Abstract
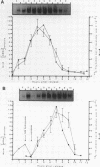
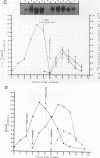
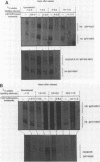
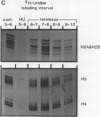
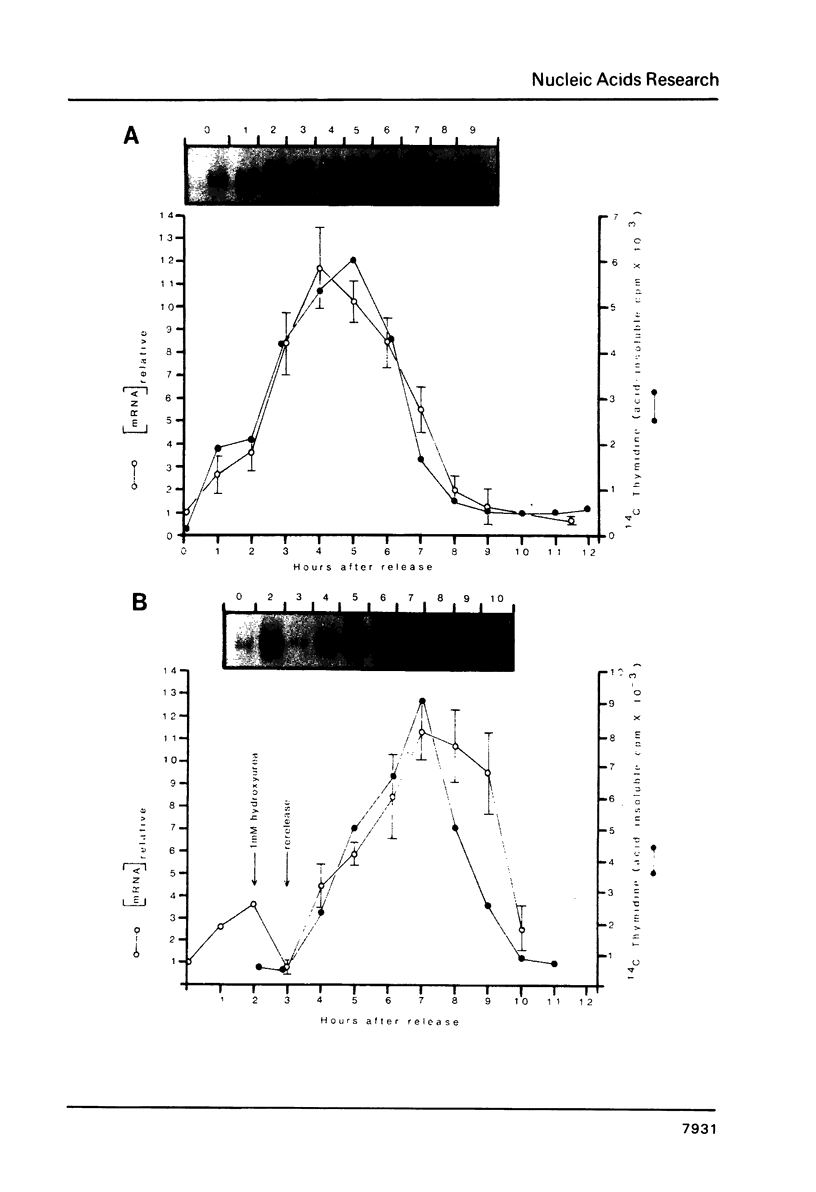
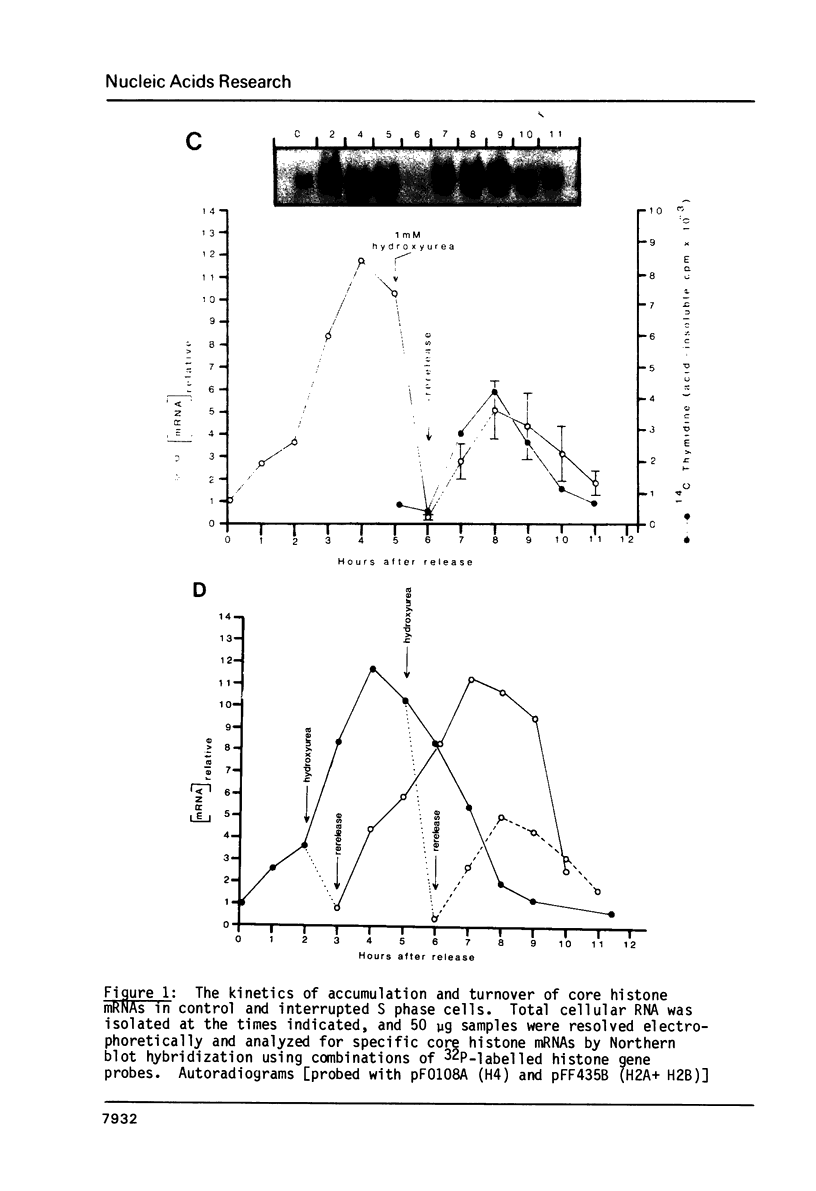
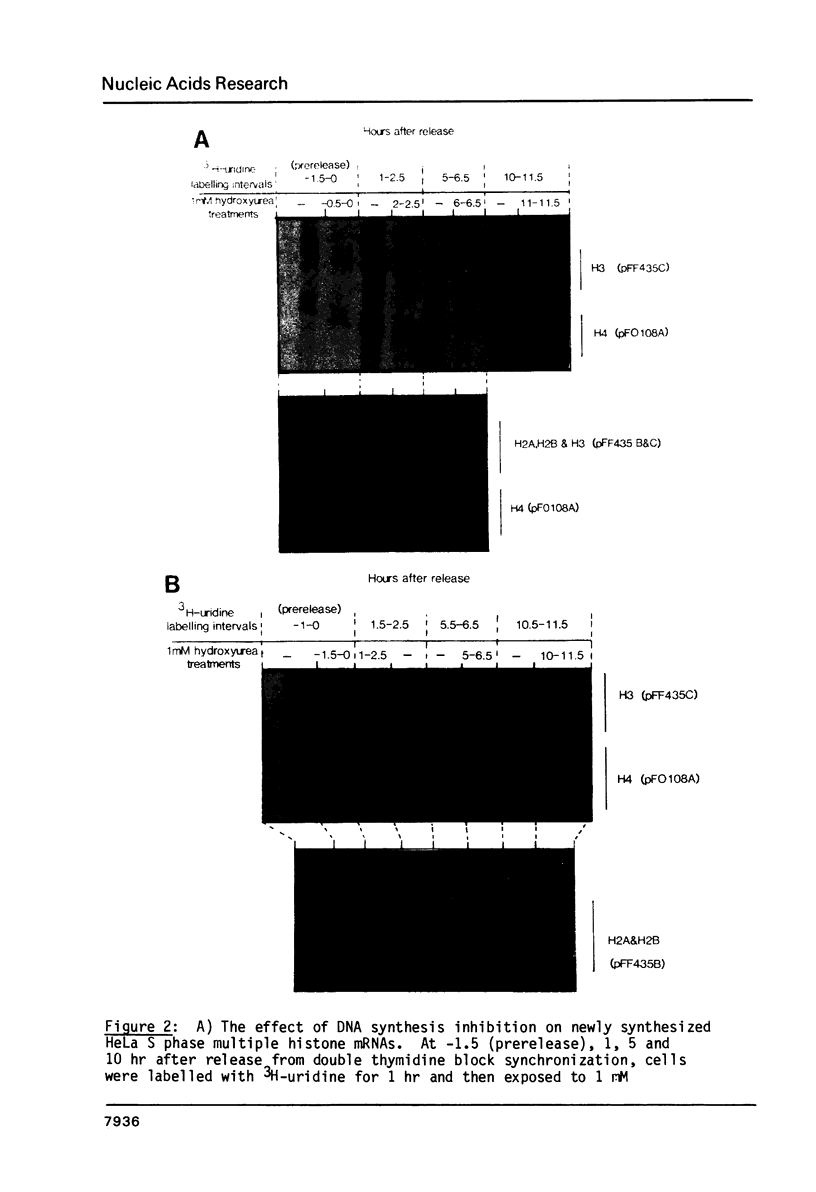
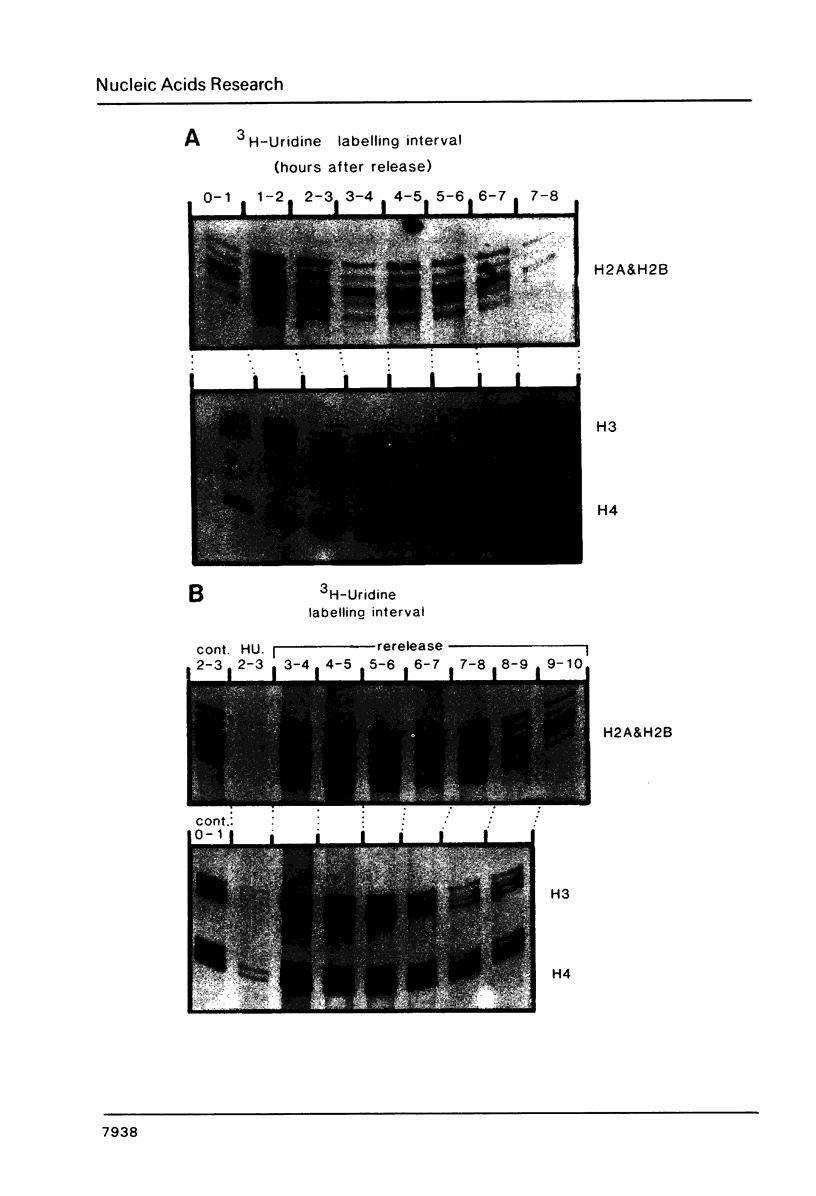
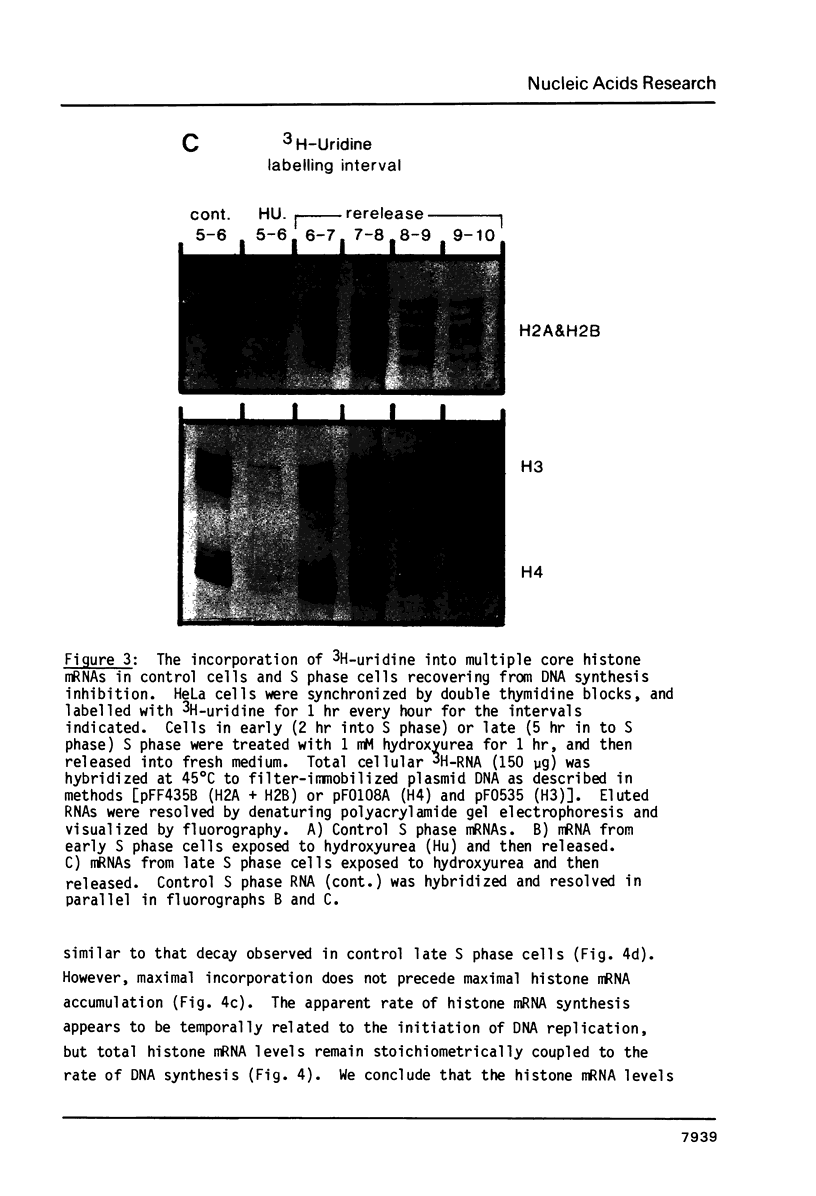
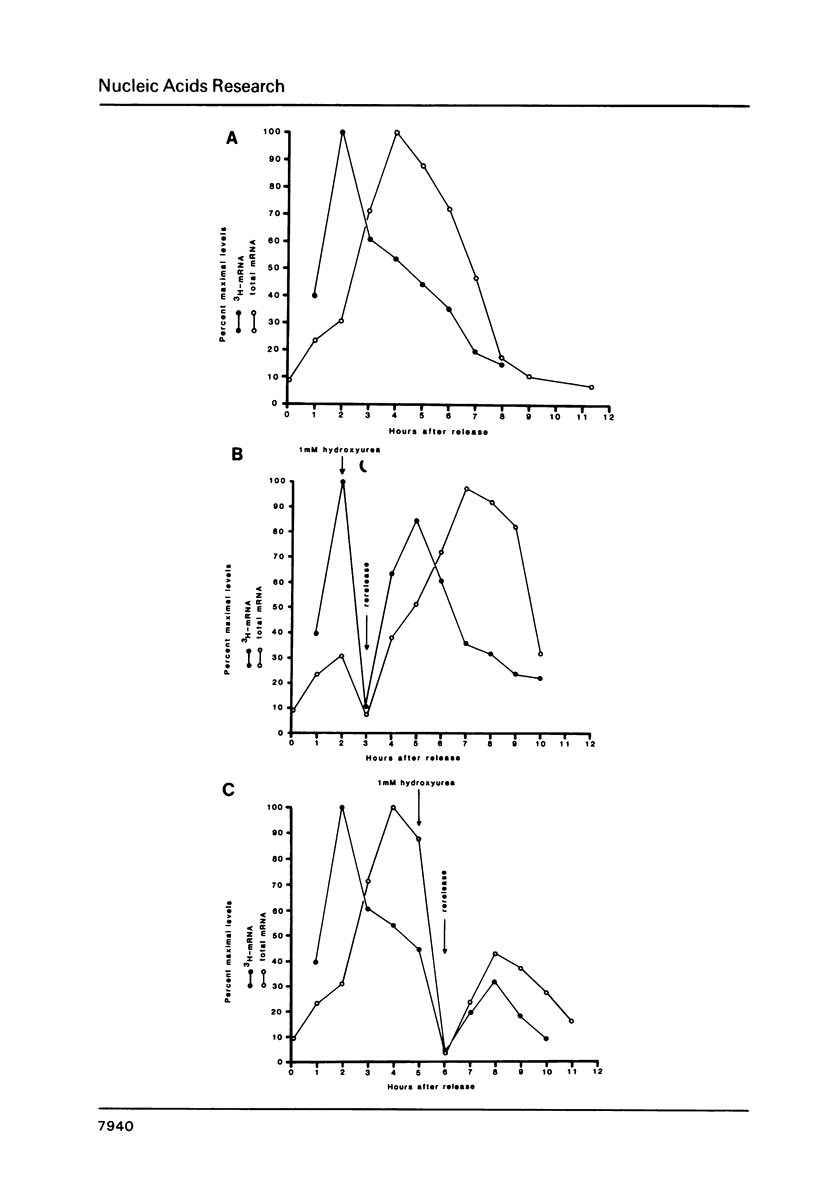
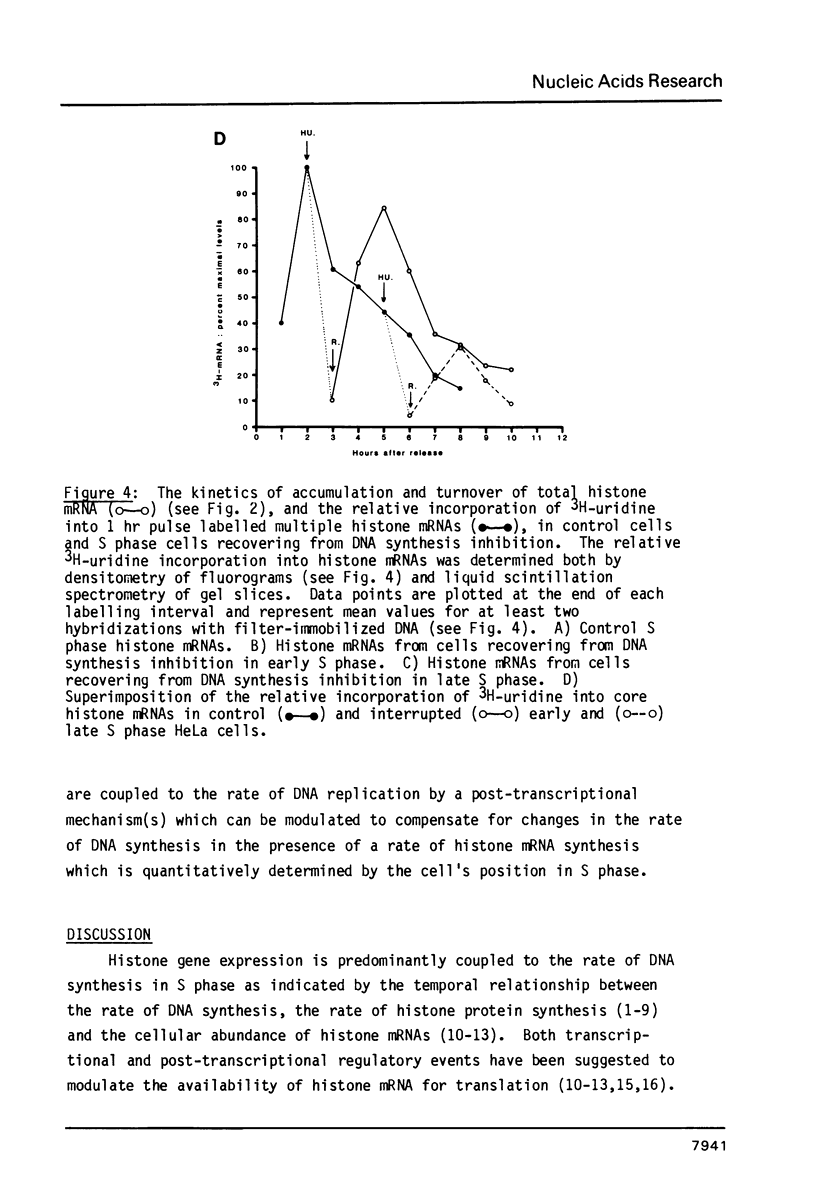
Core histone mRNA metabolism has been examined in S phase HeLa cells recovering from DNA synthesis inhibition by 1 mM hydroxyurea. Using cloned human histone genes as probes for histone mRNA quantitation, the response to and recovery from DNA synthesis inhibition is shown to depend on the position of the cell with respect to the initiation of DNA replication. The incorporation of 3H-uridine into multiple histone mRNAs in recovering cells does not exceed preinhibition levels, and as this incorporation is maximal in early S phase, the synthesis of core histone mRNA is apparently related to the ordered replication of the genome. The total histone mRNA present in interrupted S phase cells after recovery is not significantly different from that present in control cells, and a temporal and functional coupling between histone mRNA levels and the relative rate of DNA synthesis is maintained in perturbed cells.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borun T. W., Gabrielli F., Ajiro K., Zweidler A., Baglioni C. Further evidence of transcriptional and translational control of histone messenger RNA during the HeLa S3 cycle. Cell. 1975 Jan;4(1):59–67. doi: 10.1016/0092-8674(75)90134-8. [DOI] [PubMed] [Google Scholar]
- Braunstein J. D., Schulze D., DelGiudice T., Furst A., Schildkraut C. L. The temporal order of replication of murine immunoglobulin heavy chain constant region sequences corresponds to their linear order in the genome. Nucleic Acids Res. 1982 Nov 11;10(21):6887–6902. doi: 10.1093/nar/10.21.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Delegeane A. M., Lee A. S. Coupling of histone and DNA synthesis in the somatic cell cycle. Science. 1982 Jan 1;215(4528):79–81. doi: 10.1126/science.7053561. [DOI] [PubMed] [Google Scholar]
- Fangman W. L., Hice R. H., Chlebowicz-Sledziewska E. ARS replication during the yeast S phase. Cell. 1983 Mar;32(3):831–838. doi: 10.1016/0092-8674(83)90069-7. [DOI] [PubMed] [Google Scholar]
- Groppi V. E., Jr, Coffino P. G1 and S phase mammalian cells synthesize histones at equivalent rates. Cell. 1980 Aug;21(1):195–204. doi: 10.1016/0092-8674(80)90127-0. [DOI] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N., Zernik M., Roeder R. G. The structure of the human histone genes: clustered but not tandemly repeated. Cell. 1981 Jun;24(3):661–668. doi: 10.1016/0092-8674(81)90092-1. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Osley M. A., Ludwig T. R., 2nd, McLaughlin C. S. Cell-cycle regulation of yeast histone mRNA. Cell. 1981 May;24(2):367–375. doi: 10.1016/0092-8674(81)90326-3. [DOI] [PubMed] [Google Scholar]
- Hereford L., Bromley S., Osley M. A. Periodic transcription of yeast histone genes. Cell. 1982 Aug;30(1):305–310. doi: 10.1016/0092-8674(82)90036-8. [DOI] [PubMed] [Google Scholar]
- Lichtler A. C., Sierra F., Clark S., Wells J. R., Stein J. L., Stein G. S. Multiple H4 histone mRNAs of HeLa cells are encoded in different genes. Nature. 1982 Jul 8;298(5870):195–198. doi: 10.1038/298195a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marashi F., Baumbach L., Rickles R., Sierra F., Stein J. L., Stein G. S. Histone proteins in HeLa S3 cells are synthesized in a cell cycle stage specific manner. Science. 1982 Feb 5;215(4533):683–685. doi: 10.1126/science.7058333. [DOI] [PubMed] [Google Scholar]
- Moll R., Wintersberger E. Synthesis of yeast histones in the cell cycle. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1863–1867. doi: 10.1073/pnas.73.6.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb M., Stein J., Stein G. Coordinate regulation of multiple histone mRNAs during the cell cycle in HeLa cells. Nucleic Acids Res. 1983 Apr 25;11(8):2391–2410. doi: 10.1093/nar/11.8.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickles R., Marashi F., Sierra F., Clark S., Wells J., Stein J., Stein G. Analysis of histone gene expression during the cell cycle in HeLa cells by using cloned human histone genes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):749–753. doi: 10.1073/pnas.79.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard E. A., Phillips I., Davis J., Stein J. L., Stein G. S. Evidence for the resumption of DNA replication prior to histone synthesis in HeLa cells after release from treatment with hydroxyurea. FEBS Lett. 1982 Apr 19;140(2):189–192. doi: 10.1016/0014-5793(82)80891-0. [DOI] [PubMed] [Google Scholar]
- Sierra F., Lichtler A., Marashi F., Rickles R., Van Dyke T., Clark S., Wells J., Stein G., Stein J. Organization of human histone genes. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1795–1799. doi: 10.1073/pnas.79.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding J., Kajiwara K., Mueller G. C. The metabolism of basic proteins in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1535–1542. doi: 10.1073/pnas.56.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Borun T. W. The synthesis of acidic chromosomal proteins during the cell cycle of HeLa S-3 cells. I. The accelerated accumulation of acidic residual nuclear protein before the initiation of DNA replication. J Cell Biol. 1972 Feb;52(2):292–307. doi: 10.1083/jcb.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnowka M. A., Baglioni C., Basilico C. Synthesis of H1 histones by BHK cells in G1. Cell. 1978 Sep;15(1):163–171. doi: 10.1016/0092-8674(78)90092-2. [DOI] [PubMed] [Google Scholar]
- Waithe W. I., Renaud J., Nadeau P., Pallotta D. Histone synthesis by lymphocytes in G0 and G1. Biochemistry. 1983 Apr 12;22(8):1778–1783. doi: 10.1021/bi00277a006. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Melli M. Determination of the number of histone genes in human DNA. J Mol Biol. 1977 Mar 5;110(3):511–535. doi: 10.1016/s0022-2836(77)80110-1. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Bonner W. M. Separation of basal histone synthesis from S-phase histone synthesis in dividing cells. Cell. 1981 Dec;27(2 Pt 1):321–330. doi: 10.1016/0092-8674(81)90415-3. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Tsai S., Bonner W. M. Patterns of histone variant synthesis can distinguish G0 from G1 cells. Cell. 1982 Dec;31(2 Pt 1):367–374. doi: 10.1016/0092-8674(82)90130-1. [DOI] [PubMed] [Google Scholar]