Abstract
Focal cortical dysplasia (FCD) and other localized malformations of cortical development represent common causes of intractable pediatric epilepsy. Insights into the cellular and molecular pathogenesis of focal cortical malformations may reveal information about associated mechanisms of epileptogenesis and suggest new therapies for seizures caused by these developmental lesions. In animal models and human studies of FCD and the related disease of Tuberous Sclerosis Complex (TSC), the mammalian target of rapamycin (mTOR) pathway has been implicated in mediating cellular and molecular changes leading to the formation of the cortical malformations and the expression of epilepsy. The use of mTOR inhibitors may represent a rational therapeutic strategy for treating or even preventing epilepsy due to FCD and TSC.
Keywords: Epilepsy, seizure, malformation of cortical development, tuberous sclerosis complex
Epilepsy in Focal Cortical Dysplasia and Related Focal Cortical Malformations
Malformations of cortical development represent a common cause of pediatric epilepsy (Kuzniecky, 2006; Sisodiya, 2004). A variety of different cortical malformations may cause epilepsy, ranging from diffuse or multifocal abnormalities (e.g. lissencephaly, band heterotopia) to isolated, discrete focal lesions (e.g. focal cortical dysplasia). With improving neuroimaging technology, focal cortical malformations, in particular focal cortical dysplasia (FCD), have been increasing recognized as causes of intractable epilepsy in children, accounting for up to 25% of cases of medically-refractory partial epilepsy (Bast et al., 2006; Blumke et al. 2009). Furthermore, the actual prevalence of FCD causing epilepsy may be underestimated, as pathological diagnoses of FCD are often made retrospectively in surgical specimens removed from patients with intractable epilepsy that were previously labelled as having “cryptogenic” or non-lesional (i.e. MRI-negative) epilepsy. From a therapeutic standpoint, epilepsy caused by FCD is often refractory to available seizure medications. Surgical resection of FCD leads to seizure-freedom in about 50–70% of patients (Chern et al., 2010; Cohen-Gadol et al., 2004; Tassi et al., 2002), but a significant proportion of epilepsy patients related to FCD continue to have seizures despite all available medical and surgical options. Thus, novel therapies for FCD, including potential disease-modifying or antiepileptogenic treatments, are clearly needed, which will depend on obtaining a better understanding of the pathogenesis of theses developmental lesions and the associated mechanisms of epileptogenesis.
Insights into the pathogenesis of FCD may be gleaned from other related disorders in which the molecular pathophysiology is better understood. In particular, Tuberous Sclerosis Complex (TSC), an autosomal dominant genetic disease due to mutation of either the TSC1 or TSC2 gene, typically involves multifocal cortical lesions (tubers) that have cellular and histopathological features closely resembling some types of FCD (see below). Similar to FCD, epilepsy is a very common manifestation of TSC, occurring in up to 90% of TSC patients in some series (Chu-Shore et al., 2010). Furthermore, the majority of TSC patients have medically-intractable epilepsy (Chu-Shore et al., 2010; Sparagana et al., 2003). Although a subset of TSC patients may benefit substantially from epilepsy surgery (Madhavan et al., 2007; Weiner et al., 2006) or other non-medical options such as ketogenic diet (Kossoff et al., 2005), many patients are not considered good candidates for epilepsy surgery and continue to have long-term, disabling seizures. Thus, similar to FCD, better therapeutic options and disease-modifying approaches need to be developed for epilepsy in TSC.
Classification and Pathological Features of Focal Cortical Dysplasia and Related Focal Cortical Malformations
Significant progress has been made in identifying and classifying the pathological features of FCD and related focal cortical malformations. The terminology and classification of FCD has evolved and has sometimes caused confusion in the literature. From a semantic standpoint, the term “cortical dysplasia” has often been used broadly to refer to any malformation of cortical development, but strictly-applied, FCD refers to a particular type of focal cortical malformation based on specific imaging, pathological and molecular genetic criteria (Barkovich et al., 2005). Pathologically, FCD has been subdivided into at least two major subtypes: FCD Type I, characterized primarily by a focal disruption of normal intracortical lamination and columnar organization, and FCD Type II, involving both cortical dyslamination and abnormal cellular features, particularly dysmorphic neurons (Palmini et al., 2004). FCD Type II is further subdivided into those without (Type IIA) and with (Type IIB) balloon cells. Furthermore, recently an additional category of FCD Type III has been proposed, to identify cases of FCD that occurred in combination with other types of lesions, such as hippocampal sclerosis, tumors, or vascular malformations (Blumcke et al., 2011).
Striking pathological similarities have been noted between one type of FCD (Type IIB) and cortical tubers of TSC. In fact, FCD Type IIB has sometimes been proposed to represent a “forme fruste” of TSC. FCD Type IIB and tubers of TSC have been classified together as cortical malformations that are likely caused by abnormal cell proliferation (Barkovich et al., 2005). Both malformations exhibit focal cortical dyslamination and dysmorphic neurons, as well as distinctive cytomegalic cells (balloon cells in FCD Type IIB, “giant” cells in TSC). In addition, these two lesions may both involve other abnormal cellular processes along a spectrum between glia and neurons, including maloriented neurons, dysplastic neurons and glia, and reactive astrocytes. A leading hypothesis about the developmental pathogenesis of FCD Type IIB and tubers of TSC is that both lesions arise during early brain development due to abnormal cell proliferation, perhaps involving embryonic neuroglial progenitor cells. Accordingly, balloon cells and giant cells may represent immature, undifferentiated cells originating from such embryonic stem cells (Yasin et al., 2010). On the molecular level, support for this hypothesis derives from studies identifying immature markers of cortical development in both FCD and TSC, such as CD34 and doublecortin-like protein (Boer et al., 2009; Fauser et al., 2004; Lamparello et al., 2007; Lee et al., 2003; Mizuguchi et al., 2002). If FCD and TSC do share a common developmental pathogenesis, it would follow that mechanisms of epileptogenesis and targeted therapeutic strategies for epilepsy may also overlap between these two disorders.
Role of the mTOR Pathway in the Pathogenesis of Focal Cortical Dysplasia and Tuberous Sclerosis
The mammalian target of rapamycin (mTOR) pathway is a cellular signalling pathway that could represent a primary pathogenic mechanism causing the cortical lesions of FCD and TSC. mTOR is a protein kinase that normally serves as a central regulator of a number of important physiological functions, including cell growth and proliferation, metabolism, autophagy, and cellular survival and death (Sarabassov et al., 2005; Crino et al., 2006; Wong, 2010). mTOR acts as the catalytic subunit within two distinct complexes, mTOR complex 1 (mTORC1) and mTORC2, which differ in their downstream targets and their sensitivity to rapamycin. mTORC1 is rapamycin-sensitive and modulates a number of downstream signalling mechanisms that directly promote protein synthesis, such as the ribosomal S6 kinase/S6 protein pathway and the eukaryotic initiation factor 4E binding protein-1 (4E-BP1)/eukaryotic initiation factor eIF4E pathway. mTORC2 is largely rapamycin-insensitive and activates a number of other kinases, including Akt and protein kinase C, as well as cytoskeletal regulators. Conversely, the mTOR pathway, particularly mTORC1, can be activated or inhibited by a number of upstream signaling mechanisms in response to environmental cues or metabolic demands, such as the insulin/phosphoinositide 3-kinase (PI3K)/Akt pathway or the adenosine monophosphate-activated protein kinase (AMPK) pathway (Fig. 1A). The mTOR pathway is involved in a diversity of physiological processes that might contribute to various histopathological abnormalities of cortical malformations, but the involvement of mTOR in regulating cell growth and proliferation seems especially appropriate for accounting for the abnormal cellular phenotypes observed in FCD and TSC.
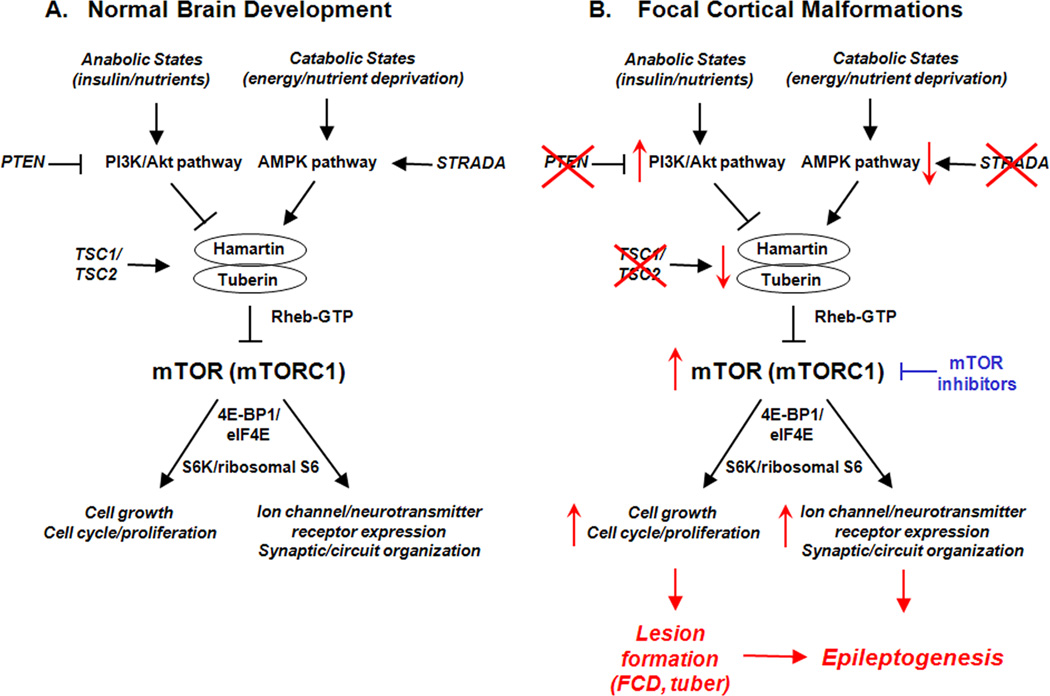
Figure 1.
Putative role of the mTOR pathway in pathogenesis and epileptogenesis of focal cortical malformations. A. During normal brain development, the mTOR pathway, particularly mTORC1, regulates a number of important physiological functions, such as cell growth, proliferation, ion channel expression, and synaptic and circuit plasticity, primarily via activation of protein synthesis mechanisms, such as through S6K/ribosomal S6 protein and eukaryotic initiation factor 4E binding protein-1 (4E-BP1)/eukaryotic initiation factor eIF4E pathways. In turn, in response to different physiological conditions and stimuli, mTORC1 may be activated or inhibited by various upstream signaling pathways, such as the insulin/phosphoinositide 3-kinase (PI3K)/Akt pathway or the adenosine monophosphate-activated protein kinase (AMPK) pathway. B. In focal malformations of cortical development, the mTOR pathway may become hyperactivated, caused in some cases by known mutations in upstream regulators of mTOR (e.g. TSC1 or TSC2 genes in the disease TSC directly affecting hamartin or tuberin expression; STRADA gene in PMSE syndrome, leading to decreased AMPK pathway activation; PTEN inactivation in an animal model of FCD, leading to increased PI3K/Akt pathway activity) or in other cases by unknown mechanisms (e.g. most cases of isolated FCD in people). Regardless of the initial upstream trigger, disinhibition or hyperactivation of the mTOR pathway can lead to abnormally increased cell growth and proliferation, which may account for the focal lesion formation of FCD and tubers. The gross structural lesions themselves, as well as non-structural molecular and cellular changes in ion channel expression and synaptic organization triggered by altered mTOR-mediated protein synthesis, may promote epileptogenesis in these disorders. Finally, mTOR inhibitors may represent a rational therapy for FCD and TSC by reversing mTORC1 hyperactivation and the downstream mechanisms of epileptogenesis.
The role of the mTOR pathway in the molecular pathogenesis of focal cortical malformations is most clearly established in TSC. TSC is caused by mutation of either the TSC1 or TSC2 genes, which encode the proteins hamartin and tuberin, respectively (Crino et al., 2006). Hamartin and tuberin bind together to form a complex that normally inhibit the mTOR pathway and consequently prevent excessive cell growth and proliferation. In TSC, mutation of TSC1 or TSC2 leads to a loss or decrease in function of the hamartin-tuberin complex, resulting in disinhibition or hyperactivation of the mTOR pathway, particularly mTORC1 (Fig. 1B). Dysregulated mTORC1 activity can then cause abnormal cell growth and proliferation, which could account for various tumors that develop in TSC, as well as abnormal cellular features of cytomegaly, dysmorphism, and astrogliosis seen in tubers. Direct evidence for the involvement of the mTOR pathway in these cellular processes comes from animal models of TSC. Knock-out mice involving inactivation of the Tsc1 or Tsc2 gene in neurons or glia exhibit some of the neuropathological features of tubers, such as cortical disorganization, enlarged neurons, and astrogliosis (Meikle et al., 2008; Way et al., 2009; Zeng et al., 2008; Zeng et al., 2011). Treatment with the mTORC1 inhibitor, rapamycin, can prevent or reverse some of these cellular and histopathological abnormalities in these mice (Meikle et al., 2008; Zeng et al., 2008; Zeng et al., 2011), indicating that the mTOR pathway is necessary for these phenotypic features related to cortical malformations. Extending these findings to human TSC, the mTOR pathway is strongly implicated in the pathogenesis of tumors in TSC, as mTOR inhibitors can decrease growth of renal and brain tumors in TSC patients and a rapamycin analog has recently been approved for treatment of subependymal giant cell astrocytomas in TSC (Bissler et al., 2008; Krueger et al., 2010). Although cortical tubers in the postnatal brain do not exhibit the same progressive growth and proliferation as tumors in TSC, it is reasonable to extrapolate that mTOR could be involved in pathogenesis of human tubers at an early stage of brain development.
While the primary molecular genetic defects and their direct relationship to the mTOR pathway are well-established in TSC, there is also some evidence that similar mechanisms could be operative in FCD. First of all, although pathogenic genes have not been identified in causing cases of isolated FCD, some studies have suggested a potential genetic link between FCD and the TSC genes. While known disease-causing mutations for TSC have not yet been reported in FCD, an increase in allelic variants or polymorphisms in the TSC1 or TSC2 genes has been found in some series of patients with FCD (Becker et al., 2002; Schonberger et al., 2009), but not in other studies (Gumbinger et al., 2009). Regardless of whether these variations in TSC genes have any influence on the pathogenesis of FCD, there is other evidence for abnormalities of mTOR signaling elements themselves in FCD. Protein expression or subcellular distribution of the TSC gene products, hamartin and tuberin, has been found to be altered in specimens of FCD Type IIB (Grajkowska et al., 2008; Lugnier et al., 2009). Downstream mediators of the mTOR pathway, such as ribosomal S6 and eIF43, are abnormally activated in cytomegalic neurons or balloon cells from FCD tissue, although the specific patterns of activation of various mTOR elements show some differences compared to giant cells from TSC (Baybis et al., 2004; Ljungberg et al., 2006; Miyata et al., 2004; Schick et al., 2007). Unlike in TSC, the pathogenic significance of abnormal mTOR activation in FCD has not been demonstrated. Perhaps the strongest evidence supporting a causative role of mTOR signaling in FCD comes from animal models. A mouse model involving phosphatase and tensin homolog (PTEN) gene inactivation in neurons leads to neuronal hypertrophy and macrocephaly, which has been proposed to represent a model of cortical dysplasia (Ljundberg et al., 2009). PTEN normally inhibits the PI3K-Akt pathway, which is an upstream activator of mTORC1 (Fig. 1A). Thus, similar to TSC, PTEN inactivation leads to disinhibition or hyperactivation of the mTOR pathway (Fig. 1B). Accordingly, mTOR inhibitors have been shown to reverse the neuronal hypertrophy and macrocephaly in PTEN knock-out mice (Kwon et al., 2003; Ljundberg et al., 2009; Zhou et al., 2009), demonstrating the importance of the mTOR pathway in this model of cortical dysplasia. So, overall, there is significant evidence that abnormal mTOR signaling is critical for the pathogenesis of tubers in TSC, and to lesser degree, FCD. However, since the pathogenic mechanisms causing the development of the structural lesions of FCD and TSC are not necessarily the same ones producing seizures, further studies are needed specifically focusing on mechanisms of epileptogenesis in these disorders.
Role of the mTOR Pathway in Epileptogenesis of Focal Cortical Dysplasia and Tuberous Sclerosis
While the pathological features of FCD and tubers are well-described, the mechanisms by which these lesions cause seizures are incompletely understood and several key questions about epileptogenesis in FCD and TSC are often debated (Wong, 2008). First, it is unclear whether seizures start within the lesions themselves or from the normal-appearing regions surrounding the lesions. Surgical resection of FCD or tubers results in seizure-freedom in a majority of carefully-selected cases (Chern et al., 2010; Cohen-Gadol et al., 2004; Madhavan et al., 2007; Tassi et al., 2002; Weiner et al., 2006), suggesting that the lesion is likely the source of the seizures in those cases. On the other hand, a significant proportion of patients continue to have seizures despite a lesionectomy, indicating that the epileptogenic zone was not contained within the lesion. While single-cell recordings have demonstrated abnormal synaptic and electrophysiological properties of neurons within excised FCD and tubers (Cepeda et al., 2010), intracranial electrocorticography studies with subdural grids and depth electrodes in and around tubers indicate that tubers are electrically silent and that epileptiform activity arises from the peri-tuber cortex (Major et al., 2009). Regardless of the site of origin of the seizures relative to the lesion, another issue is whether epileptogenesis involves primarily circuit abnormalities or cellular/molecular defects. Potential circuit-level mechanisms include a loss of GABAergic inhibitory neurons or aberrant excitatory connections within local networks. Cellular or molecular defects promoting epileptogenesis may involve changes in expression of neurotransporter receptors or ion channels. Finally, the relative contribution of different cell populations to epileptogenesis may vary, including not only different types of neurons, but also non-neuronal cells, such as astrocytes, microglia, and vascular endothelial cells.
Despite these uncertainties about mechanisms of epileptogenesis and seizure-generation in FCD and TSC, a potentially unifying principle might involve the identification of a common, central signaling pathway that triggers multiple downstream epileptogenic mechanisms on the circuit, cell, and molecular levels in different cell types. The mTOR pathway is a rational candidate for such a pathway, as mTOR regulates a number of physiological functions, which could potentially influence epileptogenesis under pathological conditions. For example, abnormal cell growth and proliferation due to mTOR hyperactivation could contribute to excessive excitability of neuronal circuits. Through its control of protein synthesis, abnormal mTOR activity could alter expression of neurotransmitter receptors or ion channels. In TSC, there is strong evidence that the mTOR pathway promotes epileptogenesis, at least in animal models. Mice with inactivation of the Tsc1 gene primarily in astrocytes develop epilepsy and premature death associated with progressive glial proliferation, dispersed neuronal lamination, and defects in astrocyte glutamate transport (Uhlmann et al., 2002; Wong et al., 2003). Treatment with rapamycin initiated at an early stage completely prevents the development of epilepsy and the associated cellular and molecular abnormalities promoting epileptogenesis in these mice. Later treatment after the onset of epilepsy can also decrease seizure frequency (Zeng et al., 2008). In another mouse model of TSC, rapamycin can also reverse learning deficits (Ehninger et al., 2008). These results indicate that the mTOR pathway is centrally involved in triggering mechanisms of epileptogenesis and other neurological deficits in TSC and that mTOR inhibitors could have a role in treating epilepsy in TSC, as well as having potential preventive antiepileptogenic or disease-modifying effects. In fact, mTOR inhibitors have been reported to decrease seizures in TSC patients (Kreuger et al., 2010; Muncy et al., 2009), although controlled clinical trials are still needed to verify efficacy more definitively
Much less is known about specific mechanisms of epileptogenesis in FCD, but in theory there could be substantial overlap with TSC. In the PTEN knock-out model, rapamycin has also been shown to decrease seizures, along with the neuronal hypertrophy and macrocephaly (Kwon et al., 2003; Ljundberg et al., 2009; Zhou et al., 2009), indicating the importance of the mTOR pathway in epileptogenesis similar to the TSC models. However, in contrast to TSC, the use of mTOR inhibitors for epilepsy due to FCD in people has not been reported.
Conclusions
The need for more effective therapies for intractable epilepsy caused by focal malformations of cortical development have been recognized for some time, but the scientific and clinical progress for establishing such therapies has been slow. Identification of the mTOR pathway as a potential central feature in the pathophysiology of FCD and TSC may provide a unique opportunity for developing rational strategies for epilepsy related to focal cortical malformations. Abnormal mTOR signaling may account for shared histopathological features seen in these related cortical malformations, such as cytomegaly, cellular dysmorphism, proliferation, and cortical dyslamination. The same or other mechanisms related to altered mTOR-mediated protein synthesis, affecting such processes as ion channel expression and circuit reorganization, may account for epileptogenesis in this group of disorders. Furthermore, mTOR has also been implicated in other related cortical malformations and epilepsy, such as hemimegalencephaly, ganglioglioma, and polyhydramnios, megalencephaly, symptomatic epilepsy (PMSE) syndrome (Aronica et al., 2007; Orlova et al., 2010; Samadani et al., 2007). For example, in PMSE syndrome, a mutation in the STRADA gene, which normally facilitates AMPK pathway inhibition of the mTOR pathway, also leads to mTOR hyperactivation (Fig. 1B; Orlova et al., 2010). Thus, this group of focal cortical malformations has been proposed to constitute a spectrum of “TORopathies” causing epilepsy (Crino, 2007; Wong and Crino, 2011). While the primary genetic defect of TSC and PMSE are well-defined, the molecular genetic etiology of FCD and most of the other related malformations remains to be determined. Based on differential activations patterns of different mTOR pathway elements between these various entities (Baybis et al., 2004; Miyata et al., 2004; Schick et al., 2007), it is likely that other upstream modulators of the mTOR pathway, besides the TSC genes, are affected in these other malformations.
Independent of the initial molecular genetic etiology in each type of focal cortical malformation, if abnormal mTOR pathway activation is a final common pathway, the use of mTOR inhibitors could represent a rational therapy for epilepsy in these group of disorders. Data from animal models provide solid evidence that mTOR inhibitors could be beneficial for epilepsy due to TORopathies. However, while there are some encouraging reports on the effects of mTOR inhibitors on seizures in TSC patients, larger controlled clinical trials are still needed, as well as initial studies in epilepsy due to FCD, before the utility of mTOR inhibitors for these disorders can be established. Furthermore, the specific timing and conditions in which mTOR inhibitors may be effective need to be better delineated. The most common and practical indication for new seizure medications are for patients with established, intractable epilepsy. However, based on the mechanisms of action of mTOR inhibitors, it’s not clear that mTOR inhibitors should be that effective for intractable epilepsy, in which the underlying mechanisms of seizure-generation are established, and perhaps irreversible. It seems more likely that mTOR inhibitors would be more effective as potential antiepileptogenic or disease-modifying therapies in pre- or early-symptomatic patients at risk for epilepsy, by preventing mechanisms of epileptogenesis from being triggered in the first place. While antiepileptogenic drug trials are very difficult to conduct, TSC patients may represent a feasible population to target. Some people are diagnosed with TSC for non-neurological reasons at an early stage prior to seizure onset and yet they are at high risk for developing epilepsy in the future. Other TSC patients present initially with infantile spasms, which usually progress eventually into chronic intractable epilepsy. These groups of TSC patients may represent appropriate candidates for antiepileptogenic therapy. On the other hand, mTOR inhibitors could have significant long-term risks and side effects, such as immunosuppression and impaired growth. As TSC has other indications for mTOR inhibitors, such as tumor treatment, TSC patients also represent the most appropriate population to test for adverse effects of these drugs. If mTOR inhibitors are proven to be safe and effective as either symptomatic or preventative therapy for epilepsy in TSC, this could pave the way for broader applications in other TORopathies.
Highlights.
Focal cortical dysplasia and tuberous sclerosis are common causes of intractable epilepsy
These focal cortical malformations have similar pathological and cellular abnormalities
mTOR pathway signalling may promote the pathogenesis of focal cortical malformations
mTOR pathway signalling may promote epileptogenesis in focal cortical malformations
mTOR inhibitors may be a rational therapy for epilepsy in focal cortical malformations
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aronica E, Boer K, Baybis M, Yu J, Crino P. Co-expression of cyclin D1 and phosphorylated ribosomal S6 proteins in hemimegalencephaly. Acta Neuropathol. 2007;114:287–293. doi: 10.1007/s00401-007-0225-6. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. A developmental and genetic classification for malformations of cortical development. Neurology. 2005;65:1873–1887. doi: 10.1212/01.wnl.0000183747.05269.2d. [DOI] [PubMed] [Google Scholar]
- Bast T, Ramantani G, Seitz A, Rating D. Focal cortical dysplasia: prevalence, clinical presentation and epilepsy in children and adults. Act Neurol. Scand. 2006;113:72–81. doi: 10.1111/j.1600-0404.2005.00555.x. [DOI] [PubMed] [Google Scholar]
- Baybis M, Yu J, Lee A, Golden JA, Weiner H, McKhann G, Aronica E, Crino PB. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann. Neurol. 2004;56:478–487. doi: 10.1002/ana.20211. [DOI] [PubMed] [Google Scholar]
- Becker AJ, Urbach H, Scheffler BJ, Baden T, Normann S, Lahl R, Pennek HW, Tuxhorn I, Elger CE, Schramm J, Wiestler OD, Blumcke I. Focal cortical dysplasia of Taylor’s balloon cell type: mutational analysis of the TSC1 gene indicates a pathogenic relationship to Tuberous Sclerosis. Ann. Neurol. 2002;52:29–37. doi: 10.1002/ana.10251. [DOI] [PubMed] [Google Scholar]
- Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, Salibury S, Franz DN. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumcke I, Vinters HV, Armstrong D, Aronica E, Thom M, Spreafico R. Malformations of cortical development and epilepsies: neuropathological findings on focal cortical dysplasia. Epileptic Disord. 2009;11:181–193. doi: 10.1684/epd.2009.0261. [DOI] [PubMed] [Google Scholar]
- Boer K, Lucassen PJ, Spliet WG, Vreugdenhil E, van Rijen PC, Troost D, Jansen FE, Aronica E. Doublecortin-like (DCL) expression in focal cortical dysplasia and cortical tubers. Epilepsia. 2009;50:2629–2637. doi: 10.1111/j.1528-1167.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Yamazaki I, Hauptman JS, Chen JY, Vinters HV, Mathern GW, Levine MS. Comparative study of cellular and synaptic abnormalities in brain tissue samples from pediatric tuberous sclerosis complex and cortical dysplasia type II. Epilepsia. 2010;51(Suppl.3):166–170. doi: 10.1111/j.1528-1167.2010.02633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chern JJ, Patel AJ, Jea A, Curry DJ, Comair YG. Surgical outcome for focal cortical dysplasia: an analysis of recent surgical series. J. Neurosurg. Pediatrics. 2010;6:452–458. doi: 10.3171/2010.8.PEDS10145. [DOI] [PubMed] [Google Scholar]
- Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–1241. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Gadol AA, Ozduman K, Bronen RA, Kim JH, Spencer DD. Long-term outcome after epilepsy surgery for focal cortical dysplasia. J. Neurosurg. 2004;101:55–65. doi: 10.3171/jns.2004.101.1.0055. [DOI] [PubMed] [Google Scholar]
- Crino PB. Focal brain malformations: a spectrum of disorders along the mTOR cascade. Novartis Found. Symp. 2007;288:260–272. doi: 10.1002/9780470994030.ch18. [DOI] [PubMed] [Google Scholar]
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N. Engl. J. Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shiyansky C, Zhou Y, Li W, Kwaitkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat. Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser S, Becker A, Schulze-Bonhage A, Hildebrandt M, Tuxhorn I, Pannek HW, Lahl R, Schramm J, Blumcke I. CD34-immunoreactive balloon cells in cortical malformations. Acta Neuropathol. 2004;108:272–278. doi: 10.1007/s00401-004-0889-0. [DOI] [PubMed] [Google Scholar]
- Grajkowski W, Kotulska K, Matyja E, Larysz-Brysz M, Mandera M, Roszkowski M, Domanska-Pakilela D, Lewik-Kowalik J, Jozwiak S. Expression of tuberin and hamartin in tuberous sclerosis complex-associated and sporadic cortical dysplasia of Taylor’s balloon cell type. Folia Neuropathol. 2008;46:43–48. [PubMed] [Google Scholar]
- Gumbinger C, Rohsbach CB, Schulze-Bonhage A, Korinthenberg R, Zentner J, Haffner M, Fauser S. Focal cortical dysplasia: a genotype-phenotype analysis of polymorphisms and mutations in the TSC genes. Epilepsia. 2009;50:1396–1408. doi: 10.1111/j.1528-1167.2008.01979.x. [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Thiele EA, Pfeifer HH, McGrogan JR, Freeman JM. Tuberous sclerosis complex and the ketogenic diet. Epilepsia. 2005;46:1684–1686. doi: 10.1111/j.1528-1167.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- Krueger DA, Care MM, Holland K, Agricola K, Tydor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T, Franz DN. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N. Engl. J. Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- Kuzniecky RI. Malformations of cortical development and epilepsy, part 1: diagnosis and classification scheme. Rev. Neurobiol. Dis. 2006;3:151–162. [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Baker SJ. mTOR is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci USA. 2003;100:12923–12928. doi: 10.1073/pnas.2132711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamparello P, Baybis M, Pollard J, Hol EM, Eisenstat DD, Aronica E, Crino PB. Developmental lineage of cell types in cortical dysplasia with balloon cells. Brain. 2007;130:2267–2276. doi: 10.1093/brain/awm175. [DOI] [PubMed] [Google Scholar]
- Lee A, Maldanado M, Baybis M, Walsh CA, Scheithauer B, Yeung R, Parent J, Weiner HL, Crino PB. Markers of cellular proliferation are expressed in cortical tubers. Ann. Neurol. 2003;53:668–673. doi: 10.1002/ana.10579. [DOI] [PubMed] [Google Scholar]
- Ljungberg MC, Bhattacharjee MB, Lu Y, Armstrong DL, Yoshor D, Swann JW, Sheldon M, D’Arcangelo G. Activation of mammalian target of rapamycin in cytomegalic neurons of human cortical dysplasia. Ann. Neurol. 2006;60:420–429. doi: 10.1002/ana.20949. [DOI] [PubMed] [Google Scholar]
- Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D’Arcangelo G. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis. Model. Mech. 2009;2:389–398. doi: 10.1242/dmm.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugnier C, Majores M, Fassunke J, Pernhorst K, Niehusmann P, Simon M, Nellist M, Schoch S, Becker A. Hamartin variants that are frequent in focal dysplasias and cortical tubers have reduced tuberin binding and aberrant subcellular distribution in vitro. J. Neuropathol. Exp. Neurol. 2009;68:1136–1146. doi: 10.1097/NEN.0b013e3181b9a699. [DOI] [PubMed] [Google Scholar]
- Madhavan D, Schaffer S, Yankovsky A, Arzimanoglou A, Renaldo F, Zaorff CM, LaJoie J, Weiner HL, Andermann E, Franz DN, Leonard J, Connolly M, Cascino GD, Devinsky O. Surgical outcome in tuberous sclerosis complex: a multicenter survey. Epilepsia. 2007;48:1625–1628. doi: 10.1111/j.1528-1167.2007.01112.x. [DOI] [PubMed] [Google Scholar]
- Major P, Rakowski S, Simon MV, Cheng ML, Eskandar E, Baron J, Leeman BA, Frosch MP, Thiele EA. Are cortical tubers epileptogenic? Evidence from electrocorticography. Epilepsia. 2009;50:147–154. doi: 10.1111/j.1528-1167.2008.01814.x. [DOI] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J. Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H, Chiang ACY, Vinters HV. Insulin signaling pathways in cortical dysplasia and TSC-tubers: tissue microarray analysis. Ann. Neurol. 2004;56:510–519. doi: 10.1002/ana.20234. [DOI] [PubMed] [Google Scholar]
- Mizuguchi M, Yamanouchi Hl, Becker LE, Itoh M, Takshima S. Doublecortin immunoreactivity in giant cells of tuberous sclerosis and focal cortical dysplasia. Acta Neuropathol. 2002;104:418–424. doi: 10.1007/s00401-002-0575-z. [DOI] [PubMed] [Google Scholar]
- Muncy J, Butler IJ, Koenig MK. Rapamycin reduces seizure frequency in tuberous sclerosis complex. J. Child Neurol. 2009;24:477. doi: 10.1177/0883073808324535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova KA, Parker WE, Heuer GG, Tsai V, Yoon J, Baybis M, Fenning RS, Strauss K, Crino PB. STRADa deficiency results in aberrant mTORC1 signaling during corticogenesis. J. Clin. Invest. 2010;120:1591–1602. doi: 10.1172/JCI41592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, Foldvary-Schaefer N, Jackson G, Luders HO, Prayson R, Spreafico R, Vinters HV. Terminology and classification of cortical dysplasias. Neurology. 2004;62(Suppl 3):S2–S8. doi: 10.1212/01.wnl.0000114507.30388.7e. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Cur. Opin. Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Samadani U, Judkins A, Akpalu A, Aronica E, Crino PB. Differential gene expression in neurons and astrocytes in ganglioglioma. Epilepsia. 2007;48:646–653. doi: 10.1111/j.1528-1167.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- Schick V, Majores M, Engels G, Hartmann W, Elger CE, Schramm J, Schoch S, Becker AJ. Differential Pi3K-pathway activation in cortical tubers and focal cortical dysplasias with balloon cells. Brain Pathol. 2007;17:165–173. doi: 10.1111/j.1750-3639.2007.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberger A, Niehusmann P, Urbach H, Majores M, Grote A, Holthausen H, Blumcke I, Deckert M, Becker AJ. Increased frequency of distinct TSC2 alleleic variants in focal cortical dysplasias with balloon cells and mineralization. Neuropathol. 2009;29:559–565. doi: 10.1111/j.1440-1789.2009.01018.x. [DOI] [PubMed] [Google Scholar]
- Sisodiya SM. Malformations of cortical development: burdens and insights from important causes of human epilepsy. Lancet Neurol. 2004;3:29–38. doi: 10.1016/s1474-4422(03)00620-3. [DOI] [PubMed] [Google Scholar]
- Sparagana SP, Delgado MR, Batchelor LL, Roach ES. Seizure remission and antiepileptic drug discontinuation in children with tuberous sclerosis complex. Arch. Neurol. 2003;60:1286–1289. doi: 10.1001/archneur.60.9.1286. [DOI] [PubMed] [Google Scholar]
- Tassi L, Colombo N, Garbelli R, Francione S, Lo Russo G, Mai R, Cardinale F, Cossu M, Ferrario A, Galli C, Bramerio M, Citterio A, Spreafico R. Focal cortical dysplasia: neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain. 2002;125:1719–1732. doi: 10.1093/brain/awf175. [DOI] [PubMed] [Google Scholar]
- Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada KA, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann. Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- Way SW, McKenna J, 3rd, Mietzsch U, Reith RM, Wu HC, Gambello MJ. Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum. Mol. Genet. 2009;18:1252–1265. doi: 10.1093/hmg/ddp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL, Carlson C, Ridgway EB, Zaroff CM, Miles D, LaJoie J, Devinsky O. Epilepsy surgery in young children with tuberous sclerosis: results of a novel approach. Pediatrics. 2006;117:1494–1502. doi: 10.1542/peds.2005-1206. [DOI] [PubMed] [Google Scholar]
- Wong M. Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development involving abnormal glioneuronal proliferation. Epilepsia. 2008;49:8–21. doi: 10.1111/j.1528-1167.2007.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. Mammalian target of rapamycin (mTOR) inhibition as potential antiepileptogenic therapy: From tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:27–36. doi: 10.1111/j.1528-1167.2009.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Ess KE, Uhlmann EJ, Jansen LA, Li W, Crino PB, Mennerick S, Yamada KA, Gutmann DH. Impaired astrocyte glutamate transport in a mouse epilepsy model of tuberous sclerosis complex. Ann. Neurol. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- Yasin SA, Latak K, Becherini F, Ganapathi A, Miller K, Campos O, Picker SR, Bier N, Smith M, Thom M, Anderson G, Cross JH, Harkness W, Harding B, Jacques TS. Balloon cells in human cortical dysplasia and tuberous sclerosis: isolation of a pathological progenitor-like cell. Acta Neuropathol. 2010;120:85–96. doi: 10.1007/s00401-010-0677-y. [DOI] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Zhang B, Gutmann DH, Gambello MJ, Wong M. Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of Tuberous Sclerosis Complex. Hum. Mol. Genet. 2011;20:445–454. doi: 10.1093/hmg/ddq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann. Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J. Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]