Abstract
Although activity that induced tumor regression was observed and termed tumor necrosis factor (TNF) as early as the 1960s, the true identity of TNF was not clear until 1984, when Aggarwal and coworkers reported, for the first time, the isolation of 2 cytotoxic factors: one, derived from macrophages (molecular mass 17 kDa), was named TNF, and the second, derived from lymphocytes (20 kDa), was named lymphotoxin. Because the 2 cytotoxic factors exhibited 50% amino acid sequence homology and bound to the same receptor, they came to be called TNF-α and TNF-β. Identification of the protein sequences led to cloning of their cDNA. Based on sequence homology to TNF-α, now a total of 19 members of the TNF superfamily have been identified, along with 29 interacting receptors, and several molecules that interact with the cytoplasmic domain of these receptors. The roles of the TNF superfamily in inflammation, apoptosis, proliferation, invasion, angiogenesis, metastasis, and morphogenesis have been documented. Their roles in immunologic, cardiovascular, neurologic, pulmonary, and metabolic diseases are becoming apparent. TNF superfamily members are active targets for drug development, as indicated by the recent approval and expanding market of TNF blockers used to treat rheumatoid arthritis, psoriasis, Crohns disease, and osteoporosis, with a total market of more than US $20 billion. As we learn more about this family, more therapeutics will probably emerge. In this review, we summarize the initial discovery of TNF-α, and the insights gained regarding the roles of this molecule and its related family members in normal physiology and disease.
Introduction
The tumor necrosis factor (TNF) superfamily, composed of 19 ligands and 29 receptors, plays highly diversified roles in the body. The interest in TNF research has increased dramatically over the years as indicated by more than 113 000 citations on TNF-α alone, 27 000 on anti–TNF-α, 25 000 on TNF-α inhibitors, 55 000 on TNF receptors, 12 000 on TNF-mediated apoptosis, 40 000 on TNF-α-induced signals, and 9610 reviews. All members of the TNF superfamily, without exception, exhibit pro-inflammatory activity, in part through activation of the transcription factor NF-κB. Several members of the TNF superfamily exhibit proliferative activity on hematopoietic cells, in part through activation of various mitogen-activated kinases, and some members of this family play a role in apoptosis (Figure 1).1,2 Some members of the TNF superfamily have also been reported to play a role in morphogenetic changes and differentiation. Most members of the TNF superfamily have both beneficial and potentially harmful effects.3 Although TNF-α, for example, has been linked with physiologic proliferation and differentiation of B cells under steady-state conditions, it also has been linked with a wide variety of diseases, including cancer, cardiovascular, neurologic, pulmonary, autoimmune, and metabolic disorders.
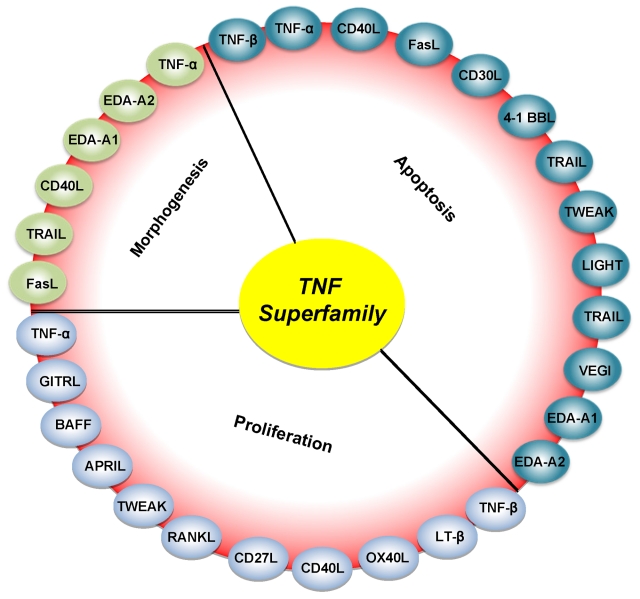
Figure 1.
Roles of various members of the TNF superfamily in inflammation, cellular proliferation, apoptosis, and morphogenesis. All members of the TNF superfamily exhibit pro-inflammatory activity, in part through activation of the transcription factor NF-κB (full red circle); OX40L, CD40L, CD27L, APRIL, and BAFF exhibit proliferative activity in part through activation of various mitogen-activated kinases (sky blue); TNF-α, TNF-β, FasL, and TRAIL control apoptosis (bluish-green); and EDA-A1, EDA-A2, TNF-α, FasL, and TRAIL regulate morphogenesis (green).
Although the fascinating history of TNF could be traced back more than one century, the name “tumor necrotizing factor” was first used in 1962 for tumor (sarcoma 37) regression activity induced in the serum of mice treated with Serratia marcescens polysaccharide; this activity was shown by Carswell et al in 1975 to be the result of TNF.4,5 Whether this TNF is the same as what our group later renamed TNF-α is unclear. In 1984 to 1985, our group structurally identified 2 different TNFs and cloned their genes. From hundreds of liters of conditioned medium collected from the human lymphoblastoid cell line RPMI 1788, we purified a protein 25 kDa in size, which initially was named lymphotoxin-α (LT-α) and later changed to TNF-β with the later discovery of sequence homology with TNF-α.6,7 Using the same cell lysis assays and antibodies against lymphotoxin, we then reported isolation of a second cytotoxic factor from hundreds of liters of conditioned supernatants of human promyelomonocytic cell line HL-60; this factor had a molecular mass approximately 17 kDa and was named human TNF-α.8 When we first performed the amino acid sequencing of the 2 proteins, it became clear that they exhibited as much as 50% sequence homology.6–8 Generation of antibodies against each molecule proved that these 2 proteins were immunologically distinct9; whereas TNF-α was produced by macrophages, TNF-β was produced by lymphocytes.10
The amino acid sequence was used to prepare and isolate the full-length cDNAs for TNF-α and TNF-β.11,12 The cloning of the cDNA for TNF-β turned out to be much more difficult than that of TNF-α, for unclear reasons; thus, for TNF-β, most of the gene actually had to be chemically synthesized on the basis of the protein sequence.12 Soon our group discovered that both TNF-α and TNF-β bind to a common high-affinity cell surface receptor present on most cell types.13 While we were finishing the isolation of the TNF-α and TNF-β from monocytes and lymphocytes, respectively, Beutler et al from Rockefeller University reported the identity of TNF with the macrophage-secreted factor cachectin,14 which was responsible for cachexia. This protein was found to be a murine counterpart of human TNF-α, based on the amino acid sequence homology to human TNF-α. Cerami gave his account of this event in a recent article.15 Although passive immunization against cachectin/TNF has been shown to protect mice from the lethal effect of endotoxin, there are reports suggesting that endotoxin-induced factor is distinct from TNF-α.14
More than 25 years later, 19 different members of the TNF superfamily have been identified based on their gene sequences (Figure 2; Table 1). TNF-α and TNF-β are the only members of this family that were first identified at the protein level, before isolation of their cDNA; all other members of this family were identified via human cDNA sequence homology. The superfamily members are TNF-α, TNF-β, lymphotoxin-β, CD40L, FasL, CD30L, 4-1BBL, CD27L, OX40L, TNF-related apoptosis-inducing ligand (TRAIL), LIGHT, receptor activator of NF-κB ligand (RANKL), TNF-related weak inducer of apoptosis (TWEAK), a proliferation-inducing ligand (APRIL), B-cell activating factor (BAFF), vascular endothelial cell-growth inhibitor (VEGI), ectodysplasin A (EDA)–A1, EDA-A2, and GITRL. These 19 members bind to 29 different receptors (Figure 2; Table 1). In addition, 3 TNF receptors (ie, TNFRSF22-mDcTRAILR2/TNFRH2, TNFRSF23-mDcTRAILR1/TNFRH1, and TNFRSF26-mTNFRH3) have been identified in mice only.16 The TNF receptor NGFR differs in its biologic activity from other members. Furthermore, the nomenclature of the TNF superfamily ligands has been standardized by an international commission as TNF superfamily (TNFSF), and the receptors as TNFRSF (Table 1). The cell types expressing each ligand or receptor are well characterized and summarized in Table 1. Both the ligands and receptors are expressed primarily by various cells of the immune system, but other cell types have been shown to express ligands as well as the receptors, under both physiologic and pathologic conditions. The importance of these proteins/cytokines in the pathophysiology of human diseases is indicated by the fact that in 2010 sales of TNF blockers exceeded US $20 billion (www.biospace.com/News).
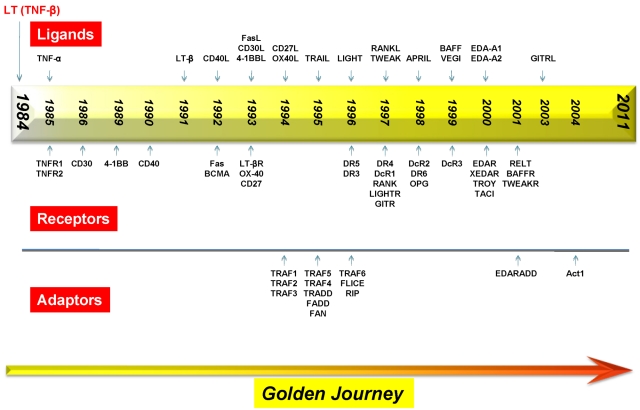
Figure 2.
Timeline for the discovery of various members of the TNF superfamily, their receptors, and the receptor-associated adaptor proteins.
Table 1.
Expression profile of ligands and receptors of human tumor necrosis factor superfamily
| Symbol | Ligand (alias) | Cellular expression | Symbol | Receptor (alias) | Cellular expression |
|---|---|---|---|---|---|
| TNFSF1 | TNF-β (LT-α) | NK, T, and B cells | TNFRSF1A | TNFR1 (DR1) | Hematopoietic and immune cells |
| TNFRSF1B | TNFR2 | Immune and endothelial cells | |||
| TNFSF2 | TNF-α | Macrophages and NK, T, and B cells | TNFRSF1A/B | TNFR1/2 | Immune and endothelial cells |
| TNFSF3 | LT-β | Activated CD4+ T cells and T, DC, and NK cells | TNFRSF3 | LT-βR | NK cells, CD4+ and CD8+ T cells |
| TNFSF4 | OX40L (CD252, gp34) | B and T cells, DCs, endothelial and smooth muscle cells | TNFRSF4 | OX40 (CD134) | Activated CD4+ T cells and neutrophils |
| TNFSF5 | CD40L (CD154, gp39) | Activated CD4+ T lymphocytes, NK cells, mast cells, basophils, and eosinophils | TNFRSF5 | CD40 (p50) | B cells, monocytes, DCs, and thymic epithelium, Reed-Sternberg cells |
| TNFSF6 | FasL (CD95L, Apo1L) | Activated splenocytes, thymocytes, nonlymphoid tissues, and NK cells | TNFRSF6 | Fas (CD95, Apo1, DR2) | Epithelial cells, hepatocytes, activated mature lymphocytes, and transformed cells |
| TNFRSF6B | DcR3 | Lung and colon cells | |||
| TNFSF7 | CD27L (CD70) | NK, T, B, and mast cells, smooth muscle and thymic epithelial cells | TNFRSF7 | CD27 | Hematopoietic progenitors, and CD4+ and CD8+T cells |
| TNFSF8 | CD30L (CD153) | Activated T cells, B cells, and monocytes, granulocytes, and medullary thymic epithelial cells | TNFRSF8 | CD30 | Reed-Sternberg cells |
| TNFSF9 | 4–1BBL | APCs (B cells, macrophages, and DCs), mast cells | TNFRSF9 | 4–1BB (CD137, ILA) | T, NK, and mast cells, and neutrophils |
| TNFSF10 | TRAIL (Apo2L) | NK and T cells, DCs | TNFRSF10A | TRAILR1 (DR4, Apo2) | Most normal and transformed cells |
| TNFRSF10B | TRAILR2 (DR5) | Most normal and transformed cells | |||
| TNFRSF10C | TRAILR3 (DcR1) | Most normal and transformed cells | |||
| TNFRSF10D | TRAILR4 (DcR2) | Most normal and transformed cells | |||
| TNFRSF11B | OPG (OCIF) | Most normal and transformed cells | |||
| TNFSF11 | RANKL (TRANCE, OPGL, ODF) | T cells, thymus, and lymph nodes | TNFRSF11A | RANK (TRANCER) | Osteoclasts, osteoblasts, and activated T cells |
| TNFRSF11B | OPG (OCIF) | Osteoclast precursors, endothelial cells, and others | |||
| TNFSF12 | TWEAK (Apo3L) | Monocytes | TNFRSF12A | TWEAKR (FN14) | Endothelial cells and fibroblasts |
| TNFSF13 | APRIL (TALL-2, TRDL-1) | Macrophages, lymphoid cells, and tumor cells | TNFRSF13A/17 | BCMA | B cells, PBLs, spleen, thymus, lymph nodes, liver, and adrenals |
| TNFRSF13B | TACI | B cells, activated T cells, PBLs, spleen, thymus, and small intestine | |||
| TNFSF13B | BAFF (BLYS, THANK) | T cells, monocytes, macrophages, and DCs | TNFRSF13B | TACI | B cells, activated T cells, PBLs, spleen, thymus, and small intestine |
| TNFRSF13C | BAFFR | B cells, resting T cells, PBLs, spleen, lymph nodes | |||
| TNFRSF17 | BCMA | B cells, resting T cells, PBLs, spleen, lymph nodes | |||
| TNFSF14 | LIGHT (HVEML, LT-γ) | T cells, granulocytes, monocytes, and DCs | TNFRSF14 | LIGHTR (HVEM) | T and B cells, monocytes, and lymphoid cells |
| TNFRSF3 | LT-βR | Nonlymphoid hematopoietic and stromal cells | |||
| TNFSF15 | VEGI (TL1A) | Endothelial cells | TNFRSF25 | DR3 | NK cells, CD4+ and CD8+ T cells |
| APCs (B cells, macrophages, and DCs) | TNFRSF6B | DcR3 | Activated T cells | ||
| TNFSF18 | GITRL | HUVECs | TNFRSF18 | GITR (AITR) | CD4+CD25+ T cells |
| EDA-A1 | Skin | EDAR | Ectodermal derivative | ||
| EDA-A2 | Skin | XEDAR | Ectodermal derivative, embryonic hair follicles | ||
| NI | TNFRSF19 | TROY (TAJ) | Embryo skin, epithelium, hair follicles, and brain | ||
| NI | TNFRSF19L | RELT | Lymphoid tissues, hematopoietic tissues | ||
| NI | TNFRSF21 | DR6 | Resting T cells | ||
| NI | TNFRSF16 | NGFR (CD271) | Neuronal axons, Schwann cells, perineural cells |
NI indicates not identified; OX40L, OX40 ligand; Fas, fibroblast-associated; TRANCE, TNF-related activation-induced cytokines; OPGL, OPG ligand; ODF, osteoclast differentiation factor; TALL, TNF- and APOL-related leukocyte expressed ligand; TRDL, TNF-related death ligand; HVEM, herpesvirus entry mediator; GITR, glucocorticoid-induced TND receptor; APCs, antigen-presenting cells; HUVECs, human umbilical vein endothelial cells; ILA, induced by lymphocyte activation; OCIF, osteoclastogenesis inhibitory factor; FN14, fibroblast growth factor-inducible immediate-early response gene 14; PBLs, peripheral blood lymphocytes; AITR, activation-inducible TNF receptor superfamily member; XEDAR, X-linked ectodysplasin receptor; TROY, TNFRSF expressed on the mouse embryo; TAJ, toxicity and JNK inducer; RELT, receptor expressed in lymphoid tissues; and NGFR, nerve growth factor receptor.
TNF-α was first identified as a factor with antitumor activity. The potent pro-inflammatory activity, however, prevents systemic administration of TNF-α to cancer patients.17,18 However, TNF-α is indeed used in the clinic. Specifically, it is used in the treatment of soft tissue sarcomas and melanomas in the extremities, using a technique called isolated limb perfusion. In this setting, TNF-α has demonstrated potent antitumor activity with an acceptable safety profile.19–21
In the sections to follow, we discuss the signaling events mediated by members of TNF superfamily and receptors with a particular emphasis on TNF-α. We describe the role of this superfamily in normal physiology and disease. Finally, we discuss rationally designed therapeutics that are based on TNF signaling.
Promiscuity and cell signaling of TNF superfamily
The mechanism by which TNF and its various family members transduce target cell signals has been studied extensively. That the 19 members of the TNF superfamily interact with 29 distinct receptors implies that at least some of the ligands must interact with more than one receptor. For instance, TNF-α is known to interact with 2 distinct receptors, TNF receptor 1 (TNFR1) and TNFR2 (Figure 3). On B cells, BAFF has been shown to bind to 3 distinct receptors, transmembrane activator and CAML interactor (TACI), B-cell maturation protein (BCMA), and BAFF receptor (BAFFR). TRAIL has been shown to bind to as many as 5 different receptors, including death receptor 4 (DR4), DR5, decoy receptor 1 (DcR1), DcR2, and osteoprotegerin (OPG).22 Whereas DR4 and DR5 transduce the signals across the cell membrane, DcR1 and DcR2 bind the ligand but do not transduce signals and OPG is primarily a soluble receptor without any transmembrane domain. Some of the cell-surface receptors bind more than one ligand, as in the case of TNF-α and TNF-β, both of which bind to the same receptor with comparable affinity.8
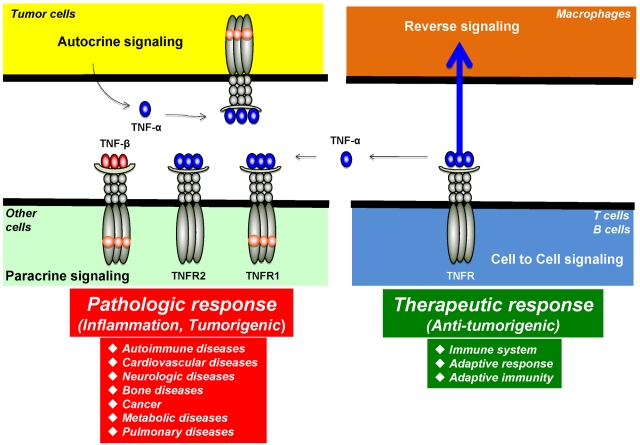
Figure 3.
Autocrine, paracrine, cell to cell, and reverse signaling pathways for TNF-α. Ligands, such as TNF-α, are expressed as both transmembrane and in soluble forms. The transmembrane form of the ligand appears to mediate therapeutic effects, but soluble ligand is linked to pathologic effects of TNF-α. Ligands, such as TNF-β, lack the transmembrane domain and thus are expressed only as a soluble protein. TNF-α, made by tumor cells, acts primarily through TNFR1 in an autocrine and paracrine manner. There are also examples of reverse signaling through TNF-α when it binds to its receptor.
Most of the receptors of the TNF superfamily can be classified into one of 2 categories: those that possess an intracellular death domain (DD) and those that do not. The DD is a region approximately 45 amino acids long that is required for recruitment of other proteins resulting in cell death. Six different receptors have been identified with DD in their intracellular domain: DR1 (also called TNFR1); DR2 (also called Fas); DR3, to which VEGI binds; DR4 and DR5, to which TRAIL binds; and DR6. No ligand has yet been identified that binds to DR6. Interestingly, a recent study indicates that DR6 is broadly expressed by developing neurons and is activated by β-amyloid precursor protein.23 The authors suggested that an extracellular fragment of β-amyloid precursor protein, acting via DR6 and caspase-6, contributes to Alzheimer disease.
The expression of various receptors of the TNF superfamily may vary significantly between cell types and tissue. Receptors that contain a DD in their intracellular domain are expressed most universally. TNF receptor 1 (TNFR1), which contains a DD, is highly promiscuous and is expressed on every cell type in the body studied to date. This may reflect the receptor's diverse functions in different cell types. Similarly, DR4 and DR5 have been shown to be expressed in most cell types. The expression of TNFR2, in comparison, is limited to cells of the immune system, endothelial cells, and nerve cells. Fas receptor expression is also highly heterogeneous and is found on epithelial cells, hepatocytes, and lymphocytes.
Cell signaling for most cytokines and growth factors is normally mediated through interaction between a soluble ligand and a transmembrane receptor. Among the TNF superfamily, however, several ligands have been identified that rarely appear as soluble ligand, including FasL, CD27L, CD30L, CD40L, OX40L, and 4-1BBL. All of these ligands are instead primarily expressed as transmembrane proteins on the cell surface and interact with cells that express the corresponding receptors. Some ligands, such as TNF-α, are expressed as both transmembrane and soluble forms, whereas ligands, such as TNF-β, lack the transmembrane domain and thus are expressed only as a soluble protein. Obviously, cell signaling effects are more restricted when a ligand is ex-pressed only as a transmembrane protein. There are reports, furthermore, that the nature of cell signaling may differ between ligands expressed as soluble protein and those expressed in transmembrane form.3
Another characteristic feature of some ligand-receptor interactions in the TNF superfamily is the phenomenon of “reverse signaling”: instead of signaling being transmitted from ligand to receptor, it is transmitted from receptor to the cell bearing the transmembrane form of the ligand (Figure 3).24 For example, one report showed that TNF-α, when activated by TNF-α antibody, induced E-selectin (CD62E) expression on activated human CD4+ T cells via an outside-to-inside signal through the membrane.25 This report indicates that membrane TNF-α can transmit bipolar signals. Reverse signaling has also been reported via the CD30 ligand.26 CD30 ligand is a type II membrane protein with a C-terminal extracellular domain that is homologous with the extracellular domains of other TNF family members. Cross-linking of CD30 ligand by a monoclonal antibody or by CD30-Fc fusion protein induced production of IL-8 by freshly isolated neutrophils. CD30 ligand, but not CD30, is expressed on neutrophils. Clearly, several TNF family members and their cognate receptors signal bidirectionally, thus blurring the distinction between ligand and receptor. How this kind of signaling is mediated is not fully understood.
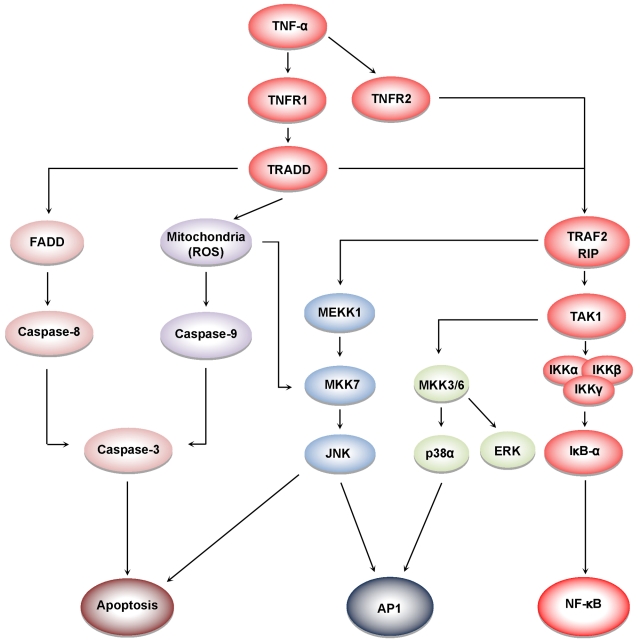
TNF-α induces at least 5 different types of signals that include activation of NF-κB, apoptosis pathways, extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (p38MAPK), and c-Jun N-terminal kinase (JNK; Figure 4). When TNF-α binds to TNFR1, it recruits a protein called TNFR-associated death domain (TRADD) through its DD.27 TRADD then recruits a protein called Fas-associated protein with death domain (FADD), which then sequentially activates caspase-8 and caspase-3, and thus apoptosis.28 Alternatively, TNF-α can activate mitochondria to sequentially release ROS, cytochrome C, and Bax, leading to activation of caspase-9 and caspase-3 and thus apoptosis.29 Paradoxically, TNF-α has also been shown to activate NF-κB, which in turn regulates the expression of proteins associated with cell survival and cell proliferation.30 NF-κB activation by TNF-α is mediated through sequential recruitment of TNFR1, TRADD, TNFR-associated factor 2 (TRAF2/TRAF5), receptor interacting protein (RIP), TGF-β–activated kinase 1 (TAK1), IκB kinase (IKK) complex, and inhibitor of nuclear factor-κBα (IκBα) phosphorylation, ubiquitination, and degradation, and finally nuclear translocation of p50 and p65 and DNA binding.31 The pro-inflammatory effect of TNF is mediated through NF-κB–regulated proteins, such as IL-6, IL-8, IL-18, chemokines, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and 5-lipoxygenase (5-LOX), all major mediators of inflammation. Indeed, TNF-α can induce expression of TNF-α itself through activation of NF-κB.3 TNF-α can also activate cellular proliferation through activation of another transcription factor, activator protein-1 (AP-1),32 which is activated by TNF-α through sequential recruitment of TNFR1, TRADD, TRAF2, MAP/ERK kinase kinase 1 (MEKK1), MAP kinase kinase 7 (MKK7), and JNK. The activation of p38MAPK by TNF-α is mediated through TRADD-TRAF2-MKK3. How TNFR2, which lacks a DD, activates cell signaling is much less clear than how TNFR1 activates cell signaling. Because TNFR2 can directly bind to TRAF2, it can activate both NF-κB and MAPK signaling quite well. Interestingly, TRADD has been reported recently to mediate cell signaling by TOLL-like receptors 3 and 4.33
Figure 4.
Cell signaling pathways activated by TNF. TNFR1 activation leads to recruitment of intracellular adaptor proteins (TRADD, FADD, TRAF, and RIP), which activate multiple signal transduction pathways. TNFR sequentially recruits TRADD, TRAF2, RIP, TAK1, and IKK, leading to the activation of NF-κB31; and the recruitment of TRADD, FADD, and caspase-8, leads to the activation of caspase-3, which in turn induces apoptosis.28 JNK is activated through the sequential recruitment of TRAF2, RIP, MEKK1, and MKK7.79 Exposure of cells to TNFα in most cases results in the generation of reactive oxygen species, leading to activation of MKK7 and JNK.29 The activation of ERK and p38MAPK is via TRADD, TRAF2, RIP, TAK1, and MKK3/6.80
Although members of the TNF superfamily have the potential to activate NF-κB, almost all of them, unlike TNF-α, activate NF-κB in a cell type-specific manner. This is restricted in part by the expression of cell surface receptors on particular cell types and in part through independent mechanisms yet to be determined. For instance, DR2, DR4, and DR5 are expressed on most cell types, but their interactions with respective ligands rarely lead to NF-κB activation. On the other hand, interaction of DR1 with its ligand leads to NF-κB activation in most cells. This ability of TNF-α to activate NF-κB nonselectively makes it the most promiscuous and universal pro-inflammatory family member in most situations. TNF-β has been shown to be a less potent pro-inflammatory agent than TNF-α.34
Although various members of the TNF superfamily are known to activate apoptosis, most cells are resistant to apoptosis induced by TNF-α alone.35 The mechanism for this resistance to apoptosis is not understood; it is not the result of lack of receptors. It seems that cell survival, proliferative, and apoptotic signals are all activated simultaneously by TNF-α and related proteins and that the balance between these signals determines whether the TNF family member induces apoptosis, proliferation, versus no effect at all. Whereas new protein synthesis is usually required for survival and proliferative signals, no protein synthesis is required for apoptosis.
Life with TNF-α
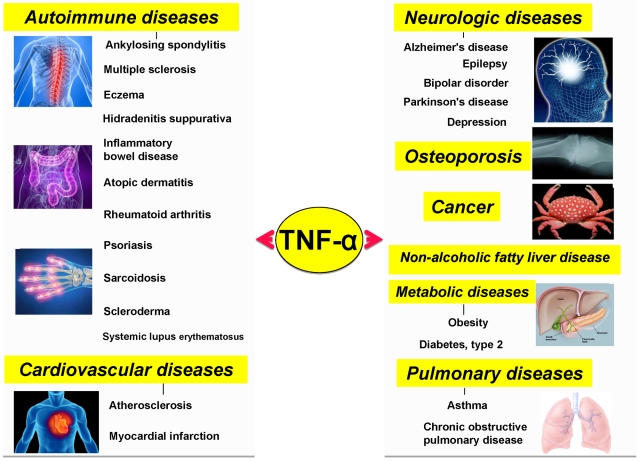
Although initially discovered as an anticancer agent, TNF-α and its family members have now been linked to an array of pathophysiologies, including cancer, neurologic diseases, cardiovascular diseases, pulmonary diseases, autoimmune diseases, and metabolic diseases.
TNF-α and cancer
The carcinogenic activities of TNF-α are mediated through its ability to activate the pro-inflammatory transcription factor NF-κB, which up-regulates the expression of genes linked to tumor cell survival, proliferation, invasion, angiogenesis, and metastasis.36 Several tumor cell types constitutively express TNF-α, including ovarian cancer, breast cancer, and others.37 Most tumor cells that express TNF-α exhibit constitutive activation of NF-κB. These tumors cells are “addicted” to NF-κB, as their survival is highly dependent on this factor.
In contrast to TNF-α, other members of the TNF superfamily exhibit more anticancer as opposed to oncogenic potential. For instance, TRAIL is being actively explored as an anticancer agent because of its preferential ability to induce apoptosis in tumorigenic or transformed cells, but not in normal cells or tissues.38 Like TNF-α, CD27L, CD30L, CD40L, APRIL, and BAFF, although essential regulators of the immune system, play a major role in hematopoietic tumorigenesis.
TNF-α and neurologic diseases
Both TNF-α and its receptors are expressed by microglial cells in the brain. Through activation of NF-κB, TNF-α plays an essential role in the survival of these cells. In the brain, TNF-α has been shown to induce pro-inflammatory signals that have been linked with depression,39 bipolar disporder,40 epilepsy,41 Alzheimer disease,42 Parkinson disease,43 and multiple sclerosis.44 Similarly, Fas ligation has been shown to induce selective expression of chemokines, IL-8, and monocyte chemoattractant protein-1 (MCP-1) in human astroglioma cells in vitro.45
TNF-α and cardiovascular diseases
TNF-α, along with other inflammatory molecules, is known to play a role in the initiation and progression of cardiovascular diseases. Although normal heart does not express TNF-α, the failing heart produces massive amounts of TNF-α. There is now growing evidence that the immune system is an important source for TNF production in failing heart. However, myocardium may also synthesize TNF-α de novo in failing heart.46 Perhaps the earliest indication that TNF-α is linked with heart failure emerged in 1990 when levels of circulating TNF-α levels were found to be elevated in sera of patients with chronic heart failure.47 Since then, numerous reports and reviews have been published on this subject.48 Evidence indicate that sustained and excessive production of TNF-α worsens the disease.49
Reports indicate that patients with chronic inflammatory disorders, such as rheumatoid arthritis (RA) and psoriasis, exhibit higher than expected rates of mortality that cannot be explained by traditional risk factors alone.50 This statement implies that inflammatory pathways might also contribute to the increased vascular risk in such diseases. The epidemiologic, physiologic, and model data suggest that TNF-α is directly involved in vascular pathophysiology. According to one published report, there are approximately 50% greater chances of cardiovascular mortality in RA patients compared with the general population.51
TNF-α and pulmonary diseases
TNF-α has been shown to play a major role in various pulmonary diseases, including asthma, chronic bronchitis, chronic obstructive pulmonary disease, acute lung injury, and acute respiratory distress syndrome.52 TNF-α is expressed in asthmatic airways and has been shown to play a role in amplifying asthmatic inflammation through the activation of NF-κB, AP-1, and other transcription factors.53 TNF-α has also been shown to induce pathologic features associated with COPD in animal models, such as an inflammatory cell infiltrate into the lungs, pulmonary fibrosis, and emphysema.54 TRAIL has also been linked to an inflammatory response in asthma.55 Interestingly, although TNF-α and TRAIL mediate lung inflammation, signaling through DR3 has been linked with expansion of Tregs, which plays a protective role against allergic lung inflammation in a mouse model of asthma.56
TNF-α, diabetes, and obesity
The first indication of increased cytokine release in obesity was provided by the identification of increased expression of TNF-α in the adipose tissue of obese mice in the early 1990s. TNF-α is expressed in and secreted by adipose tissue; its levels correlate with the degree of adiposity and the associated insulin resistance.57 The dephosphorylation of insulin receptor substrate-1 through activation of protein phosphatases has been shown to account for TNF-α-induced insulin resistance.57 Obesity is a leading cause of insulin resistance and type 2 diabetes, and targeting TNF-α and/or its receptors has been suggested as a promising treatment strategy for these conditions.58 Emerging evidence has indicated that inflammation is one of the critical processes associated with development of insulin resistance, diabetes, and related diseases, and obesity is now considered a state of chronic low-grade inflammation.59
TNF-α and autoimmune diseases
One area where TNF family members play a pivotal role is in autoimmune diseases. Uveitis, multiple sclerosis, systemic lupus, arthritis, psoriasis, and Crohn disease are all autoimmune diseases and are connected with dysregulation of various members of the TNF superfamily or their receptors as described here.60 Indeed, TNF-α blockers have been approved for the treatment of osteoarthritis, psoriasis, and Crohn disease. Among all the members of the TNF superfamily, the role of TNF-α in autoimmune diseases is best understood.
Life without TNF-α
From the studies already described, it is clear that both TNF-α and its family members play critical roles in the immune, cardiovascular, pulmonary, metabolic, and neurologic systems. Further insights into the function of these cytokines have been gained through gene deletion/gene knockout studies. The genes for the ligands and the receptors of the TNF superfamily have been deleted one by one in mice, and the resulting phenotypes have been examined (Table 2). Most commonly, defects in the immune system have been observed in these mice. For instance, deletion of TNF-β yielded a defect in development of secondary lymphoid organs and disorganized splenic architecture.61 Deletion of TNF-α, on the other hand, resulted in normal development and a complete lack of detectable morphologic or gross structural abnormalities.62 However, TNF-α-deficient mice were highly susceptible to challenge with an infectious agent and were resistant to the lethality of minute doses of lipopolysaccharide after D-galactosamine treatment.63 Amazingly, deletion of TNF-α had no effect on phagocytic activity of macrophages or on T-cell functions, as measured by proliferation, cytokine release, or cytotoxicity, suggesting redundancy with other superfamily members.
Table 2.
Effect of gene knockout on the phenotype of TNF superfamily, receptors, and receptor-associated proteins
| Gene | Phenotype |
|---|---|
| Cytokine | |
| TNF-β (LT-α) | Defects in secondary lymphoid organ development; disorganized splenic microarchitecture61 |
| TNF-α | No phenotypic abnormalities in LN; lack splenic primary B-cell follicles; disorganized FDC networks and germinal centers62 |
| LT-β | Defects in organogenesis of the lymphoid system; lymphocytosis in the circulation and peritoneal cavity; lymphocytic infiltrations in lungs and liver81 |
| OX40L | Defective T-cell responses82 |
| CD40L | Defective T-cell and IgG responses; hyper-IgM syndrome82 |
| FasL | Impaired activation-induced T-cell death; lymphoproliferation; autoimmunity82 |
| 4–1BBL | Defective T-cell responses82 |
| TRAIL | Delayed regression of retinal neovascularization64 |
| RANKL | Osteopetrosis; growth retardation of limbs, skull, and vertebrae; chondrodysplasia83 |
| APRIL | Normal immune system development 84 |
| Impaired IgA class switching85 | |
| BAFF | Impaired B-cell maturation86 |
| Low Ig serum levels; block in B-cell development at the T1 stage; absence of T2, mantle, and follicular zone B cells in the LN and spleen87 | |
| EDA-A1 | Ectodermal dysplasias88* |
| EDA-A2 | Impaired development of hair, eccrine sweat glands, and teeth89 |
| Multifocal myodegeneration90 | |
| Receptor | |
| TNFR1 | Resistant to low levels of LPS; increased susceptibility to Listeria monocytogenes infection91 |
| Impaired oval cell proliferation; reduction of tumorigenesis92 | |
| TNFR2 | Increased sensitivity to bacterial pathogens; decreased sensitivity to LPS; reduced antigen-induced T-cell |
| apoptosis82 | |
| LT-βR | Absence of LN, PP; defective GC formation82 |
| OX40 | Defective T-cell responses82 |
| CD40 | Defective Ig class switching and GC formation causing immunodeficiency82 |
| Fas | Impaired activation-induced T-cell death; lymphoproliferative syndrome; autoimmunity93 |
| Accumulation of autoreactive B cells in T cell-rich zones; production of autoantibodies94 | |
| Resistant to suppression by high doses of antigen and to apoptosis in mature CD4+ T cells95 | |
| CD27 | Defective T-cell responses82 |
| CD30 | Impaired follicular GC responses; reduced recall-memory Ab responses96 |
| 4–1BB | Enhanced T-cell response but normal T-cell development97 |
| Reduced number of NK and NKT cells; resistance to LPS-induced shock syndrome98 | |
| Increased number of myeloid progenitor and mature DCs; impaired DC function99 | |
| Reduced atherosclerosis in hyperlipidemic mice100 | |
| DR5 | Normal development with an enlarged thymus66 |
| RANK | Osteopetrosis; absence of osteoclasts and LN; PP present; abnormal B-cell development82 |
| OPG | Osteoporosis; arterial calcification82 |
| FN14 | Reduced proliferative capacity; altered myotube formation101 |
| Reduced neurogenesis in the subventricular zone102 | |
| Reduced LPC numbers; attenuated inflammation; cytokine production103 | |
| TACI | Increased B-cell accumulation; splenomegaly104 |
| BAFFR | Reduced late transitional and follicular B-cell numbers; devoid of marginal zone B cells; reduced CD21 and CD23 surface expression105 |
| DR3 | Impaired negative selection and anti–CD3-induced apoptosis106 |
| GITR | Abolished anti–CD3-induced T-cell activation107 |
| EDAR | Abnormal tooth, hair, and sweat gland formation82 |
| XEDAR | No different than wild-type littermates90 |
| TROY | No apparent defects in skin appendages108 |
| DR6 | Enhanced CD4+ T-cell expansion and Th2 differentiation; enhanced splenic GC formation109 |
| Impaired JNK activity; T-cell differentiation110 | |
| CD4+ T-cell proliferation; Th differentiation111 | |
| NGFR | Decreased sensory neuron innervation; impaired heat sensitivity112 |
| Receptor-associated proteins | |
| TRAF1 | Normal lymphocyte development113 |
| Attenuation of atherosclerosis114 | |
| TRAF2 | Died prematurely; elevated sTNF levels; hypersensitivity to TNF-induced cell death115 |
| TRAF3 | Postnatal lethality; defect in T-dependent immune responses116 |
| TRAF5 | Defect in proliferation; up-regulation of surface molecules CD23, CD54, CD80, CD86, and Fas after CD40 stimulation117 |
| TRAF6 | Osteopetrosis; perinatal and postnatal lethality118 |
| Hypohidrotic ectodermal dysplasia119 | |
| TRADD | Embryonic lethal120 |
| FADD | Embryonic lethal; cardiac failure and abdominal hemorrhage121 |
| FAN | Impaired neutral sphingomyelinase activation; cutaneous barrier repair122 |
| FLICE | Perinatal lethal123 |
| RIP | Perinatal lethal124 |
| IKK-α | Abnormal morphogenesis (limb and skeletal patterning; proliferation and differentiation of epidermal keratinocytes)125 |
| IKK-β | Died at mid-gestation126 |
| Apaf-1 | Reduced apoptosis in the brain; hyperproliferation of neuronal cells127 |
| Caspase-9 | Embryonic lethal; defect in brain development128 |
| Caspase-3 | Decreased apoptosis in the brain; premature lethality129 |
| EDARADD | Hypohidrotic ectodermal dysplasia130 |
| Act1 | B cell–mediated autoimmune phenotypes131 |
Fas indicates fibroblast-associated; FN14, fibroblast growth factor-inducible immediate-early response gene 14; GITR, glucocorticoid-induced TND receptor; EDAR, EDA receptor; XEDAR, X-linked ectodysplasin receptor; TROY, TNFRSF expressed on the mouse embryo; NGFR, nerve growth factor receptor; FAN, factor associated with neutral SMase activation; FLICE, FADD-like IL-1β-converting enzyme; Apaf-1, apoptotic protease activating factor-1; EDARADD, ectodysplasin-A receptor-associated adapter protein; LN, lymph node; FDC, follicular dendritic cell; LPS, lipopolysaccharide; PP, Peyer patches; GC, germinal center; and sTNF, serum TNF.
Study performed in human, others in mouse model.
The deletion of the TRAIL gene has been shown to affect the retinal vascularization in mice.64 Generally, deletions of a ligand and its receptor have been shown to produce related phenotypes; however, in some cases, the 2 phenotypes are quite dissimilar. For instance, TRAIL is known to bind 2 different signaling receptors, DR4 and DR5, in humans but only DR5 in mice. Whereas deletion of TRAIL affected susceptibility to tumor burden and accelerated autoimmune diseases,65 animals with deletion of DR5 developed normally but had an enlarged thymus.66 This indicates that the ligand may also function independently of the receptor.
Mutation of TNF-α and its superfamily
Various mutations in genes for TNF, its family members, and its receptors have been identified in humans (Table 3). These mutations are reflected either by a change in phenotype or a change in function. For instance, abnormalities in the CD40L gene have been linked to the X-linked immunodeficiency hyper-IgM syndrome. This human disease is characterized by elevated concentrations of serum IgM and decreased amounts of all other immunoglobulin isotypes. The CD40L protein produced in these patients was incapable of binding to CD40 and thus was unable to induce proliferation or IgE secretion from normal B cells. Activated T cells from some of these affected patients failed to express functional CD40L.
Table 3.
Diseases caused by mutation of TNF superfamily, receptors, and adaptors
| Gene | Study | Disease |
|---|---|---|
| Cytokine | ||
| TNF-β (LT-α) | Human | Cerebral infarction68 |
| TNF-α | Human | Cerebral infarction68 |
| CD40L | Human | X-linked hyper-IgM syndrome132 |
| FasL | Mouse | Generalized lymphoproliferative disease133 |
| EDA-A1 | Human | Ectodermal dysplasias88 |
| EDA-A2 | Dog | X-linked hypohidrotic ectodermal dysplasia134 |
| Receptor | ||
| TNFR1 | Human | TNFR1-associated periodic syndrome135 |
| Human | TRAPS associated with SLE67 | |
| Human | Crohn disease136 | |
| TNFR2 | Human | Crohn disease136 |
| Fas | Mouse | Autoimmune lymphoproliferative syndrome137 |
| Human | Generalized lymphoproliferative disease133 | |
| RANK | Human | Familial expansile osteolysis138 |
| OPG | Human | Idiopathic hyperphosphatasia139 |
| Human | Juvenile Paget disease140 | |
| TACI | Human | Common variable immunodeficiency141 |
| BAFFR | Mouse | Lupus-like syndrome (B cell−mediated autoimmunity)142 |
| EDAR | Human | Hypohidrotic ectodermal dysplasia143 |
| Adaptors | ||
| TRAF3 | Human | Herpes simplex encephalitis144 |
| TRAF6 | Mouse | Hypohidrotic ectodermal dysplasia119 |
| EDARADD | Mouse | Hypohidrotic ectodermal dysplasia130 |
| Act1 | Mouse | B cell−mediated autoimmune phenotypes131 |
RANK indicates receptor activator of NF-κB; OPG, osteoprotegerin; EDAR, EDA receptor; and EDARADD, ectodysplasin-A receptor-associated adapter protein.
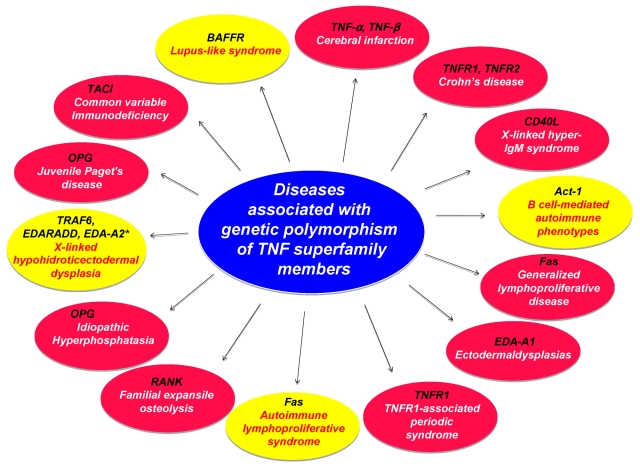
TNFR1-associated periodic syndrome (TRAPS) is the result of a mutation in the TNF receptor gene and is associated with systemic lupus erythematosus (SLE).67 Two biallelic polymorphisms in TNF-α (TNF-α–308) and TNF-β (TNF-β +252) genes have been associated with TNF production and susceptibility to inflammatory diseases.68 Two genetic polymorphisms in the TNF locus (TNF-α–308 G → A and TNF-β +252 A → G) were found to be risk factors for cerebral infarction.68 These mutations provide novel insights into the function of various members of the TNF superfamily.
TNF-α–based therapeutics
TNF superfamily members play a role in the pathogenesis of various human diseases (Figures 5 and 6). Thus, this superfamily represents an active target for drug development. Various antagonists against TNF family members, and their receptors have been approved by FDA and some are in clinical trials (Table 4). These agents are effective against RA, psoriatic arthritis, psoriasis, ulcerative colitis, and Crohn disease. Approved therapeutics are primarily one of 2 types of antagonist, either a soluble receptor that binds circulating TNF-α and acts as a “sink,” preventing interaction with cell surface receptors, or antibodies against TNF-α. Golimumab is a fully humanized TNF-α monoclonal antibody that is specific for human TNF-α and was approved for RA, psoriatic arthritis, and active ankylosing spondylitis.69 Similarly, Certolizumab pegol, a PEGylated Fab′ fragment of a humanized TNF antibody, is a monoclonal antibody directed against human TNF-α. Certolizumab pegol was approved for RA and Crohn disease.70 A human antibody against RANKL (denosumab, Prolia) was recently approved for the treatment of osteoporosis.
Figure 5.
Various diseases that have been closely linked to TNF-α and members of its family.
Figure 6.
Diseases caused by mutation of genes in members of the TNF superfamily, its receptors, and adaptor proteins. Red circle represents studies in human; and yellow circle, studies in mice. Asterisk (*) within yellow circle indicates diseases in dogs.
Table 4.
Selected FDA-approved drugs and those in development targeting the TNF superfamily
| Cytokine | Drug | Mechanism | Disease | Status |
|---|---|---|---|---|
| TNF-α | Infliximab | mAbs | RA, PA, psoriasis, ALS, ulcerative colitis, | Approved |
| Crohn disease | ||||
| Etanercept | RD | RA, PA, psoriasis, ALS, juvenile RA | Approved | |
| Adalimumab | mAbs | RA, PA, psoriasis, ALS, juvenile RA, Crohn disease | Approved | |
| Certolizumab | mAbs | RA, Crohn disease | Approved | |
| Golimumab | mAbs | RA, PA, AS | Approved | |
| TNF-α kinoid | Vaccine* | RA, Crohn disease | Phase 2 | |
| ESBA105 | SC antibody | Anterior uveitis | Phase 2 | |
| ART621 | mAbs | RA, psoriasis | Phase 2 | |
| ATN-103 | Nanobody | RA | Phase 1 | |
| LT-β | Baminercept α | LT-βR-Ig | RA | Phase 2 |
| OX40L | huMAb OX40L | mAbs | Asthma | Phase 2‡ |
| CD40L | Dacetuzumab | mAbs | Multiple myeloma | Phase 1 |
| Lymphoma, large B-cell, diffuse lymphoma, NHL | Phase 2 | |||
| HCD122 | mAbs | Multiple myeloma | Phase 1 | |
| Follicular lymphoma | Phase 2‡ | |||
| FasL | APO010 | Fusion protein† | Solid tumors | Phase 1 |
| CD27L | MDX-1411 | mAbs | Kidney cancer | Phase 1‡ |
| MDX-1203 | ADC | Renal cell carcinoma, NHL | Phase 1 | |
| CD30L | XmAb2513 | mAbs | Hodgkin lymphoma, anaplastic large-cell lymphoma | Phase 1 |
| SGN-35 | ADC | Hodgkin lymphomas | Phase 3‡ | |
| Lymphoma, large-cell, anaplastic lymphoma, NHL | Phase 2 | |||
| MDX-1401 | mAbs | Hodgkin lymphomas | Phase 1 | |
| 4–1BBL | BMS-663513 | mAbs | Advanced cancer | Phase 1 |
| Melanoma | Phase 2 | |||
| TRAIL | Mapatumumab | mAbs | Colorectal, NHL, non−small-cell lung cancer | Phase 2 |
| Lexatumumab | mAbs | Kidney cancer, lymphoma, neuroblastoma, sarcoma | Phase 1 | |
| CS-1008 | mAbs | Malignancies, lymphoma | Phase 1 | |
| Colorectal neoplasms, pancreatic, lung cancer | Phase 2 | |||
| RANKL | Denosumab | mAbs | Low bone mass, low bone mineral density, osteopenia, osteoporosis | Phase 3‡ |
| TWEAK | BIIB023 | mAbs | RA | Phase 1 |
| APRIL | Atacicept | Fusion protein† | RA | Phase 2 |
| SLE | Phase 3‡ | |||
| BAFF | Atacicept | Fusion protein† | RA | Phase 2 |
| Benlysta | mAbs | Arthritis, rheumatoid, SLE | Phase 2 | |
| LY2127399 | mAbs | RA | Phase 2 | |
| LIGHT | Baminercept α | LT-βR-Ig | RA | Phase 2 |
RD indicates receptor derivative; SC, single-chain; PA, psoriatic arthritis; ALS, amyotrophic lateral sclerosis; AS, ankylosing spondylitis; ADC, antibody-drug conjugate; and NHL, non-Hodgkin lymphoma.
Vaccine composed of a keyhole limpet hemocyanin-hTNFα heterocomplex immunogen.
Recombinant fusion protein.
Ongoing study.
Several other products are currently in clinical trials. Atacicept is a human recombinant fusion protein designed to inhibit B cells, thereby suppressing autoimmune disease. This fusion protein composes the binding portion of a receptor for both BLyS and APRIL. Atacicept blocks activation of B cells by TACI, a transmembrane receptor protein found predominantly on the surface of B cells, and thus production of autoantibodies. The efficacy of atacicept in animal models of autoimmune disease and the biologic activity of atacicept in patients with SLE and RA has been demonstrated.71 Two recent phase 2 clinical trials evaluated the safety, efficacy, and biologic activity of atacicept in patients with active RA and an inadequate response to methotrexate. Despite the biologic effects of atacicept, the proportion of patients meeting the primary efficacy endpoint did not differ significantly in the atacicept groups and the placebo group.72,73 Another phase 2 clinical trial was designed to assess the safety and tolerability of atacicept and its effects on central nervous system inflammation in relapsing multiple sclerosis (IMP28063, www.clinicaltrials.gov, #NCT00642902). Surprisingly, an increase in inflammatory disease activity was reported that led to a suspension of all atacicept trials in multiple sclerosis.
One of the major problems with most of these TNF blockers is that they produce numerous side effects. For example, infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira) are immunosuppressants and carry black-box warning labels regarding the increased risk of serious infections (such as tuberculosis). These drugs also carry a warning label regarding an increased risk of central nervous system demyelinating disorders. Adverse reactions in patients receiving infliximab have been studied and reported. Risks include serious and sometimes fatal blood disorders, serious infections, lymphoma, and solid tumors. There are reports of serious liver injury, reactivation of hepatitis B, hepatosplenic T-cell lymphoma, and drug-induced lupus. Cases of leukopenia, neutropenia, thrombocytopenia, and pancytopenia (some fatal) have also been reported with infliximab. Besides safety issues, most of the TNF blockers are highly expensive. For instance, infliximab can cost as much as $22 000 a year per patient. Thus, alternatives that are safer and more affordable, yet effective, are needed.
TNF-α and the golden spice
Attempts have been made over the past several years for the identification of agents that are safe, efficacious, and inexpensive. Agents derived from natural sources have gained considerable attention because of their ability to suppress TNF expression and TNF signaling. Curcumin (diferuloylmethane) is one such agent that was isolated more than a century ago from the rhizomes of the golden spice turmeric (Curcuma longa). While searching for a TNF blocker, our group was the first to demonstrate that curcumin can block the activity of TNF-α,74 and another group showed that it can also suppress TNF production.75 Since then, we and others have shown its ability to inhibit the growth of a wide variety of tumor cells through suppression of TNF signaling. Curcumin has also been shown to inactivate TNF-α by direct binding.76 In one study, curcumin docked at the receptor-binding sites of TNF-α and exhibited direct interaction by both noncovalent and covalent interactions.77 Curcumin is currently in clinical trials for all those diseases for which TNF blockers have been approved. In addition, several other natural products are also known to suppress both the production and action of various members of the TNF superfamily.78
Future of TNF research
From the studies described here, it is clear that TNF-α and its family members constitute a very active area of research and thus has opened up numerous possibilities for treatment of variety of human diseases, including osteoporosis, psoriasis, arthritis, and Crohn disease. The number of publications on this superfamily, their involvement in a wide variety of human diseases, their potential to treat human disease, and the interest in and sales of products based on this family all are indicators of the future potential of these agents. However, this research is just beginning and will probably continue for decades to come. It is becoming increasingly evident that, although under controlled circumstances acute inflammation has therapeutic potential, when out of control, chronic inflammation can mediate most chronic diseases. Members of the TNF superfamily are major mediators of chronic inflammation and thus need to be regulated. More understanding is required of the pathways activated by these ligands and how to interrupt these pathways selectively for the benefit of mankind.
In conclusion, the golden journey of TNF started almost 25 years ago with the discovery of the 2 proteins, discovery of their amino acid sequence, cloning of their genes, and identification of their receptors. A great deal has been learned about cell signaling by these proteins, the cellular responses, their physiologic and pathologic roles, up-regulation and down-regulation, and genetic alterations. Most of the information in hand is about TNF-α, however; and very little is known about TNF-β, which binds to the same receptor as TNF-α. For instance, is TNF-β as pro-inflammatory as TNF-α, and if not, why not? What is the true physiologic role of TRAIL? Is this cytokine designed to kill tumor cells or is its true role yet to be determined? What is the role of VEGI? How does it signal? What is the ligand for DR6 or DR3, and how do they signal? Almost 50 different proteins constitute ligands of the TNF superfamily, ligand receptors, and receptor-associated proteins. Much is yet to be learned about them, and we expect this information to be forthcoming.
Acknowledgments
The authors thank Kathryn Hale for carefully proofreading the manuscript and providing valuable comments.
This work was supported by the National Institutes of Health (Cancer Center Core grant CA-16672 and Program Project grant CA-124787-01A2) and the Center for Targeted Therapy at The University of Texas M. D. Anderson Cancer Center. B.B.A. is the Ransom Horne Jr Professor of Cancer Research.
Personal account of corresponding author: In May 1980, Dr Aggarwal left the Hormone Research Laboratory of the University of California, San Francisco, to join a start-up company in South San Francisco called Genentech. Soon after joining Genentech, he was approached by the founder and president of the company, the young entrepreneur Bob Swanson, who was obsessed with gene cloning. Bob walked into Dr Aggarwal's office and asked him to find a “cure for cancer.” When Dr Aggarwal asked how he was supposed to do that, the immediate answer of Bob was that lymphokines are a cure for cancer and if he could clone this “sucker” he'll have a cure. After extensive reading, attending a conference in Dallas, and discussions with Dr Gale Granger, a pioneer in the lymphotoxin field, Dr Aggarwal learned that lymphokines are nothing but superatants from activated lymphocytes, and were being used at the time for the treatment of cancer. With this background, Dr Aggarwal got started in an area he had known nothing about.
Authorship
Contribution: B.B.A. and J.H.K. reviewed the literature; J.H.K. wrote portions of the tables and figures; B.B.A. and S.C.G. wrote portions of the manuscript; and B.B.A. had overall editorial responsibility for the manuscript, tables, and figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bharat B. Aggarwal, Department of Experimental Therapeutics, The University of Texas M. D. Anderson Cancer Center, 1901 East Rd, Unit 1950, Houston, TX 77054; e-mail: aggarwal@mdanderson.org.
References
- 1.Bhardwaj A, Aggarwal BB. Receptor-mediated choreography of life and death. J Clin Immunol. 2003;23(5):317–332. doi: 10.1023/a:1025319031417. [DOI] [PubMed] [Google Scholar]
- 2.Gaur U, Aggarwal BB. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. 2003;66(8):1403–1408. doi: 10.1016/s0006-2952(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3(9):745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 4.O'Malley Action of bacterial polysaccharide on tumors: II. Damage of Sarcoma 37 by serum of mice treated with Serratia marcescens polysaccharide, and induced tolerance. J Natl Cancer Inst. 1962;29:1169–1175. doi: 10.1111/j.1753-4887.1988.tb05376.x. [DOI] [PubMed] [Google Scholar]
- 5.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal BB, Moffat B, Harkins RN. Human lymphotoxin: production by a lymphoblastoid cell line, purification, and initial characterization. J Biol Chem. 1984;259(1):686–691. [PubMed] [Google Scholar]
- 7.Aggarwal BB, Henzel WJ, Moffat B, Kohr WJ, Harkins RN. Primary structure of human lymphotoxin derived from 1788 lymphoblastoid cell line. J Biol Chem. 1985;260(4):2334–2344. [PubMed] [Google Scholar]
- 8.Aggarwal BB, Kohr WJ, Hass PE, et al. Human tumor necrosis factor: production, purification, and characterization. J Biol Chem. 1985;260(4):2345–2354. [PubMed] [Google Scholar]
- 9.Bringman TS, Aggarwal BB. Monoclonal antibodies to human tumor necrosis factors alpha and beta: application for affinity purification, immunoassays, and as structural probes. Hybridoma. 1987;6(5):489–507. doi: 10.1089/hyb.1987.6.489. [DOI] [PubMed] [Google Scholar]
- 10.Kelker HC, Oppenheim JD, Stone-Wolff D, et al. Characterization of human tumor necrosis factor produced by peripheral blood monocytes and its separation from lymphotoxin. Int J Cancer. 1985;36(1):69–73. doi: 10.1002/ijc.2910360112. [DOI] [PubMed] [Google Scholar]
- 11.Pennica D, Nedwin GE, Hayflick JS, et al. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- 12.Gray PW, Aggarwal BB, Benton CV, et al. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature. 1984;312(5996):721–724. doi: 10.1038/312721a0. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal BB, Eessalu TE, Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985;318(6047):665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- 14.Beutler B, Greenwald D, Hulmes JD, et al. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- 15.Cerami A. The value of failure: the discovery of TNF and its natural inhibitor erythropoietin. J Intern Med. 2011;269(1):8–15. doi: 10.1111/j.1365-2796.2010.02319.x. [DOI] [PubMed] [Google Scholar]
- 16.Schneider P, Olson D, Tardivel A, et al. Identification of a new murine tumor necrosis factor receptor locus that contains two novel murine receptors for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J Biol Chem. 2003;278(7):5444–5454. doi: 10.1074/jbc.M210783200. [DOI] [PubMed] [Google Scholar]
- 17.Moore RJ, Owens DM, Stamp G, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5(7):828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 18.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25(3):409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 19.Deroose JP, Eggermont AM, van Geel AN, et al. Long-term results of tumor necrosis factor α- and melphalan-based isolated limb perfusion in locally advanced extremity soft tissue sarcomas. J Clin Oncol. 2011;29(30):4036–4044. doi: 10.1200/JCO.2011.35.6618. [DOI] [PubMed] [Google Scholar]
- 20.Deroose JP, Eggermont AM, van Geel AN, de Wilt JH, Burger JW, Verhoef C. 20 years experience of TNF-based isolated limb perfusion for in-transit melanoma metastases: TNF dose matters [published online ahead of print August 31, 2011]. Ann Surg Oncol. doi: 10.1245/s10434-011-2030-7. doi: 10.1245/s10434-011-2030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunhagen DJ, de Wilt JH, van Geel AN, Verhoef C, Eggermont AM. Isolated limb perfusion with TNF-alpha and melphalan in locally advanced soft tissue sarcomas of the extremities. Recent Results Cancer Res. 2009;179:257–270. doi: 10.1007/978-3-540-77960-5_16. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal BB, Bhardwaj U, Takada Y. Regulation of TRAIL-induced apoptosis by ectopic expression of antiapoptotic factors. Vitam Horm. 2004;67:453–483. doi: 10.1016/S0083-6729(04)67023-3. [DOI] [PubMed] [Google Scholar]
- 23.Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457(7232):981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Watts AD, Hunt NH, Wanigasekara Y, et al. A casein kinase I motif present in the cytoplasmic domain of members of the tumour necrosis factor ligand family is implicated in “reverse signalling.”. EMBO J. 1999;18(8):2119–2126. doi: 10.1093/emboj/18.8.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harashima S, Horiuchi T, Hatta N, et al. Outside-to-inside signal through the membrane TNF-alpha induces E-selectin (CD62E) expression on activated human CD4+ T cells. J Immunol. 2001;166(1):130–136. doi: 10.4049/jimmunol.166.1.130. [DOI] [PubMed] [Google Scholar]
- 26.Wiley SR, Goodwin RG, Smith CA. Reverse signaling via CD30 ligand. J Immunol. 1996;157(8):3635–3639. [PubMed] [Google Scholar]
- 27.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81(4):495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 28.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84(2):299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 29.Morgan MJ, Liu ZG. Reactive oxygen species in TNFalpha-induced signaling and cell death. Mol Cells. 2010;30(1):1–12. doi: 10.1007/s10059-010-0105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6(3):203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Devin A, Cook A, Lin Y, Rodriguez Y, Kelliher M, Liu Z. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12(4):419–429. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- 32.Natoli G, Costanzo A, Moretti F, Fulco M, Balsano C, Levrero M. Tumor necrosis factor (TNF) receptor 1 signaling downstream of TNF receptor-associated factor 2: nuclear factor kappaB (NFkappaB)-inducing kinase requirement for activation of activating protein 1 and NFkappaB but not of c-Jun N-terminal kinase/stress-activated protein kinase. J Biol Chem. 1997;272(42):26079–26082. doi: 10.1074/jbc.272.42.26079. [DOI] [PubMed] [Google Scholar]
- 33.Ermolaeva MA, Michallet MC, Papadopoulou N, et al. Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat Immunol. 2008;9(9):1037–1046. doi: 10.1038/ni.1638. [DOI] [PubMed] [Google Scholar]
- 34.Desch CE, Dobrina A, Aggarwal BB, Harlan JM. Tumor necrosis factor-alpha exhibits greater proinflammatory activity than lymphotoxin in vitro. Blood. 1990;75(10):2030–2034. [PubMed] [Google Scholar]
- 35.Sugarman BJ, Aggarwal BB, Hass PE, Figari IS, Palladino MA, Jr, Shepard HM. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- 36.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9(5):361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 37.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72(11):1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 38.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3(6):673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 39.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 40.Brietzke E, Kapczinski F. TNF-alpha as a molecular target in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1355–1361. doi: 10.1016/j.pnpbp.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Murashima YL, Suzuki J, Yoshii M. Role of cytokines during epileptogenesis and in the transition from the interictal to the ictal state in the epileptic mutant EL mouse. Gene Regul Syst Bio. 2008;2:267–274. [PMC free article] [PubMed] [Google Scholar]
- 42.Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer's disease. Biol Psychiatry. 2010;68(10):930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Nagatsu T, Sawada M. Inflammatory process in Parkinson's disease: role for cytokines. Curr Pharm Des. 2005;11(8):999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- 44.Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J Immunol. 2010;184(12):6891–6900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 45.Choi C, Gillespie GY, Van Wagoner NJ, Benveniste EN. Fas engagement increases expression of interleukin-6 in human glioma cells. J Neuro-oncol. 2002;56(1):13–19. doi: 10.1023/a:1014467626314. [DOI] [PubMed] [Google Scholar]
- 46.Kapadia S, Lee J, Torre-Amione G, Birdsall HH, Ma TS, Mann DL. Tumor necrosis factor-alpha gene and protein expression in adult feline myocardium after endotoxin administration. J Clin Invest. 1995;96(2):1042–1052. doi: 10.1172/JCI118090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323(4):236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 48.Feldman AM, Combes A, Wagner D, et al. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol. 2000;35(3):537–544. doi: 10.1016/s0735-1097(99)00600-2. [DOI] [PubMed] [Google Scholar]
- 49.Diwan A, Tran T, Misra A, Mann DL. Inflammatory mediators and the failing heart: a translational approach. Curr Mol Med. 2003;3(2):161–182. doi: 10.2174/1566524033361537. [DOI] [PubMed] [Google Scholar]
- 50.McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol. 2009;6(6):410–417. doi: 10.1038/nrcardio.2009.57. [DOI] [PubMed] [Google Scholar]
- 51.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59(12):1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 52.Matera MG, Calzetta L, Cazzola M. TNF-alpha inhibitors in asthma and COPD: we must not throw the baby out with the bath water. Pulm Pharmacol Ther. 2010;23(2):121–128. doi: 10.1016/j.pupt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Kips JC, Tavernier JH, Joos GF, Peleman RA, Pauwels RA. The potential role of tumour necrosis factor alpha in asthma. Clin Exp Allergy. 1993;23(4):247–250. doi: 10.1111/j.1365-2222.1993.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 54.Lundblad LK, Thompson-Figueroa J, Leclair T, et al. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med. 2005;171(12):1363–1370. doi: 10.1164/rccm.200410-1349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collison A, Foster PS, Mattes J. Emerging role of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) as a key regulator of inflammatory responses. Clin Exp Pharmacol Physiol. 2009;36(11):1049–1053. doi: 10.1111/j.1440-1681.2009.05258.x. [DOI] [PubMed] [Google Scholar]
- 56.Schreiber TH, Wolf D, Tsai MS, et al. Therapeutic Treg expansion in mice by TNFRSF25 prevents allergic lung inflammation. J Clin Invest. 2010;120(10):3629–3640. doi: 10.1172/JCI42933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 58.Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun. 2010;11:145–156. doi: 10.1159/000289203. [DOI] [PubMed] [Google Scholar]
- 59.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 60.Vinay DS, Kwon BS. The tumour necrosis factor/TNF receptor superfamily: therapeutic targets in autoimmune diseases. Clin Exp Immunol. 2011;164:145–157. doi: 10.1111/j.1365-2249.2011.04375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banks TA, Rouse BT, Kerley MK, et al. Lymphotoxin-alpha-deficient mice: effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155(4):1685–1693. [Google Scholar]
- 62.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184(4):1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marino MW, Dunn A, Grail D, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci U S A. 1997;94(15):8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hubert KE, Davies MH, Stempel AJ, Griffith TS, Powers MR. TRAIL-deficient mice exhibit delayed regression of retinal neovascularization. Am J Pathol. 2009;175(6):2697–2708. doi: 10.2353/ajpath.2009.090099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lamhamedi-Cherradi SE, Zheng SJ, Maguschak KA, Peschon J, Chen YH. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL−/− mice. Nat Immunol. 2003;4(3):255–260. doi: 10.1038/ni894. [DOI] [PubMed] [Google Scholar]
- 66.Finnberg N, Gruber JJ, Fei P, et al. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol Cell Biol. 2005;25(5):2000–2013. doi: 10.1128/MCB.25.5.2000-2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ida H, Kawasaki E, Miyashita T, et al. A novel mutation (T61I) in the gene encoding tumour necrosis factor receptor superfamily 1A (TNFRSF1A) in a Japanese patient with tumour necrosis factor receptor-associated periodic syndrome (TRAPS) associated with systemic lupus erythematosus. Rheumatology (Oxford) 2004;43(10):1292–1299. doi: 10.1093/rheumatology/keh320. [DOI] [PubMed] [Google Scholar]
- 68.Um JY, An NH, Kim HM. TNF-alpha and TNF-beta gene polymorphisms in cerebral infarction. J Mol Neurosci. 2003;21(2):167–171. doi: 10.1385/JMN:21:2:167. [DOI] [PubMed] [Google Scholar]
- 69.Boyce EG, Halilovic J, Stan-Ugbene O. Golimumab: review of the efficacy and tolerability of a recently approved tumor necrosis factor-alpha inhibitor. Clin Ther. 2010;32(10):1681–1703. doi: 10.1016/j.clinthera.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Schreiber S, Rutgeerts P, Fedorak RN, et al. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology. 2005;129(3):807–818. doi: 10.1053/j.gastro.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 71.Hartung HP, Kieseier BC. Atacicept: targeting B cells in multiple sclerosis. Ther Adv Neurol Disord. 2010;3(4):205–216. doi: 10.1177/1756285610371146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Genovese MC, Kinnman N, de La Bourdonnaye G, Pena Rossi C, Tak PP. Atacicept in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor antagonist therapy: results of a phase II, randomized, placebo-controlled, dose-finding trial. Arthritis Rheum. 2011;63(7):1793–1803. doi: 10.1002/art.30373. [DOI] [PubMed] [Google Scholar]
- 73.van Vollenhoven RF, Kinnman N, Vincent E, Wax S, Bathon J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 2011;63(7):1782–1792. doi: 10.1002/art.30372. [DOI] [PubMed] [Google Scholar]
- 74.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. 1995;270(42):24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 75.Chan MM. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem Pharmacol. 1995;49(11):1551–1556. doi: 10.1016/0006-2952(95)00171-u. [DOI] [PubMed] [Google Scholar]
- 76.Gupta SC, Prasad S, Kim JH, et al. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011;28(12):1937–1955. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wua ST, Suna JC, Leeb KJ, Sunc YM. Docking prediction for tumor necrosis factor-α and five herbal inhibitors. Intl J Eng Sci Technol. 2010;2:4263–4277. [Google Scholar]
- 78.Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Front Biosci. 2008;13:5094–5107. doi: 10.2741/3066. [DOI] [PubMed] [Google Scholar]
- 79.Song HY, Regnier CH, Kirschning CJ, Goeddel DV, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci U S A. 1997;94(18):9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuasa T, Ohno S, Kehrl JH, Kyriakis JM. Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38: germinal center kinase couples TRAF2 to mitogen-activated protein kinase/ERK kinase kinase 1 and SAPK while receptor interacting protein associates with a mitogen-activated protein kinase kinase kinase upstream of MKK6 and p38. J Biol Chem. 1998;273(35):22681–22692. doi: 10.1074/jbc.273.35.22681. [DOI] [PubMed] [Google Scholar]
- 81.Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, et al. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc Natl Acad Sci U S A. 1997;94(17):9302–9307. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 83.Kim N, Odgren PR, Kim DK, Marks SC, Jr, Choi Y. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci U S A. 2000;97(20):10905–10910. doi: 10.1073/pnas.200294797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Varfolomeev E, Kischkel F, Martin F, et al. APRIL-deficient mice have normal immune system development. Mol Cell Biol. 2004;24(3):997–1006. doi: 10.1128/MCB.24.3.997-1006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Castigli E, Scott S, Dedeoglu F, et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A. 2004;101(11):3903–3908. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gross JA, Dillon SR, Mudri S, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15(2):289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 87.Schiemann B, Gommerman JL, Vora K, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293(5537):2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 88.Monreal AW, Zonana J, Ferguson B. Identification of a new splice form of the EDA1 gene permits detection of nearly all X-linked hypohidrotic ectodermal dysplasia mutations. Am J Hum Genet. 1998;63(2):380–389. doi: 10.1086/301984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schneider P, Street SL, Gaide O, et al. Mutations leading to X-linked hypohidrotic ectodermal dysplasia affect three major functional domains in the tumor necrosis factor family member ectodysplasin-A. J Biol Chem. 2001;276(22):18819–18827. doi: 10.1074/jbc.M101280200. [DOI] [PubMed] [Google Scholar]
- 90.Newton K, French DM, Yan M, Frantz GD, Dixit VM. Myodegeneration in EDA-A2 transgenic mice is prevented by XEDAR deficiency. Mol Cell Biol. 2004;24(4):1608–1613. doi: 10.1128/MCB.24.4.1608-1613.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rothe J, Lesslauer W, Lotscher H, et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364(6440):798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 92.Knight B, Yeoh GC, Husk KL, et al. Impaired preneoplastic changes and liver tumor formation in tumor necrosis factor receptor type 1 knockout mice. J Exp Med. 2000;192(12):1809–1818. doi: 10.1084/jem.192.12.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rieux-Laucat F, Le Deist F, Hivroz C, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268(5215):1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 94.Jacobson BA, Panka DJ, Nguyen KA, Erikson J, Abbas AK, Marshak-Rothstein A. Anatomy of autoantibody production: dominant localization of antibody-producing cells to T cell zones in Fas-deficient mice. Immunity. 1995;3(4):509–519. doi: 10.1016/1074-7613(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 95.Singer GG, Abbas AK. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1(5):365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 96.Gaspal FM, Kim MY, McConnell FM, Raykundalia C, Bekiaris V, Lane PJ. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J Immunol. 2005;174(7):3891–3896. doi: 10.4049/jimmunol.174.7.3891. [DOI] [PubMed] [Google Scholar]
- 97.Kwon BS, Hurtado JC, Lee ZH, et al. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol. 2002;168(11):5483–5490. doi: 10.4049/jimmunol.168.11.5483. [DOI] [PubMed] [Google Scholar]
- 98.Vinay DS, Choi BK, Bae JS, Kim WY, Gebhardt BM, Kwon BS. CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J Immunol. 2004;173(6):4218–4229. doi: 10.4049/jimmunol.173.6.4218. [DOI] [PubMed] [Google Scholar]
- 99.Lee SW, Park Y, So T, et al. Identification of regulatory functions for 4-1BB and 4-1BBL in myelopoiesis and the development of dendritic cells. Nat Immunol. 2008;9(8):917–926. doi: 10.1038/ni.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jeon HJ, Choi JH, Jung IH, et al. CD137 (4-1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation. 2010;121(9):1124–1133. doi: 10.1161/CIRCULATIONAHA.109.882704. [DOI] [PubMed] [Google Scholar]
- 101.Girgenrath M, Weng S, Kostek CA, et al. TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. EMBO J. 2006;25(24):5826–5839. doi: 10.1038/sj.emboj.7601441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scholzke MN, Rottinger A, Murikinati S, Gehrig N, Leib C, Schwaninger M. TWEAK regulates proliferation and differentiation of adult neural progenitor cells. Mol Cell Neurosci. 2011;46(1):325–332. doi: 10.1016/j.mcn.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 103.Tirnitz-Parker JE, Viebahn CS, Jakubowski A, et al. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology. 2010;52(1):291–302. doi: 10.1002/hep.23663. [DOI] [PubMed] [Google Scholar]
- 104.Yan M, Wang H, Chan B, et al. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol. 2001;2(7):638–643. doi: 10.1038/89790. [DOI] [PubMed] [Google Scholar]
- 105.Yan M, Brady JR, Chan B, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11(19):1547–1552. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 106.Wang EC, Thern A, Denzel A, Kitson J, Farrow SN, Owen MJ. DR3 regulates negative selection during thymocyte development. Mol Cell Biol. 2001;21(10):3451–3461. [Google Scholar]
- 107.Stephens GL, McHugh RS, Whitters MJ, et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173(8):5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 108.Pispa J, Pummila M, Barker PA, Thesleff I, Mikkola ML. Edar and Troy signalling pathways act redundantly to regulate initiation of hair follicle development. Hum Mol Genet. 2008;17(21):3380–3391. doi: 10.1093/hmg/ddn232. [DOI] [PubMed] [Google Scholar]
- 109.Schmidt CS, Liu J, Zhang T, et al. Enhanced B cell expansion, survival, and humoral responses by targeting death receptor 6. J Exp Med. 2003;197(1):51–62. doi: 10.1084/jem.20020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao H, Yan M, Wang H, Erickson S, Grewal IS, Dixit VM. Impaired c-Jun amino terminal kinase activity and T cell differentiation in death receptor 6-deficient mice. J Exp Med. 2001;194(10):1441–1448. doi: 10.1084/jem.194.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu J, Na S, Glasebrook A, et al. Enhanced CD4+ T cell proliferation and Th2 cytokine production in DR6-deficient mice. Immunity. 2001;15(1):23–34. doi: 10.1016/s1074-7613(01)00162-5. [DOI] [PubMed] [Google Scholar]
- 112.Lee KF, Li E, Huber LJ, et al. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69(5):737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- 113.Tsitsikov EN, Laouini D, Dunn IF, et al. TRAF1 is a negative regulator of TNF signaling. enhanced TNF signaling in TRAF1-deficient mice. Immunity. 2001;15(4):647–657. doi: 10.1016/s1074-7613(01)00207-2. [DOI] [PubMed] [Google Scholar]
- 114.Missiou A, Kostlin N, Varo N, et al. Tumor necrosis factor receptor-associated factor 1 (TRAF1) deficiency attenuates atherosclerosis in mice by impairing monocyte recruitment to the vessel wall. Circulation. 2010;121(18):2033–2044. doi: 10.1161/CIRCULATIONAHA.109.895037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nguyen LT, Duncan GS, Mirtsos C, et al. TRAF2 deficiency results in hyperactivity of certain TNFR1 signals and impairment of CD40-mediated responses. Immunity. 1999;11(3):379–389. doi: 10.1016/s1074-7613(00)80113-2. [DOI] [PubMed] [Google Scholar]
- 116.Xu Y, Cheng G, Baltimore D. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity. 1996;5(5):407–415. doi: 10.1016/s1074-7613(00)80497-5. [DOI] [PubMed] [Google Scholar]
- 117.Nakano H, Sakon S, Koseki H, et al. Targeted disruption of Traf5 gene causes defects in CD40- and CD27-mediated lymphocyte activation. Proc Natl Acad Sci U S A. 1999;96(17):9803–9808. doi: 10.1073/pnas.96.17.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lomaga MA, Yeh WC, Sarosi I, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13(8):1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Naito A, Yoshida H, Nishioka E, et al. TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc Natl Acad Sci U S A. 2002;99(13):8766–8771. doi: 10.1073/pnas.132636999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goeddel DV. Signal transduction by tumor necrosis factor: the Parker B. Francis Lectureship. Chest. 1999;116(1 suppl):69S–73S. doi: 10.1378/chest.116.suppl_1.69s. [DOI] [PubMed] [Google Scholar]
- 121.Yeh WC, Pompa JL, McCurrach ME, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279(5358):1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 122.Kreder D, Krut O, Adam-Klages S, et al. Impaired neutral sphingomyelinase activation and cutaneous barrier repair in FAN-deficient mice. EMBO J. 1999;18(9):2472–2479. doi: 10.1093/emboj/18.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Varfolomeev EE, Schuchmann M, Luria V, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9(2):267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 124.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8(3):297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 125.Hu Y, Baud V, Delhase M, et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284(5412):316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 126.Li ZW, Chu W, Hu Y, et al. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189(11):1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yoshida H, Kong YY, Yoshida R, et al. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94(6):739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- 128.Hakem R, Hakem A, Duncan GS, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94(3):339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 129.Kuida K, Zheng TS, Na S, et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384(6607):368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 130.Headon DJ, Emmal SA, Ferguson BM, et al. Gene defect in ectodermal dysplasia implicates a death domain adapter in development. Nature. 2001;414(6866):913–916. doi: 10.1038/414913a. [DOI] [PubMed] [Google Scholar]
- 131.Li X. Act1 modulates autoimmunity through its dual functions in CD40L/BAFF and IL-17 signaling. Cytokine. 2008;41(2):105–113. doi: 10.1016/j.cyto.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 132.Allen RC, Armitage RJ, Conley ME, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993;259(5097):990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 133.Takahashi T, Tanaka M, Brannan CI, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76(6):969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 134.Casal ML, Scheidt JL, Rhodes JL, Henthorn PS, Werner P. Mutation identification in a canine model of X-linked ectodermal dysplasia. Mamm Genome. 2005;16(7):524–531. doi: 10.1007/s00335-004-2463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McDermott MF, Aksentijevich I, Galon J, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97(1):133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 136.Waschke KA, Villani AC, Vermeire S, et al. Tumor necrosis factor receptor gene polymorphisms in Crohn's disease: association with clinical phenotypes. Am J Gastroenterol. 2005;100(5):1126–1133. doi: 10.1111/j.1572-0241.2005.40534.x. [DOI] [PubMed] [Google Scholar]
- 137.Magerus-Chatinet A, Neven B, Stolzenberg MC, et al. Onset of autoimmune lymphoproliferative syndrome (ALPS) in humans as a consequence of genetic defect accumulation. J Clin Invest. 2011;121(1):106–112. doi: 10.1172/JCI43752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hughes AE, Ralston SH, Marken J, et al. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat Genet. 2000;24(1):45–48. doi: 10.1038/71667. [DOI] [PubMed] [Google Scholar]
- 139.Cundy T, Hegde M, Naot D, et al. A mutation in the gene TNFRSF11B encoding osteoprotegerin causes an idiopathic hyperphosphatasia phenotype. Hum Mol Genet. 2002;11(18):2119–2127. doi: 10.1093/hmg/11.18.2119. [DOI] [PubMed] [Google Scholar]
- 140.Middleton-Hardie C, Zhu Q, Cundy H, et al. Deletion of aspartate 182 in OPG causes juvenile Paget's disease by impairing both protein secretion and binding to RANKL. J Bone Miner Res. 2006;21(3):438–445. doi: 10.1359/JBMR.051104. [DOI] [PubMed] [Google Scholar]
- 141.Salzer U, Chapel HM, Webster AD, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37(8):820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 142.Mayne CG, Amanna IJ, Nashold FE, Hayes CE. Systemic autoimmunity in BAFF-R-mutant A/WySnJ strain mice. Eur J Immunol. 2008;38(2):587–598. doi: 10.1002/eji.200737817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shimomura Y, Sato N, Miyashita A, Hashimoto T, Ito M, Kuwano R. A rare case of hypohidrotic ectodermal dysplasia caused by compound heterozygous mutations in the EDAR gene. J Invest Dermatol. 2004;123(4):649–655. doi: 10.1111/j.0022-202X.2004.23405.x. [DOI] [PubMed] [Google Scholar]
- 144.Perez de Diego R, Sancho-Shimizu V, Lorenzo L, et al. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity. 2010;33(3):400–411. doi: 10.1016/j.immuni.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]