes standardized to wild-type pax2a at 100%. Percentage of embryos with reduced pax2a is seen as below 100% (as seen in ponzr1VP16-injected embryos) and expanded pax2a expression is seen above 100% (as seen in ponzr1EnR-injected embryos). Both ponzr1-VP16 and ponzr1-EnR are significantly different from wild type. ***P<0.001
DISCUSSION
The mysterious glomerulus
The conserved regulatory gene, pax2, plays a conserved role in kidney biology from flies to humans. However, because pax2 is a key kidney regulatory gene, it is found in both glomerular and aglomerular vertebrates, presenting a conundrum regarding the mechanism underlying differences in kidney biology between vertebrate lineages. The glomerulus is a complex organ structure whose development requires multiple cell types of diverse origin (Ditrich, 2005; Kramer-Zucker et al., 2005). Over the past few years, great strides have been made in discovering the molecular signals for tubular and ductal development (Drummond, 2004; Drummond et al., 1998; Vize et al., 1997) as well as identifying signals directing individual cell types, such as the vasculature (Eremina and Quaggin, 2004; Kitamoto et al., 1997; Majumdar and Drummond, 1999) and podocytes (Majumdar and Drummond, 2000; Quaggin and Kreidberg, 2008), to migrate into the glomerulus. However, localized, cell-type-specific development of a centralized glomerulus requires coordination through global signals that remain largely unidentified. For example, VEGF has been implicated in glomerular vascularization, but what regulates VEGF in this developmental setting is unknown (Eremina and Quaggin, 2004; Kitamoto et al., 1997). We show that in zebrafish, ponzr1 is needed to organize the filtration apparatus into a single, central glomerular structure. How ponzr1, a likely transcription factor or co-factor, regulates these cell movements and other processes underlying zebrafish kidney biology is a key next step to furthering our understanding of the role of ponzr1 in organogenesis.
ponzr1 as a mediator and modifier of pax2a
The data presented here implicate ponzr1 as a downstream modulator of anterior pax2a expression in zebrafish kidney specification (Fig. 4). pax2a is able to specify pronephric tubule and duct fates; however, pax2a alone is insufficient to specify the glomerulus. We demonstrate that the zebrafish embryo deploys ponzr1 to provide an additional layer of complexity beyond the regulatory gene pax2a, thus facilitating the formation of a glomerular pronephric kidney (Fig. 5). Therefore, we propose an addition to the current kidney development model: pax2a signals to downstream genes for the pronephric ducts but acts with ponzr1 in the zebrafish to develop additional filtration capabilities by the formation of a glomerulus (Fig. 9).
Fig. 9.
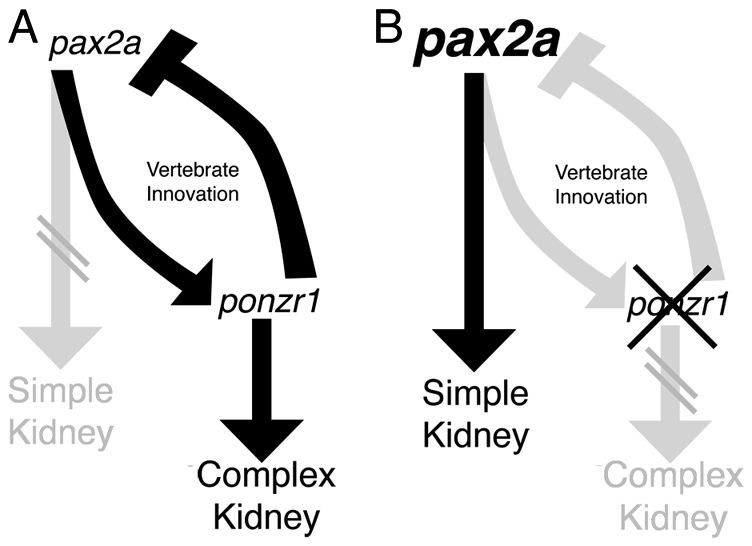
ponzr1 integrates with pax2a signaling in zebrafish kidney formation. (A) pax2a normally signals through ponzr1 to form a more complex kidney, including a feedback loop through ponzr1. (B) Without ponzr1, the zebrafish embryo is unable to form the more complex kidney type. However, pax2a can still signal to form a simplified, tubular kidney.
wt1 is thought to antagonize pax2 function in kidney development (Ryan et al., 1995). ponzr1 morphants, however, display distinct effects on pax2a compared with wt1. ponzr1 morphants show altered pax2a expression; however, the overall expression of wt1 appears largely unaffected. The net result – ectopic pax2a but normal wt1 – appears to separate the interplay between pax2 and wt1 in the zebrafish during kidney formation.
What is the cellular response to this separation between wt1 and pax2 function? One hypothesis is that co-expression of pax2 and wt1 in the developing glomerulus prevents the glomerular cells from differentiating properly and, therefore, from forming a functioning glomerulus. Within such a model, our data argue that the podocyte markers do not require this repression effect, but vascular invasion into the glomerulus does.
Transcription factor ponzr1?
We have shown evidence that ponzr1 can act as a transcription factor or co-factor (Fig. 8). Can ponzr1 act as a DNA-binding protein? ponzr1 lacks any overt homology to known DNA-binding proteins. Alternatively, ponzr1 could bind to a DNA-binding protein and interact with the transcription machinery. This complex would enable ponzr1 to function with established, conserved proteins to influence the development of the glomerulus.
ponzr1 and pax2a and the kidney GRN model
GRNs help visualize and conceptualize the dynamic interplay of the numerous genes and regulatory sequences needed to make an organ (Davidson, 2010; Davidson and Erwin, 2006). Our simplified pax2a/ponzr1 model is easily incorporated into the GRN model of organ development and innovation. Using this hybrid model, ponzr1 would be a component of a pronephric-specific plug-in that interacts with and regulates the pronephric kernel driven by pax2 and related, conserved regulatory genes. Using plug-ins that are lineage-specific or even species-specific provides another way to encode for diversity.
ponzr1 as a pronephric gene
Studies in mammals as diverse as humans, rabbits and sheep have described the pronephros as ‘rudimentary’ (Moritz et al., 2008; Rouiller and Muller, 1969), and it is believed to be nonfunctional in these animals (Moritz et al., 2008; Rouiller and Muller, 1969). Furthermore, the most complete mammalian genomes, namely the human and mouse, do not yield any apparent ortholog to ponzr1 (supplementary material Fig. S1). However, the genome of an animal with a functional pronephros, X. tropicalis, does encode a putative ortholog (supplementary material Fig. S1). One intriguing question is whether ponzr1 is a zebrafish-specific gene or is needed for all vertebrates with a functioning pronephric glomerulus. Functional assessments of ponzr1 in diverse organisms like X. tropicalis will distinguish between these possibilities for ponzr1 function.
Evolutionarily dynamic gene families
New in-depth sequencing of the genomes of diverse organisms and improved predictive and comparative bioinformatics approaches have facilitated the discovery of evolutionarily dynamic gene families (Boutet et al., 2010; Katsube et al., 2009) (this paper). How these families arose, their molecular functions and the biological capacities in which they serve have yet to be characterized. One mechanism for protein genesis includes de novo origination from non-coding sequences followed by subsequent gene duplication to give rise to a lineage-specific protein family (Cai et al., 2008; Knowles and McLysaght, 2009; Levine et al., 2006). Regardless of the molecular origin of these novel genes, however, examining a variety of model organisms at key points along the evolutionary timeline will be crucial to experimentally determine how these lineage-specific gene families operate in the context of well-studied ancient conserved pathways.
Do ancient pathways and lineage-specific genes lead to new structures?
The data presented in this study represent functional evidence that members of evolutionarily dynamic gene families can provide an additional pathway to innovation. We find that one member of a dynamic family, ponzr1, is integrated into the ancient pax2 pathway, facilitating innovation in the pronephros through the formation of the glomerulus. Whether other Ponzr family members or additional dynamic gene families – only now being discovered through the extensive new comparative genomics projects – are responsible for diversity in additional organ systems is unknown. Do other lineage-specific genome additions function in ancient pathways to encode for innovation?
Supplementary Material
Acknowledgments
We thank Dr Englert for the Tg(wt1b:EGFP) fish line, Dr Drummond for the Tg(atp1a1a.4:GFP) fish line, Dr Pack for the Tg(enpep.4:GFP) fish line, and Stephanie Westcot and Dr Keith Cheng for key manuscript revisions.
Footnotes
Funding
This work was funded by National Institute of General Medical Sciences (NIGMS) [GM63904]; National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) [1F30DK083219-01]; and National Institute on Drug Abuse (NIDA) [DA14546]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.071720/-/DC1
References
- Beyenbach K. W. (2004). Kidneys sans glomeruli. Am. J. Physiol. Renal Physiol. 286, F811–F827 [DOI] [PubMed] [Google Scholar]
- Bolouri H., Davidson E. H. (2002). Modeling DNA sequence-based cis-regulatory gene networks. Dev. Biol. 246, 2–13 [DOI] [PubMed] [Google Scholar]
- Boutet A., Comai G., Schedl A. (2010). The WTX/AMER1 gene family: evolution, signature and function. BMC Evol. Biol. 10, 280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M., Heisenberg C. P., Jiang Y. J., Beuchle D., Lun K., Furutani-Seiki M., Granato M., Haffter P., Hammerschmidt M., Kane D. A., et al. (1996). Mutations in zebrafish genes affecting the formation of the boundary between midbrain and hindbrain. Development 123, 179–190 [DOI] [PubMed] [Google Scholar]
- Brickman J. M., Jones C. M., Clements M., Smith J. C., Beddington R. S. (2000). Hex is a transcriptional repressor that contributes to anterior identity and suppresses Spemann organiser function. Development 127, 2303–2315 [DOI] [PubMed] [Google Scholar]
- Cadigan K. M., Nusse R. (1997). Wnt signaling: a common theme in animal development. Genes Dev. 11, 3286–3305 [DOI] [PubMed] [Google Scholar]
- Cagan R. (2003). The signals that drive kidney development: a view from the fly eye. Curr. Opin. Nephrol. Hypertens. 12, 11–17 [DOI] [PubMed] [Google Scholar]
- Cai J., Zhao R., Jiang H., Wang W. (2008). De novo origination of a new protein-coding gene in Saccharomyces cerevisiae. Genetics 179, 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. B. (2005). Endless Forms Most Beautiful: the New Science of Evo Devo and the Making of the Animal Kingdom. New York: Norton; [Google Scholar]
- Chen E., Hermanson S., Ekker S. C. (2004). Syndecan-2 is essential for angiogenic sprouting during zebrafish development. Blood 103, 1710–1719 [DOI] [PubMed] [Google Scholar]
- Clark K. J., Balciunas D., Pogoda H. M., Ding Y., Westcot S. E., Bedell V. M., Greenwood T. M., Urban M. D., Skuster K. J., Petzold A. M., et al. (2011). In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nat. Methods 8, 506–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. J., Tickle C. (1999). Developmental basis of limblessness and axial patterning in snakes. Nature 399, 474–479 [DOI] [PubMed] [Google Scholar]
- Conlon F. L., Sedgwick S. G., Weston K. M., Smith J. C. (1996). Inhibition of Xbra transcription activation causes defects in mesodermal patterning and reveals autoregulation of Xbra in dorsal mesoderm. Development 122, 2427–2435 [DOI] [PubMed] [Google Scholar]
- Dantzler W. H. (1989). Comparative Physiology of the Vertebrate Kidney. Berlin: Springer-Verlag; [Google Scholar]
- Darwin C. (1890). The Origin of Species by Means of Natural Selection. New York: D. Appleton and Company; [Google Scholar]
- Davidson E. H. (2010). Emerging properties of animal gene regulatory networks. Nature 468, 911–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. H., Erwin D. H. (2006). Gene regulatory networks and the evolution of animal body plans. Science 311, 796–800 [DOI] [PubMed] [Google Scholar]
- Demuth J. P., De Bie T., Stajich J. E., Cristianini N., Hahn M. W. (2006). The evolution of mammalian gene families. PLoS ONE 1, e85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditrich H. (2005). Renal Structure and Function in Vertebrates. Enfield, NH: Science Publishers; [Google Scholar]
- Dressler G. R. (2006). The cellular basis of kidney development. Annu. Rev. Cell Dev. Biol. 22, 509–529 [DOI] [PubMed] [Google Scholar]
- Drummond I. A. (2004). Zebrafish kidney development. Methods Cell Biol. 76, 501–530 [DOI] [PubMed] [Google Scholar]
- Drummond I. A., Majumdar A. (2003). The pronephric glomerulus. In The Kidney From Normal Development to Congenital Disease (ed. Vize P. D., Woolf A. S., Bard J. B. L.), pp. 61–73 San Diego: Academic Press; [Google Scholar]
- Drummond I. A., Majumdar A., Hentschel H., Elger M., Solnica-Krezel L., Schier A. F., Neuhauss S. C., Stemple D. L., Zwartkruis F., Rangini Z., et al. (1998). Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125, 4655–4667 [DOI] [PubMed] [Google Scholar]
- Dyce K. M., Sack W. O., Wensing C. J. G. (1995). Textbook of Veterinary Anatomy. Philadelphia: Saunders; [Google Scholar]
- Eremina V., Quaggin S. E. (2004). The role of VEGF-A in glomerular development and function. Curr. Opin. Nephrol. Hypertens. 13, 9–15 [DOI] [PubMed] [Google Scholar]
- Force A., Lynch M., Pickett F. B., Amores A., Yan Y. L., Postlethwait J. (1999). Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151, 1531–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandson R. D., Wilke W. L., Fails A. D. (2009). Anatomy and Physiology of Farm Animals. Ames, Iowa: Wiley-Blackwell; [Google Scholar]
- Gilbert S. F. (2000). Developmental Biology. Sunderland, MA: Sinauer Associates; [Google Scholar]
- Gilchrist M., Thorsson V., Li B., Rust A. G., Korb M., Roach J. C., Kennedy K., Hai T., Bolouri H., Aderem A. (2006). Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature 441, 173–178 [DOI] [PubMed] [Google Scholar]
- Hersh B. M., Carroll S. B. (2005). Direct regulation of knot gene expression by Ultrabithorax and the evolution of cis-regulatory elements in Drosophila. Development 132, 1567–1577 [DOI] [PubMed] [Google Scholar]
- Howland R. B. (1921). Experiments on the effect of removal of the pronephros of Amblystoma punctatum. J. Exp. Zool. 32, 355–396 [Google Scholar]
- Hyatt T. M., Ekker S. C. (1999). Vectors and techniques for ectopic gene expression in zebrafish. Methods Cell Biol. 59, 117–126 [DOI] [PubMed] [Google Scholar]
- Jaynes J. B., O’Farrell P. H. (1991). Active repression of transcription by the engrailed homeodomain protein. EMBO J. 10, 1427–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsube K., Sakamoto K., Tamamura Y., Yamaguchi A. (2009). Role of CCN, a vertebrate specific gene family, in development. Dev. Growth Differ. 51, 55–67 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- Kingsley D. M. (1994). The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 8, 133–146 [DOI] [PubMed] [Google Scholar]
- Kitamoto Y., Tokunaga H., Tomita K. (1997). Vascular endothelial growth factor is an essential molecule for mouse kidney development: glomerulogenesis and nephrogenesis. J. Clin. Invest. 99, 2351–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles D. G., McLysaght A. (2009). Recent de novo origin of human protein-coding genes. Genome Res. 19, 1752–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Zucker A. G., Wiessner S., Jensen A. M., Drummond I. A. (2005). Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev. Biol. 285, 316–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S., Johansen T., Korzh V., Fjose A. (1991). Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development 113, 1193–1206 [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Weinstein B. M. (2002). In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307–318 [DOI] [PubMed] [Google Scholar]
- Levine M. T., Jones C. D., Kern A. D., Lindfors H. A., Begun D. J. (2006). Novel genes derived from noncoding DNA in Drosophila melanogaster are frequently X-linked and exhibit testis-biased expression. Proc. Natl. Acad. Sci. USA 103, 9935–9939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. B. (1978). A gene complex controlling segmentation in Drosophila. Nature 276, 565–570 [DOI] [PubMed] [Google Scholar]
- Liu Y., Pathak N., Kramer-Zucker A., Drummond I. A. (2007). Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development 134, 1111–1122 [DOI] [PubMed] [Google Scholar]
- Lun K., Brand M. (1998). A series of no isthmus (noi) alleles of the zebrafish pax2.1 gene reveals multiple signaling events in development of the midbrain-hindbrain boundary. Development 125, 3049–3062 [DOI] [PubMed] [Google Scholar]
- Majumdar A., Drummond I. A. (1999). Podocyte differentiation in the absence of endothelial cells as revealed in the zebrafish avascular mutant, cloche. Dev. Genet. 24, 220–229 [DOI] [PubMed] [Google Scholar]
- Majumdar A., Drummond I. A. (2000). The zebrafish floating head mutant demonstrates podocytes play an important role in directing glomerular differentiation. Dev. Biol. 222, 147–157 [DOI] [PubMed] [Google Scholar]
- Majumdar A., Lun K., Brand M., Drummond I. A. (2000). Zebrafish no isthmus reveals a role for pax2.1 in tubule differentiation and patterning events in the pronephric primordia. Development 127, 2089–2098 [DOI] [PubMed] [Google Scholar]
- Marshall E. K. J. (1930). A comparison of the function of the glomerular and aglomerular kidney. Am. J. Physiol. 94, 1–10 [Google Scholar]
- Moritz K. M., Wintour-Coghlan M., Bertram J. F., Black M. J., Caruana G. (2008). Factors Influencing Mammalian Kidney Development: Implications for Health in Adult Life. Berlin: Springer-Verlag; [DOI] [PubMed] [Google Scholar]
- Nasevicius A., Ekker S. C. (2000). Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 26, 216–220 [DOI] [PubMed] [Google Scholar]
- Obbard D. J., Welch J. J., Little T. J. (2009). Inferring selection in the Anopheles gambiae species complex: an example from immune-related serine protease inhibitors. Malar. J. 8, 117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. (1968). Nephrons and Kidneys; a Quantitative Study of Developmental and Evolutionary Mammalian Renal Architectonics. New York: Hoeber Medical Division; [Google Scholar]
- Perner B., Englert C., Bollig F. (2007). The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Dev. Biol. 309, 87–96 [DOI] [PubMed] [Google Scholar]
- Peter I. S., Davidson E. H. (2009). Modularity and design principles in the sea urchin embryo gene regulatory network. FEBS Lett. 583, 3948–3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold A. M., Balciunas D., Sivasubbu S., Clark K. J., Bedell V. M., Westcot S. E., Myers S. R., Moulder G. L., Thomas M. J., Ekker S. C. (2009). Nicotine response genetics in the zebrafish. Proc. Natl. Acad. Sci. USA 106, 18662–18667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold A. M., Bedell V. M., Boczek N. J., Essner J. J., Balciunas D., Clark K. J., Ekker S. C. (2010). SCORE imaging: specimen in a corrected optical rotational enclosure. Zebrafish 7, 149–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart M. A., Klee E. W., Nielsen A. L., Sivasubbu S., Mendenhall E. M., Bill B. R., Chen E., Eckfeldt C. E., Knowlton M., Robu M. E., et al. (2006). Genome-wide reverse genetics framework to identify novel functions of the vertebrate secretome. PLoS ONE 1, e104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G. C. (1910). The structure and function of the adult head kidney of Bdellostoma stouti. J. Exp. Zool. 9, 849–864 [Google Scholar]
- Punzo C., Seimiya M., Flister S., Gehring W. J., Plaza S. (2002). Differential interactions of eyeless and twin of eyeless with the sine oculis enhancer. Development 129, 625–634 [DOI] [PubMed] [Google Scholar]
- Quaggin S. E., Kreidberg J. A. (2008). Development of the renal glomerulus: good neighbors and good fences. Development 135, 609–620 [DOI] [PubMed] [Google Scholar]
- Rouiller C., Muller A. F. (1969). The Kidney: Morphology, Biochemistry, Physiology. New York: Academic Press; [Google Scholar]
- Ryan G., Steele-Perkins V., Morris J. F., Rauscher F. J., 3rd, Dressler G. R. (1995). Repression of Pax-2 by WT1 during normal kidney development. Development 121, 867–875 [DOI] [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. (1988). GAL4-VP16 is an unusually potent transcriptional activator. Nature 335, 563–564 [DOI] [PubMed] [Google Scholar]
- Sanyanusin P., Schimmenti L. A., McNoe L. A., Ward T. A., Pierpont M. E., Sullivan M. J., Dobyns W. B., Eccles M. R. (1995). Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat. Genet. 9, 358–364 [DOI] [PubMed] [Google Scholar]
- Schimmenti L. A., Cunliffe H. E., McNoe L. A., Ward T. A., French M. C., Shim H. H., Zhang Y. H., Proesmans W., Leys A., Byerly K. A., et al. (1997). Further delineation of renal-coloboma syndrome in patients with extreme variability of phenotype and identical PAX2 mutations. Am. J. Hum. Genet. 60, 869–878 [PMC free article] [PubMed] [Google Scholar]
- Seiler C., Pack M. (2011). Transgenic labeling of the zebrafish pronephric duct and tubules using a promoter from the enpep gene. Gene Expr. Patterns 11, 118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S. J., Rebay I. (2005). Signaling circuitries in development: insights from the retinal determination gene network. Development 132, 3–13 [DOI] [PubMed] [Google Scholar]
- Smith H. W. (1937). The Physiology of the Kidney. New York: Oxford University Press; [Google Scholar]
- Thisse C., Thisse B. (2008). High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69 [DOI] [PubMed] [Google Scholar]
- Torres M., Gomez-Pardo E., Dressler G. R., Gruss P. (1995). Pax-2 controls multiple steps of urogenital development. Development 121, 4057–4065 [DOI] [PubMed] [Google Scholar]
- Toyama R., Dawid I. B. (1997). lim6, a novel LIM homeobox gene in the zebrafish: comparison of its expression pattern with lim1. Dev. Dyn. 209, 406–417 [DOI] [PubMed] [Google Scholar]
- Toyama R., O’Connell M. L., Wright C. V., Kuehn M. R., Dawid I. B. (1995). Nodal induces ectopic goosecoid and lim1 expression and axis duplication in zebrafish. Development 121, 383–391 [DOI] [PubMed] [Google Scholar]
- Traver D., Paw B. H., Poss K. D., Penberthy W. T., Lin S., Zon L. I. (2003). Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol. 4, 1238–1246 [DOI] [PubMed] [Google Scholar]
- Vize P. D., Smith H. W. (2004). A Homeric view of kidney evolution: A reprint of H.W. Smith’s classic essay with a new introduction. Evolution of the kidney. 1943. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 277, 344–354 [DOI] [PubMed] [Google Scholar]
- Vize P. D., Seufert D. W., Carroll T. J., Wallingford J. B. (1997). Model systems for the study of kidney development: use of the pronephros in the analysis of organ induction and patterning. Dev. Biol. 188, 189–204 [DOI] [PubMed] [Google Scholar]
- Wagner R., Tulk A. (1845). Elements of the Comparative Anatomy of the Vertebrate Animals, Designed Especially for the Use of Students. London: Longman, Brown, Green and Longmans; [Google Scholar]
- Walker M. B., Kimmel C. B. (2007). A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech. Histochem. 82, 23–28 [DOI] [PubMed] [Google Scholar]
- Wingert R. A., Selleck R., Yu J., Song H. D., Chen Z., Song A., Zhou Y., Thisse B., Thisse C., McMahon A. P., et al. (2007). The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 3, 1922–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.