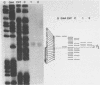
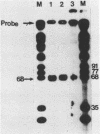
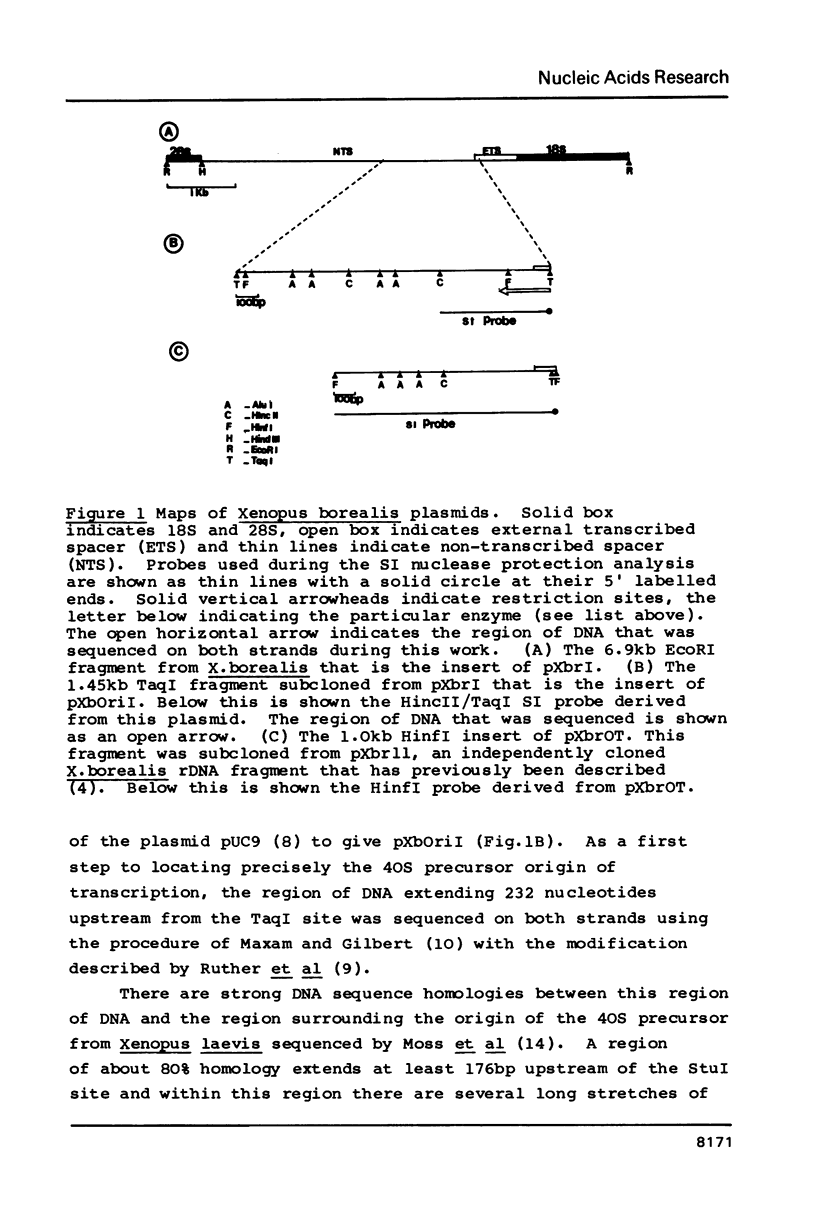
Abstract
We have determined the origin of the major transcript of Xenopus borealis rDNA by the use of an SI nuclease protection assay. The DNA surrounding the origin of this transcript was sequenced, and the region upstream of the origin was shown to have strong sequence homology with that region from X.laevis rDNA. We have also demonstrated faithful transcription from this origin using cloned X.borealis rDNA in an extract derived from X. laevis culture cells. This in vitro transcription was insensitive to 100 micrograms/ml alpha-amanatin, suggesting that it was mediated by RNA polymerase 1.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach R., Allet B., Crippa M. Sequence organization of the spacer in the ribosomal genes of Xenopus clivii and Xenopus borealis. Nucleic Acids Res. 1981 Oct 24;9(20):5311–5330. doi: 10.1093/nar/9.20.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Boseley P., Moss T., Mächler M., Portmann R., Birnstiel M. Sequence organization of the spacer DNA in a ribosomal gene unit of Xenopus laevis. Cell. 1979 May;17(1):19–31. doi: 10.1016/0092-8674(79)90291-5. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Wensink P. C., Jordan E. A comparison of the ribosomal DNA's of Xenopus laevis and Xenopus mulleri: the evolution of tandem genes. J Mol Biol. 1972 Jan 14;63(1):57–73. doi: 10.1016/0022-2836(72)90521-9. [DOI] [PubMed] [Google Scholar]
- Coen E. S., Dover G. A. Multiple Pol I initiation sequences in rDNA spacers of Drosophila melanogaster. Nucleic Acids Res. 1982 Nov 11;10(21):7017–7026. doi: 10.1093/nar/10.21.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsheit A. B., Davidson N., Brown D. D. An electron microscope heteroduplex study of the ribosomal DNAs of Xenopus laevis and Xenopus mulleri. J Mol Biol. 1974 Dec 5;90(2):301–314. doi: 10.1016/0022-2836(74)90375-1. [DOI] [PubMed] [Google Scholar]
- Garoff H., Ansorge W. Improvements of DNA sequencing gels. Anal Biochem. 1981 Aug;115(2):450–457. doi: 10.1016/0003-2697(81)90031-2. [DOI] [PubMed] [Google Scholar]
- Grummt I., Roth E., Paule M. R. Ribosomal RNA transcription in vitro is species specific. Nature. 1982 Mar 11;296(5853):173–174. doi: 10.1038/296173a0. [DOI] [PubMed] [Google Scholar]
- Grummt I. Specific transcription of mouse ribosomal DNA in a cell-free system that mimics control in vivo. Proc Natl Acad Sci U S A. 1981 Feb;78(2):727–731. doi: 10.1073/pnas.78.2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Reeder R. H. Preferential transcription of Xenopus laevis ribosomal RNA in interspecies hybrids between Xenopus laevis and Xenopus mulleri. J Mol Biol. 1973 Oct 25;80(2):217–228. doi: 10.1016/0022-2836(73)90168-x. [DOI] [PubMed] [Google Scholar]
- Kohorn B. D., Rae P. M. Accurate transcription of truncated ribosomal DNA templates in a Drosophila cell-free system. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1501–1505. doi: 10.1073/pnas.79.5.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn B. D., Rae P. M. Nontranscribed spacer sequences promote in vitro transcription of Drosophila ribosomal DNA. Nucleic Acids Res. 1982 Nov 11;10(21):6879–6886. doi: 10.1093/nar/10.21.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Volpe A., Taggart M., McStay B., Bird A. DNaseI-hypersensitive sites at promoter-like sequences in the spacer of Xenopus laevis and Xenopus borealis ribosomal DNA. Nucleic Acids Res. 1983 Aug 25;11(16):5361–5380. doi: 10.1093/nar/11.16.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod D., Bird A. DNAase I sensitivity and methylation of active versus inactive rRNA genes in xenopus species hybrids. Cell. 1982 May;29(1):211–218. doi: 10.1016/0092-8674(82)90105-2. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Synthesis of ribosomal RNA in synkaryons and heterokaryons formed between human and rodent cells. J Cell Sci. 1975 Mar;17(3):307–325. doi: 10.1242/jcs.17.3.307. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Arnheim N. Identification of the in vivo and in vitro origin of transcription in human rDNA. Nucleic Acids Res. 1982 Jul 10;10(13):3933–3949. doi: 10.1093/nar/10.13.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. A., Dev V. G., Tantravahi R., Miller O. J. Suppression of human nucleolus organizer activity in mouse-human somatic hybrid cells. Exp Cell Res. 1976 Sep;101(2):235–243. doi: 10.1016/0014-4827(76)90373-6. [DOI] [PubMed] [Google Scholar]
- Miller J. R., Hayward D. C., Glover D. M. Transcription of the 'non-transcribed' spacer of Drosophila melanogaster rDNA. Nucleic Acids Res. 1983 Jan 11;11(1):11–19. doi: 10.1093/nar/11.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Sollner-Webb B. Transcription of mouse rRNA genes by RNA polymerase I: in vitro and in vivo initiation and processing sites. Cell. 1981 Nov;27(1 Pt 2):165–174. doi: 10.1016/0092-8674(81)90370-6. [DOI] [PubMed] [Google Scholar]
- Miller O. J., Miller D. A., Dev V. G., Tantravahi R., Croce C. M. Expression of human and suppression of mouse nucleolus organizer activity in mouse-human somatic cell hybrids. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4531–4535. doi: 10.1073/pnas.73.12.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. J., Stephens D. L., Mertz J. E. Kinetics of accumulation and processing of simian virus 40 RNA in Xenopus laevis oocytes injected with simian virus 40 DNA. Mol Cell Biol. 1982 Dec;2(12):1581–1594. doi: 10.1128/mcb.2.12.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss T. A transcriptional function for the repetitive ribosomal spacer in Xenopus laevis. Nature. 1983 Mar 17;302(5905):223–228. doi: 10.1038/302223a0. [DOI] [PubMed] [Google Scholar]
- Moss T., Birnstiel M. L. The putative promoter of a Xenopus laevis ribosomal gene is reduplicated. Nucleic Acids Res. 1979 Aug 24;6(12):3733–3743. doi: 10.1093/nar/6.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss T., Boseley P. G., Birnstiel M. L. More ribosomal spacer sequences from Xenopus laevis. Nucleic Acids Res. 1980 Feb 11;8(3):467–485. doi: 10.1093/nar/8.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss T. Transcription of cloned Xenopus laevis ribosomal DNA microinjected into Xenopus oocytes, and the identification of an RNA polymerase I promoter. Cell. 1982 Oct;30(3):835–842. doi: 10.1016/0092-8674(82)90288-4. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Schibler U., Huebner K., Croce C. M. Selective suppression of the transcription of ribosomal genes in mouse-human hybrid cells. J Cell Physiol. 1979 Mar;98(3):553–559. doi: 10.1002/jcp.1040980313. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Wilkinson J., Bakken A., Morgan G., Busby S. J., Roan J., Sollner-Webb B. Evidence for two functional regions in the Xenopus laevis RNA polymerase I promoter. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):867–871. doi: 10.1101/sqb.1983.047.01.099. [DOI] [PubMed] [Google Scholar]
- Rüther U., Koenen M., Otto K., Müller-Hill B. pUR222, a vector for cloning and rapid chemical sequencing of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4087–4098. doi: 10.1093/nar/9.16.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A., de Falco A., Macino G., Boncinelli E. Sequence organization of the ribosomal spacer of D.melanogaster. Nucleic Acids Res. 1982 Dec 20;10(24):8263–8272. doi: 10.1093/nar/10.24.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B., McKnight S. L. Accurate transcription of cloned Xenopus rRNA genes by RNA polymerase I: demonstration by S1 nuclease mapping. Nucleic Acids Res. 1982 Jun 11;10(11):3391–3405. doi: 10.1093/nar/10.11.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]