Summary
Background and objectives
The incidence of ESRD is higher in African Americans than in whites, despite reports of a similar or lower prevalence of CKD.
Design, setting, participants, & measurements
This study compared the incidence of CKD among young African-American and white adults over 20 years of follow-up in the community-based Coronary Artery Risk Development in Young Adults study. Participants included 4119 adults, 18–30 years of age, with an estimated GFR (eGFR) ≥60 ml/min per 1.73 m2 at baseline. Incident CKD was defined as an eGFR <60 ml/min per 1.73 m2 and a ≥25% decline in eGFR at study visits conducted 10, 15, and 20 years after baseline.
Results
At baseline, the mean age of African Americans and whites was 24 and 26 years, respectively (P<0.001), and 56% and 53% of participants, respectively, were women (P=0.06). There were 43 incident cases of CKD during follow-up, 29 (1.4%) among African Americans and 14 (0.7%) among whites (P=0.02). The age- and sex-adjusted hazard ratio (HR) for incident CKD comparing African Americans to whites was 2.56 (95% confidence interval [95% CI], 1.35–5.05). After further adjustment for body mass index, systolic BP, fasting plasma glucose, and HDL cholesterol, the HR was 2.51 (95% CI, 1.25–5.05). After multivariable adjustment including albuminuria at year 10, the HR for CKD at year 15 or 20 was 1.12 (95% CI, 0.52–2.41).
Conclusions
In this study, the 20-year CKD incidence was higher among African Americans than whites, a difference that is explained in part by albuminuria.
Introduction
Recent estimates suggest that 15 million US adults have moderate to severe CKD, defined as an estimated GFR (eGFR) of 15–59 ml/min per 1.73 m2 (1). A higher prevalence of moderate to severe CKD in whites compared with African Americans has been reported in several cross-sectional studies (2–5). In contrast, the incidence of ESRD is over three times higher in African Americans than in whites (6).
Many explanations have been proposed for the higher incidence of ESRD among African Americans compared with whites despite their lower prevalence of moderate CKD. For example, because disease prevalence is a function of its incidence and duration, it has been suggested that the lower CKD prevalence among African Americans is due to faster progression to ESRD (shorter duration) compared with whites (6–8). It has also been proposed that the equations used to estimate GFR overadjust for differences in creatinine production between African Americans and whites (9). Although the equations used to estimate GFR were developed and validated in a pooled data set that included both whites and African Americans (10,11), these have not been validated in healthy, young African Americans. Understanding whether differences in the incidence of CKD exist between whites and African Americans may elucidate reasons for differences in the prevalence of moderate to severe CKD and of ESRD incidence between these two groups. Data on racial differences in the incidence of CKD remain limited, especially in younger age groups. Therefore, we sought to examine whether differences in CKD incidence exist between African-American and white young adults over 20 years of follow-up in the Coronary Artery Risk Development in Young Adults (CARDIA) study.
Materials and Methods
Study Population
CARDIA is a prospective cohort study sponsored by the National Heart, Lung, and Blood Institute (12). Briefly, the cohort was recruited at four sites in the United States (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California). Recruitment of 5115 African-American and white men and women, aged 18–30 years, for a baseline (year 0) examination occurred in 1985 and 1986. Of relevance to the current analysis, study visits with serum creatinine (SCr) measurements were conducted in 1985–1986, 1995–1996, 2000–2001, and 2005–2006 (referred to as year 0, 10, 15, and 20 examinations, respectively). Of the 5115 study participants, 65 had missing SCr data at baseline, 6 had an eGFR <60 ml/min per 1.73 m2 at baseline, and 211 reported having kidney disease at baseline. In addition, 89 participants were deceased by the year 10 study visit and 625 were alive but did not attend the year 10, 15, or 20 study visits. These participants were excluded from the present analyses. None of the 89 deaths were attributed to kidney disease. Of the remaining sample of 4119 participants, 3662, 3427, and 3332 participants attended and had valid SCr measurements at the year 10, 15, and 20 study visits, respectively. Of the 217 participants excluded for self-report of kidney disease or an eGFR <60 ml/min per 1.73 m2 at baseline, 109 (50%) were African American and 108 (50%) were white. This study and all of its components (e.g., each study visit and follow-up methods) were approved by the institutional review boards at the participating institutions. In addition, all participants provided informed consent before participation at each study visit.
Data Collection
Demographic information was assessed using a questionnaire. At baseline, three BP measurements were taken by centrally trained staff and the mean of the last two measurements was calculated. Hypertension was defined as a systolic BP ≥140 mmHg, a diastolic BP ≥90 mmHg, or the use of antihypertensive medications. Blood was collected at each study visit and stored at –70°C until measurements were performed. Both fasting glucose and a fasting lipid profile (triglycerides, total cholesterol, and HDL cholesterol) were measured using a standard laboratory technique. LDL cholesterol was estimated using the Friedewald equation (13). The presence of diabetes was defined as fasting blood glucose ≥126 mg/dl or the use of insulin and/or oral hypoglycemic agents.
CKD
At baseline and at years 10, 15, and 20 of study visits, SCr was measured by nephelometry. Seventeen years after baseline, a random sample of baseline samples were re-run using the method used at year 15 (n=103). At 23 years after baseline, stored samples from years 10 (n=197), 15 (n=198), and 20 (n=187) were re-run, calibrated to National Institute of Standards and Technology standards as recommended by the National Kidney Disease Education Program Laboratory Working Group (14). Using these data, SCr was re-calibrated and eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) estimating equation (10). For the main analyses, CKD was defined as the presence of an eGFR <60 ml/min per 1.73 m2 at years 10, 15, or 20 and a ≥25% decline in eGFR from the baseline visit (15). The same cases were identified when incident CKD was defined as an eGFR <60 ml/min per 1.73 m2 during follow-up (16).
The occurrence of ESRD among study participants was ascertained using outpatient, hospitalization, and mortality data collected over the 20-year follow-up period. Only seven patients had ESRD, six of whom were African American and one was white. The six participants who developed ESRD met the definition for CKD outlined above, whereas one participant had an eGFR <60 ml/min per 1.73 m2 at baseline. Thus, including ESRD as part of the CKD case definition did not influence the calculation of CKD incidence.
Statistical Analyses
Baseline characteristics of study participants were calculated for African Americans and whites separately. The proportion of African Americans and whites developing CKD, overall, and at each follow-up visit was calculated with the statistical significance of differences by race assessed with the Fisher exact chi-squared test. The hazard ratio for developing CKD for African Americans versus whites during follow-up was then determined. Because the exact date that a participant developed CKD is not known (i.e., CKD was assessed at prespecified study visits), we used interval-censored regression models to calculate the hazard ratios (17,18). Interval-censored data arise when individuals in a study are inspected at set time periods and the incidence of events occurs between two successive times. Participants who did not develop CKD were right censored on the date of their last follow-up visit and those developing CKD were left or interval censored, as appropriate. Hazard ratios were calculated unadjusted, after age and sex adjustment, and also in a model that included additional adjustment for baseline levels of body mass index (BMI), systolic BP, and fasting plasma glucose. We confined adjustment for baseline eGFR to secondary analyses, because it is in the causal pathway leading to incident CKD, and race was associated with eGFR at baseline in CARDIA. It has been suggested that adjusting for baseline eGFR in analyses of incident CKD could introduce bias (19). Because of the low number of cases that occurred during follow-up and no statistically significant differences in CKD incidence by field center (data not shown), we did not adjust for field center in the regression models.
Several additional secondary analyses were conducted. First, the incidence and hazard ratio for persistent CKD was determined. To be categorized as having persistent CKD, those with CKD as defined above had to have CKD at the last study visit attended. Following this approach, five individuals with CKD at the year 10 or year 15 visits without CKD at subsequent visits were considered not to have persistent CKD. Because some have questioned whether estimating equations overcorrect for racial differences in GFR at the same SCr level, a second sensitivity analysis was conducted in which eGFR was calculated using a race coefficient of 1.12 instead of 1.159. This value was chosen on the basis of previous analyses in this study suggesting that the difference in creatinine excretion between African-American and white adults is 12% based on three timed 24-hour urine collections in 835 participants (20). For additional sensitivity analyses, the incidence and hazard ratio for doubling of calibrated SCr and an increase in calibrated SCr ≥0.4 mg/dl from the baseline visit were determined. Finally, hazard ratios for the progression of CKD, defined as a reduction of eGFR ≥3 ml/min per 1.73 m2 per year were calculated.
Data on albuminuria were not available from the baseline study visit. However, albuminuria, based on a random spot albumin-to-creatinine ratio (ACR), was assessed at the year 10 visit. In a post hoc analysis, we calculated the hazard ratio for incident CKD (eGFR <60 ml/min per 1.73 m2 and an at least 25% decline in eGFR) at the year 15 and year 20 study visits for African Americans compared with whites after adjustment for ACR, modeled in milligrams per gram as a log-transformed continuous variable. Data on ACR were missing for 817 (20.4%) of the 4011 participants included in this analysis. Therefore, multiple imputation with five data sets was used to fill in these data (21). All analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC).
Results
At baseline, the mean age for African-American and white participants included in the current analysis was 24 and 26 years, respectively (Table 1). The prevalence of hypertension was higher in African Americans compared with whites. African Americans had higher levels of BMI, systolic BP, and HDL and LDL cholesterol, as well as lower levels of fasting glucose and triglycerides. Mean eGFR was 131 and 116 ml/min per 1.73 m2 among African Americans and whites, respectively.
Table 1.
Baseline characteristics of participants in the CARDIA study (N=4119) by race: 1985–1986
| African American (n=2027) | White (n=2092) | P Value | |
|---|---|---|---|
| Age, yr | 24 (4) | 26 (3) | <0.001 |
| Women | 56 | 53 | 0.06 |
| Body mass index, kg/m2 | 25 (6) | 24 (4) | <0.001 |
| Systolic BP, mmHg | 112 (11) | 109 (11) | <0.001 |
| Diastolic BP, mmHg | 69 (10.0) | 68 (9.1) | 0.12 |
| Hypertension | 3.3 | 2.1 | 0.02 |
| Fasting glucose, mg/dl | 81 (11) | 83 (12) | <0.001 |
| Diabetes | 0.6 | 0.5 | 0.48 |
| Total cholesterol, mg/dl | 178 (34) | 176 (32) | 0.08 |
| HDL cholesterol, mg/dl | 54 (13) | 52 (13) | <0.001 |
| LDL cholesterol, mg/dl | 111 (32) | 109 (30) | 0.04 |
| Triglycerides, mg/dl | 57 (43–77) | 66 (48–90) | <0.001 |
| Estimated GFR, ml/min per 1.73 m2 | 131 (15) | 116 (12) | <0.001 |
Data are mean (SD) or percentage except for triglycerides, which are median (25th–75th percentiles). CARDIA, Coronary Artery Risk Development in Young Adults.
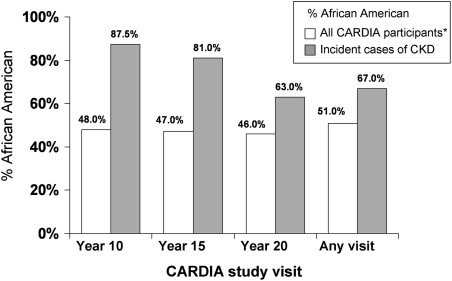
Over 20 years of follow-up, 43 participants developed CKD (Table 2). The mean eGFR for cases, at the visit in which incident CKD was detected, was 37.9 ml/min per 1.73 m2 (range, 5.5–59.7 ml/min per 1.73 m2). Of the 43 cases, 13 (30%) had an eGFR <30 ml/min per 1.73 m2 during follow-up. The proportion of participants developing CKD was higher for African Americans compared with whites (1.4% versus 0.7%; P=0.02). At the follow-up visits conducted at 10, 15, and 20 years after baseline, approximately 45%–50% of study participants were African American,. However, 88%, 81%, and 63% of participants with CKD at these three visits, respectively, were African American (Figure 1).
Table 2.
Number of prevalent cases of CKD for CARDIA study participants examined at each study visit and 20-year incident cases, overall and by race: 1985–2006
| Follow-Up Visit | African American | White | P Valuea | ||
|---|---|---|---|---|---|
| At Risk, n | Patients with CKD, n (%) | At Risk, n | Patients with CKD, n (%) | ||
| Year 10 | 1756 | 7 (0.4) | 1904 | 1 (0.05) | 0.03 |
| Year 15 | 1593 | 13 (0.8) | 1834 | 3 (0.2) | 0.006 |
| Year 20 | 1541 | 19 (1.2) | 1791 | 11 (0.6) | 0.07 |
| Any follow-up visit | 2027 | 29 (1.4) | 2092 | 14 (0.7) | 0.02 |
Data in the first three rows reflect prevalence at examination year, and data in the last row reflect 20-year incidence. CARDIA, Coronary Artery Risk Development in Young Adults.
P value comparing the incidence of CKD between African Americans and whites.
Figure 1.
The percentage of all CARDIA study participants who are African American, as well as the percentage who are African American among CARDIA participants developing CKD. CARDIA, Coronary Artery Risk Development in Young Adults. *All CARDIA participants who attended each respective visit (n=3662, n=3427, and n=3332 at the year 10, 15, and 20 visits, respectively, and n=4119 for any visit).
The unadjusted hazard ratio of developing CKD for African Americans versus whites was 2.23 (95% confidence interval [95% CI], 1.18–4.22; Table 3). After adjustment for age and sex, the hazard ratio was 2.56 (95% CI, 1.35–4.86). This association remained present after further multivariable adjustment. The hazard ratio was larger at 3.63 (95% CI, 1.17–7.70) after additional adjustment for baseline eGFR.
Table 3.
Hazard ratios for incident CKD for African-American and white CARDIA study participants: 1985–2006
| Adjustment Factor | Primary CKD Definition (n=43) | Persistent CKD (n=38) | CKD Using Adjusted Race Coefficient (n=47) | Doubling of SCr (n=31) | Increase in SCr ≥0.4 mg/dl (n=114) | eGFR Decline ≥3 ml/min per 1.73 m2 per year (n=36) |
|---|---|---|---|---|---|---|
| Unadjusted | 2.23 (1.18–4.22) | 2.33 (1.18–4.62) | 2.54 (1.36–4.74) | 3.69 (1.59–8.56) | 2.16 (1.46–3.20) | 2.15 (1.07–4.30) |
| Age and sex | 2.56 (1.35–4.86) | 2.69 (1.35–5.35) | 2.91 (1.55–5.45) | 4.19 (1.80–9.76) | 2.43 (1.64–3.60) | 2.49 (1.24–4.99) |
| Full adjustment | 2.51 (1.25–5.05) | 2.66 (1.25–5.69) | 3.06 (1.55–6.04) | 4.64 (1.80–11.9) | 2.54 (1.68–3.84) | 2.35 (1.09–5.07) |
| Full adjustment with eGFR | 3.63 (1.17–7.70) | 3.41 (1.49–7.77) | 4.35 (2.10–9.00) | 3.28 (1.11–9.71) | 1.38 (0.83–2.31) | 3.11 (1.35–7.12) |
Data are shown as hazard ratio (95% confidence interval). Full adjustment includes age, sex, BMI, systolic BP, fasting plasma glucose, and HDL cholesterol. The primary definition of CKD is an estimated GFR (eGFR) <60 ml/min per 1.73 m2 and a ≥25% reduction in eGFR. See Bash et al. for additional details (15). Persistent CKD was defined as having an eGFR <60 ml/min per 1.73 m2 at the last visit attended during follow-up. Adjusted eGFR race coefficient replaces the 16% race coefficient in the Chronic Kidney Disease Epidemiology Collaboration equation with a coefficient of 12%, and CKD is defined using the above-listed primary definition. Doubling of calibrated serum creatinine and increase in calibrated serum creatinine ≥0.4 mg/dl represent changes from baseline. CARDIA, Coronary Artery Risk Development in Young Adults; SCr, serum creatinine; eGFR, estimated GFR.
Sensitivity Analyses
During follow-up, 38 participants developed persistent CKD (1.3% of African Americans and 0.7% of whites; P=0.02) and 47 developed CKD defined as an eGFR <60 ml/min per 1.73 m2 at years 10, 15, or 20 using the CKD-EPI equation with a race-adjusted coefficient adjusted to 1.12 (1.6% of African Americans and 0.7% of whites; P=0.005). In addition, 31 participants experienced a doubling of SCr (1.2% of African Americans and 0.3% of whites; P=0.002), 114 had an increase in SCr ≥0.4 mg/dl (3.6% of African Americans and 1.8% of whites; P<0.001), and 36 had a decline in eGFR ≥3 ml/min per 1.73 m2 (1.2% of African Americans and 0.6% of whites; P<0.001). The hazard ratio of African Americans to whites for each outcome was significantly >1 before and after multivariable adjustment (Table 3). Results were consistent after further adjustment for baseline eGFR except for the outcome of increase in SCr ≥0.4 mg/dl, in which the hazard ratio was 1.38 (95% CI, 0.83–2.31).
Adjustment for Albuminuria
At the year 10 visit, the geometric mean albuminuria level was 8.2 mg/g (95% CI, 7.8–8.5) and 6.6 mg/g (95% CI, 6.4–6.8) among African Americans and whites, respectively. The age- and sex-adjusted hazard ratio for developing CKD at year 15 or 20 for African Americans versus whites was 2.14 (95% CI, 1.07–4.25). After further adjustment for BMI, systolic BP, fasting plasma glucose, and HDL cholesterol from the year 10 study visit, the hazard ratio for incident CKD at year 15 or 20 was 1.50 (95% CI, 0.71–3.16). Further adjustment for year 10 ACR reduced the hazard ratio to 1.12 (95% CI, 0.52–2.41). Results were similar when individuals missing ACR were excluded from the analyses (data not shown).
Discussion
Several population-based studies have shown a higher prevalence of moderate to severe CKD among whites compared with African Americans. These studies provide important data on racial differences in the burden of CKD. However, differences in disease prevalence could be the result of several factors and may not accurately reflect disease risk. This study utilized 20 years of follow-up to ascertain the risk for CKD among young white and African-American adults. In this study, African Americans had a two- to three-fold increased risk for developing CKD compared with whites. The higher incidence of CKD was not explained by systolic BP or fasting plasma glucose. Post hoc analyses suggest that higher levels of albuminuria may be an explanatory factor for the increased risk for CKD among African Americans.
It is well established that African Americans have a higher incidence of ESRD compared with whites (22–26). According to recent data from the United States Renal Data System, African Americans are four times more likely to develop ESRD compared with whites (6). Reasons explaining these observations remain uncertain. Our study findings suggest that incidence of earlier stages of kidney disease also occurs more frequently among African Americans. These findings are in accordance with prior studies including the Bogalusa Heart Study, in which African-American children were more likely to develop ESRD in early adulthood than their white counterparts (27). In addition, the incidence of an increase in SCr ≥0.4 mg/dl was explored in the Atherosclerosis Risk in Communities study among participants with diabetes (28). Over 3 years of follow-up, African Americans were more likely than whites to develop CKD. In contrast to findings from the Atherosclerosis Risk in Communities study, adjustment for traditional risk factors did not attenuate the hazard ratio for incident CKD in this study.
Several prior studies have reported a higher burden of moderate to severe CKD among whites compared with African Americans (2–4). Inferences on racial differences in CKD prevalence from cross-sectional studies must be undertaken with caution (29). The prevalence of a disease is related to both its incidence and duration (30). Among adults aged <65 years with CKD, African Americans have a higher mortality risk compared with whites (31). It is possible that African Americans have more severe kidney disease that progresses to ESRD more quickly (32). It is noteworthy that six of the seven participants with ESRD in this study were African American. The increased risk for CKD among African Americans present in this study highlights the need for prospective studies when evaluating racial differences in CKD.
The overcorrection for African-American race in GFR estimating equations has been proposed as an explanation for the higher burden of CKD among whites (9). The CKD-EPI equation uses a race-correction factor of 15.9% and previous authors have suggested that this may be too large. In an analysis of the Third National Health and Nutrition Examination Survey (NHANES III), metabolic consequences of reduced eGFR were more common in African Americans, compared with non-Hispanic Whites (32). In addition, the risk for ESRD is higher for African Americans compared with whites with CKD (25,33). In a sensitivity analysis, we adjusted the race-correction factor in the CKD-EPI equation by 33% (from 1.159 to 1.12). The 12% correction factor was selected to reflect the observed ratio of 24-hour urinary creatinine excretion in African Americans versus white after adjustment for sex differences, and this adjustment factor is consistent with prior studies (20,34). It is noteworthy that using this race-correction factor, the hazard ratio for CKD comparing African Americans to whites was even higher (3.06 [95% CI, 1.55–6.04]) than without the correction (2.51 [95% CI, 1.25–5.05]).
Racial differences in ACR have been reported for participants in several studies (1,3,35). For example, the prevalence ACR ≥30 mg/g was 12.1% among non-Hispanic blacks and 9.3% among non-Hispanic whites in NHANES 1999–2000 (35). In addition, higher levels of ACR among African Americans have been reported to explain a substantial proportion of their increased ESRD risk. In the Reasons for Geographic and Racial Differences in Stroke study, the African American/white hazard ratios for ESRD were 4.01 (95% CI, 2.78–5.89) after adjustment for age and sex and 1.81 (95% CI, 1.21–2.71) after further adjustment for log-transformed ACR. The results of this study are consistent with the data from the Reasons for Geographic and Racial Differences in Stroke study; the African American/white hazard ratio for incident CKD was substantially attenuated after adjustment for log-transformed ACR. Reducing ACR, especially in African Americans, may reduce the racial disparity in ESRD present in the United States.
This study has several strengths, including well characterized participants from the CARDIA study, rigorously measured outcomes and covariables, and long duration of follow-up. In addition, we used the CKD-EPI equation to estimate GFR. This formula has less bias than the Modification of Diet in Renal Disease equation among whites and African Americans (36). However, there are some important limitations. Few cases of CKD occurred during follow-up. Given the low burden of CKD in younger adults, this is not surprising. Because CKD was defined on the basis of SCr measurements at study visits, participants who did not return for these visits were excluded from this analysis. Because African-American men were the race-sex group most likely to be lost to follow-up, we may have underestimated the incidence of CKD in this group. Another limitation is that data were not available on access to healthcare. In addition, albuminuria was not assessed at baseline. Post hoc analyses suggest that higher levels of albuminuria at the year 10 follow-up may contribute, in part, to the increased risk for incident CKD among African Americans compared with whites. Further investigation of this finding in other populations is warranted.
In this study, African Americans experienced a substantially increased risk for developing CKD compared with whites. This finding provides an important contrast to cross-sectional studies reporting a higher CKD prevalence among whites compared with African Americans. Much of this increased risk may be explained by the higher prevalence of albuminuria among African Americans. Future studies are needed to confirm this finding, to identify the mediators of the increased incidence of CKD among African Americans, and to assess effective interventions for reducing this health disparity.
Disclosures
None.
Acknowledgment
Work for this manuscript was supported by the CARDIA contract (N01-HC-48047–N01-HC-48050 and N01-HC-95095) from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Kramer H, Palmas W, Kestenbaum B, Cushman M, Allison M, Astor B, Shlipak M: Chronic kidney disease prevalence estimates among racial/ethnic groups: The Multi-Ethnic Study of Atherosclerosis. Clin J Am Soc Nephrol 3: 1391–1397, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClellan W, Warnock DG, McClure L, Campbell RC, Newsome BB, Howard V, Cushman M, Howard G: Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol 17: 1710–1715, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Islam TM, Fox CS, Mann D, Muntner P: Age-related associations of hypertension and diabetes mellitus with chronic kidney disease. BMC Nephrol 10: 17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 6.United States Renal Data System (USRDS): USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006 [Google Scholar]
- 7.Hsu CY, Vittinghoff E, Lin F, Shlipak MG: The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med 141: 95–101, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Norris K, Mehrotra R, Nissenson AR: Racial differences in mortality and ESRD. Am J Kidney Dis 52: 205–208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clase CM, Garg AX, Kiberd BA: Prevalence of low glomerular filtration rate in nondiabetic Americans: Third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol 13: 1338–1349, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ: CARDIA: Study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 41: 1105–1116, 1988 [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 14.Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH. National Kidney Disease Education Program Laboratory Working Group: Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 52: 5–18, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Bash LD, Coresh J, Köttgen A, Parekh RS, Fulop T, Wang Y, Astor BC: Defining incident chronic kidney disease in the research setting: The ARIC Study. Am J Epidemiol 170: 414–424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.KDOQI Working Group: Definition and classification of stages of chronic kidney disease. Am J Kidney Dis 39: S46–S75, 2002 [Google Scholar]
- 17.Zhang Z, Sun J: Interval censoring. Stat Methods Med Res 19: 53–70, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allison P: Estimating parametric regression models with PROC LIFEREG. In: Survival Analysis Using SAS: A Practical Guide, Cary, NC, SAS Publishing, 2008, pp 61–110 [Google Scholar]
- 19.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM: When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol 162: 267–278, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Jacobs DR, Jr, Murtaugh MA, Steffes M, Yu X, Roseman J, Goetz FC: Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: The Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol 155: 1114–1119, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Rubin DB: Multiple Imputation for Nonresponse in Surveys, New York, John Wiley & Sons, Inc, 1987 [Google Scholar]
- 22.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J: End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 277: 1293–1298, 1997 [PubMed] [Google Scholar]
- 23.Whittle JC, Whelton PK, Seidler AJ, Klag MJ: Does racial variation in risk factors explain black-white differences in the incidence of hypertensive end-stage renal disease? Arch Intern Med 151: 1359–1364, 1991 [PubMed] [Google Scholar]
- 24.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O’Hare AM: White/black racial differences in risk of end-stage renal disease and death. Am J Med 122: 672–678, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newsome BB, McClellan WM, Allison JJ, Eggers PW, Chen SC, Collins AJ, Kiefe CI, Coffey CS, Warnock DG: Racial differences in the competing risks of mortality and ESRD after acute myocardial infarction. Am J Kidney Dis 52: 251–261, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, Brancati FL: Excess risk of chronic kidney disease among African-American versus white subjects in the United States: A population-based study of potential explanatory factors. J Am Soc Nephrol 13: 2363–2370, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Muntner P, Arshad A, Morse SA, Patel DA, Manapatra PD, Reisin E, Aguilar EA, Chen W, Srinivasan S, Berenson GS: End-stage renal disease in young black males in a black-white population: Longitudinal analysis of the Bogalusa Heart Study. BMC Nephrol 10: 40, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krop JS, Coresh J, Chambless LE, Shahar E, Watson RL, Szklo M, Brancati FL: A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: The Atherosclerosis Risk in Communities study. Arch Intern Med 159: 1777–1783, 1999 [DOI] [PubMed] [Google Scholar]
- 29.McFarland DD, Cobb S: Causal interpretations from cross-sectional data. An examination of the stochastic processes involved in the relationship between a personality characteristic and coronary heart disease. J Chronic Dis 20: 393–406, 1967 [DOI] [PubMed] [Google Scholar]
- 30.Gordis L: Epidemiology, 1st Ed., Philadelphia, W.B. Saunders Company, 1997 [Google Scholar]
- 31.Mehrotra R, Kermah D, Fried L, Adler S, Norris K: Racial differences in mortality among those with CKD. J Am Soc Nephrol 19: 1403–1410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foley RN, Wang C, Ishani A, Collins AJ: NHANES III: Influence of race on GFR thresholds and detection of metabolic abnormalities. J Am Soc Nephrol 18: 2575–2582, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Xue JL, Eggers PW, Agodoa LY, Foley RN, Collins AJ: Longitudinal study of racial and ethnic differences in developing end-stage renal disease among aged medicare beneficiaries. J Am Soc Nephrol 18: 1299–1306, 2007 [DOI] [PubMed] [Google Scholar]
- 34.James GD, Sealey JE, Alderman M, Ljungman S, Mueller FB, Pecker MS, Laragh JH: A longitudinal study of urinary creatinine and creatinine clearance in normal subjects. Race, sex, and age differences. Am J Hypertens 1: 124–131, 1988 [DOI] [PubMed] [Google Scholar]
- 35.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH: Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 16: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, Hamm LL, Lewis JB, Mauer M, Navis GJ, Steffes MW, Eggers PW, Coresh J, Levey AS: Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 56: 486–495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]