Abstract
K+ channels with two-pore domain (K2p) form a large family of hyperpolarizing channels. They produce background currents that oppose membrane depolarization and cell excitability. They are involved in cellular mechanisms of apoptosis, vasodilatation, anesthesia, pain, neuroprotection and depression. This review focuses on TREK-1, TREK-2 and TRAAK channels subfamily and on the mechanisms that contribute to their molecular heterogeneity and functional regulations. Not only the number of genes determines their molecular diversity but also by alternative splicing and alternative initiation of translation. These channels are sensitive to a wide array of biophysical parameters that affect their activity such as unsaturated fatty acids, intra- and extracellular pH, membrane stretch, temperature and intracellular signaling pathways. They interact with partner proteins that influence their activity and their plasma membrane expression. Molecular heterogeneity, regulatory mechanisms and protein partners are all expected to contribute to cell specific functions of TREK currents in many tissues.
Key words: background K+ channels, K2p, TREK-TRAAK, molecular regulations
Introduction
Potassium channels form the largest family of ion channels. They are expressed in virtually every cell of the organism. They drive K+ efflux under its electrochemical gradient and as such are important for K+ equilibrium that plays a key role in many cellular processes. They are essential for cell volume regulation and therefore are important factors in cell proliferation, necrosis and apoptosis. K+ channels are also key players in cell excitability. The outward K+ current flowing through these channels hyperpolarizes the plasma membrane to the negative equilibrium potential for K+, usually at −90 mV. Hence K+ channels regulate electrical activity at the plasma membrane opposing depolarization by all other ion channels. Therefore, they are essential regulators for the many cells that use ion channels for signalization. They control neuronal information coding and muscular contraction. They are important regulators of calcium-triggered secretion of neurotransmitters and hormones. The opening of K+ channels in branches of large dendrites lowers membrane resistance, which can shunt the transmission of electrical signals from distant synapses to the cell body. It is clear that K+ channels do not accomplish these functions passively. Intracellular messengers, regulatory partner proteins and biophysical parameters such as pH, temperature and membrane curvature actively regulate them.
There are three major families of K+ channels in mammals, shaker type voltage-gated (Kv), inward rectifier (Kir) and K+) channels with two-pore domains (K2p).1–3 Voltage-gated Kv and calcium-sensitive KCa channels are opened by membrane depolarization and/or intracellular calcium and accelerate repolarization of the membrane. In some circumstances, they facilitate electrical activity of the cells by increasing the speed of membrane repolarization ready for further electrical activity. α-subunits of Kv and KCa channels have six or seven transmembrane domains (TMD) and one pore-forming loop (P). The depolarization of the plasma membrane is sensed by positive charges in TMD4 coupled to the activation gate of the channel. Inwardly rectifying K+ channels have two TMD and one P loop. They are more conductive at negative membrane potential below K+ equilibrium potential than at depolarized membrane potential. The inward rectification is due to the intracellular block of the channel by Mg2+ and polyamines at depolarized potential. Some members of the Kir channel family are leak channels constitutively open at rest, while others like G protein-coupled inward rectifier channels (GIRK) and ATP-sensitive K+ channels (KATP) are gated by intracellular ligands. Kv, KCa and Kir pore forming subunits assemble in tetramers to form functional K+ selective channels. The third family of K+ channels was discovered 15 years ago. TWIK-1 (Tandem of pore domains in a Weak Inward rectifying K+ channels) was the first representative of these K+ channel subunits with two pore domains (K2p).4 This family has 15 members that are subdivided in six distinct subfamilies, TWIK, TREK (TWIK RElated K+ channels), TASK (TWIK related Acid-Sensitive K+ channels), TALK (TWIK related ALkaline pH-activated K+ channels), THIK (Tandem pore domain Halothane Inhibited K+ channels) and TRESK (TWIK RElated Spinal cord K+ channel).5 Besides conserved K+ channel signature sequence T-X-G-X-G in the pore loop, the sequence homology between K2p channels is moderate, usually as low as about 20%, with exception for THIK-1 and THIK-2, TASK-3 and TASK-5 and the TREK and TRAAK subfamilies that have higher sequence homology. All K2p channels have identical topology. Each subunit has two pore-forming loops, P1 and P2, arranged in tandem with four TMDs. A characteristic extracellular loop with a short α-helix extend between TMD1 and P1.6 This TMD1-P1 loop is a coiled-coiled domain promoting dimerization.7,8 This unique topology with two-P loops has given its family name to K2p channels. Subunits arrange as dimers with additional bilateral symmetry such that two P1 and two P2 loops form the K+ selective pore with identical P loops probably facing each other diagonally across the central pore.9–11 Recent studies have demonstrated that TREK and TRAAK channels play a key role in neuroprotection, anesthesia, pain and depression.5,12–16
Heterogeneity of the TREK Channels Subfamily
Gene heterogeneity.
TREK-1 (K2p2.1),6 TREK-2 (K2p10.1),17,18 and TRAAK (TWIK Related Arachidonic acid Activated K+ channel) (K2p4.1),19 compose the TREK subfamily of K2p channels. TREK-1 shares 63% identity and 78% homology with TREK-2. The identity falls to 45% and homology to 69% with TRAAK and to 50–55% homology with the other K2P subunits.17,18 Recent findings have revealed a significant diversity in the TREK channels subfamily with major and unexpected functional implications. The diversity of background K+ currents observed in diverse cell types is determined not only by the number of channel genes but also by alternative splicing and alternative initiation of translation.
Heterogeneity introduced by alternative splicing.
TREK-1, TREK-2 and TRAAK share the same overall gene organization with seven exons encoding the open reading frame.18 Alternative splicing of the first exon has been described for the three genes producing amino-terminal (N-ter) variants of the corresponding channel subunits. Alternative splicing of exon 1 in TREK-1 produces two isoforms of 411 and 426 residues.6,20 The importance of these N-ter variants is still unknown because most biophysical properties and regulatory mechanisms were found identical between both isoforms.21 For TREK-2, three alternative splice variants on the first exon have been described in references 18 and 22. These variants, called hTREK-2a-c, differ significantly in their 5′-UTR and in the coding region for the first 30 amino acids. hTREK-2a is a predicted 538 amino-acid poly-peptide,18 TREK-2b and -2c are 508 amino-acid polypeptides.22 Interestingly, TREK-2b has a PKC phosphorylation site in the N-ter part that is not present in the other two splice variants.22 The human isoforms of TREK-2 share the main channel properties but have distinct expression patterns. TREK-2a is strongly expressed in brain, pancreas and kidney, to a lower level in testis and intestinal smooth muscle.18 TREK-2b is expressed in kidney, primarily in the proximal tubule and pancreas. TREK-2c is mainly expressed in brain.22 For human TRAAK, two isoforms have been reported, a 393 amino-acid long TRAAKa and a 419-amino-acid long TRAAKb with a 26 amino acid N-ter extension.24,25
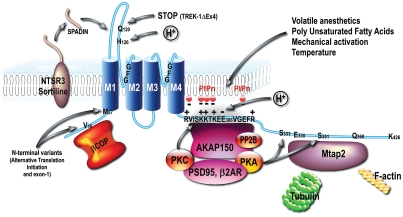
Beside N-ter alternative splice variants, other shorter isoforms of TREK-1 and TRAAK have been reported. TREK-1ΔEx4 is only 141 amino-acid long.26 It retains the cytoplasmic N-ter, the first transmembrane domain and an extracellular tail (Fig. 1). It has no channel activity by itself but its robust expression in rodent brain suggested some functional role. TREK-1ΔEx4 has dominant negative activity. It selectively reduced membrane trafficking of the full-length TREK-1 channel decreasing whole cell current.26 TREK-1ΔEx4 association with TREK-1 might hinder dimerization of the full-length subunits and consecutive association with a protein partner that might prevent trafficking to the plasma membrane.27 The TRAAK counterpart of TREK-1ΔEx4 is TRAAKt (TRAAK truncated).19 TRAAKt is 67 amino acid long, identical to mouse TRAAK from residues 1 to 63, which comprises TMD1 with the first part of the TMD1-P1 extracellular loop. Therefore TRAAKt lacks a P domain and, as might be expected, does not form a functional channel. However, unlike TREK-1ΔEx4, TRAAKt does not seem to alter functional expression of the full-length channel isoform.19 The functional relevance of TRAAKt is still unknown.
Figure 1.
Partners, regulatory domains and alternative variants of TREK-1 channel.
Heterogeneity introduced by alternative translation initiation.
The transcript encoding the 426-residue TREK-1 isoform has a weak natural Kozak sequence that may be skipped by ribosomes because of lower than optimal context of the sequences near the initiation codon AUG. Alternative translation can start from an in-frame AUG codon corresponding to methionine 57. This mechanism of alternative translation initiation produces TREK-1 channel that lacks the first 56 residues (Δ1-56 TREK-1).28 Δ1-56 TREK-1 and full-length TREK-1 are differentially expressed in a regional and developmental manner in the rat central nervous system. Although the amino-terminus is far from the selectivity filter of the channel, some important biophysical properties of TREK-1 are altered in the truncated isoform. The open channel probability of Δ1-56 TREK-1 is reduced thus outward current carried by Δ1-56 TREK-1 is about 5-fold smaller than the current of full-length TREK-1. Significantly, Δ1-56 TREK-1 has reduced ion selectivity for K+ allowing partial Na+ permeation (PNa/PK ∼ 0.18). The Δ1-56 TREK-1 current has a reversal potential near −60 mV in physiological saline which is significantly depolarized compared to −90 mV reversal potential for the K+ selective TREK-1 current (PNa/PK ∼ 0.02). Accordingly, hippocampal neurons transfected with Δ1-56 TREK-1 have depolarized resting membrane potential.28 How the N-ter of TREK-1 controls channel permeability is still unknown but it can be expected that neuronal excitation-thresholds would decrease with relative expression of Δ1-56 TREK-1 in cortex and hypothalamus, whereas almost exclusive expression of full-length TREK-1 in cerebellum and spinal cord would increase excitation-thresholds.
As with TREK-1, alternative translation initiation increases the heterogeneity of TREK-2. Three translation initiation sites on TREK-2 gene produce long, intermediate and short isoforms that are missing the first 54 and 66 amino acids.29 However, unlike TREK-1, the short splice variants of TREK-2 conserve their selectivity for K+ over Na+. N-ter isoforms of TREK-2 have similar regulation properties but different unitary conductances. Full length channels have low unitary conductance near 52 pS, while short isoforms obtained from the second and third initiation sites have large conductances between 185 and 224 pS.29
Expression Patterns of TREK and TRAAK Channels
TREK-1, TREK-2 and TRAAK channels expression has been carefully mapped in rodent and human.22,23,30–32 TREK-1 and TREK-2 expression is particularly high in the embryonic and immature mouse brain. In the adult central nervous system, TREK-1 is mainly expressed in cortex, striatum, hypothalamus and corticolimbic structures including hippocampus and amygdala.6,23,31–33 Mouse and rat TREK-2 expression, corresponding to human TREK-2a and -2c isoforms, is weak in the cortex but stronger in hippocampus, striatum and olfactory bulb.18,22,23,34 TREK-2 has also a strong expression in granular cells layer of the cerebellum. TREK-1 and TREK-2 expression is not restricted to neurons, indeed, cortical astrocytes have TREK-1 and TREK-2 currents that contribute to their characteristic large leak K+ currents.35,36 TRAAK channel expression was deemed to be essentially restricted to the nervous system.19 Its expression is low in the mouse brain embryo and increases after birth with a peak around P7.31 In adult, TRAAK is essentially expressed in cortex, spinal cord and retina.19
TREK and TRAAK channels have a broad distribution in the peripheral nervous system. They have a high level of expression in somatosensory neurons where they are believed to be important for sensory perception.15,16,37 They are found in large and small diameter neurons of dorsal root ganglia and in neurons of trigeminal ganglia that are associated with perception of thermal, mechanical and chemical stimuli.15,16,32,38,39 Recently, the expression of TREK-1 and TRAAK channels has been found in vagal afferent neurons of the nodose ganglia.40 These afferences carry sensory information required for proper internal functions such as cardiac reflex, airway dynamic and gastrointestinal regulation. The distribution of TREK-1 and TRAAK channels in specific populations of nerve sensory afferences suggests that they may be involved in transduction of different perception modalities. Finally, in autonomic nervous system, TREK and TRAAK currents have been characterized in sympathetic superior cervical ganglia.41 These recent findings expand the impact of TREK and TRAAK channels to the entire nervous system.
TREK-1 and TREK-2 channels are also expressed outside the nervous system. TREK-1 is largely expressed in muscle cells of visceral hollow organs in which it increases muscle relaxation by hyperpolarizing the plasma membrane, and contributes to adaptive relaxation.42 It was found in the muscular wall of the bowel, bladder, myometrium and basilar, mesenteric, cutaneous and pulmonary arteries.12,42–47 Interestingly, TREK-1 was not detected in carotid and femoral arteries.46 In the cardiovascular system, it is also expressed in atrial and ventricular cardiomyocytes where it was proposed to play a major role in mechanoelectrical feedback mechanism that regulates myocytes contraction through the myocardium wall.21,48–50 In epithelia, TREK-1 is involved in the different aspects of epithelial-cells physiology that involve K+ homeostasis. TREK-1 was associated with transmembrane ion fluxes, cell volume regulation, membrane polarization and perception of shear stress caused by fluids flowing through vessels. TREK-1 is expressed in vascular endothelium of mesenteric arteries and cutaneous microvessels where it plays a major role in endothelial derived pressure induced vasorelaxation.12,46 It is also expressed in lung, kidney and adrenocortical cells.51,52 In adrenal glands, TREK-1 inactivation leads to membrane depolarization and calcium influx through voltage-activated channels that initiate aldosterone and cortisol secretion.53,54 Recent findings have correlated TREK-1 expression to cell proliferation in prostate cancer, which suggested that TREK-1 could be a molecular target for the treatment of prostate cancer.55
TREK-2 is expressed in spleen, testis, pancreas and kidney. It is found at lower levels in placenta, lung, liver, colon, small intestine and atrium of the heart.17,18,21,22,51,56 TRAAK expression was believed to be restricted to the nervous system. But recently, TRAAK was detected with TREK-1 in human myometrial smooth muscle cells.44,45 Background activity of TRAAK and TREK-1 channels opposes uterus contraction during pregnancy, whereas downregulation of TREK-1 during labor enhances contractility of myometrium smooth-muscle cells.45,57
Regulatory Properties of TREK and TRAAK Channels
TREK and TRAAK pharmacology.
TREK and TRAAK channels are insensitive to the classical K+ channels blockers 4-aminopyridine, tetraethyl ammonium, quinidine or Cs+. They are only inhibited by high concentrations of tetraethyl ammonium and partially inhibited by Ba2+. TREK-1 and TREK-2, but not TRAAK, are opened by clinical concentration of volatile anesthetics isoflurane, halothane and chloroform.58 TREK-1 knockout mice present a decreased sensitivity to volatile anesthetics.14 TREK-1 is also opened by xenon, nitrous oxide and cyclopropane,59 and inhibited by local anesthetics bupivacaine and lidocaine.60,61 TREK and TRAAK channels are activated by riluzole,62 a neuroprotective drug used to protect motoneurons in amyotrophic lateral sclerosis. Selective serotonin reuptake inhibitors (SSRIs) paroxetine and fluoxetine and antipsychotic drug chlorpromazine inhibit TREK-1 and TREK-2.13,63–66
TREK and TRAAK are mechanosensitive channels.
TREK and TRAAK are mechanoactivated channels that sense shear stress and negative membrane pressure.17,64,67,68 They generate progressive and non-inactivating current when negative pressure is applied to the plasma membrane as seen with cell swelling or when the membrane is pulled inside a patch-pipette. It was then proposed that negative curvature of the membrane open TREK and TRAAK channels. Indeed, trinitrophenol, an anionic amphipath crenator that inserts into the outer leaflet of the membrane to generate convex curvature like negative pressure does, opens TREK-1. Conversely, TREK-1 is inhibited by cationic amphipaths such as chlorpromazine and tetracain that insert into the inner leaflet of the membrane to generate concave curvature.64,69,70 Interestingly, mechanosensitivity is preserved in isolated patches of membrane in the inside-out configuration of the patch-clamp technique.17,24,64 This argues that TREK and TRAAK channels are mechanotransducers on their own. The extremity of the fourth transmembrane domain and the initial segment of the carboxy-terminal (C-ter) domain interacting with phospholipids are essential for mechanogating.71–74
Temperature activation of TREK and TRAAK channels.
TREK and TRAAK channels are gradually activated by temperature.16,39,75 TREK and TRAAK background currents increase 7- to 20-fold between 14° and 42°C. This is a significantly higher thermosensitivity for TREK and TRAAK channels than other K2p channels. At temperatures above 42°C, TREK and TRAAK channels activity progressively decreases. The maximal temperature activation of TREK and TRAAK channels is in the range between 30°C and 42°C. Therefore, at physiological body temperature TREK and TRAAK channels are well activated and their control over membrane potential is near optimal.
Partial truncation of TREK-1 intracellular C-ter or swapping of this segment by the corresponding C-ter of TASK-1, a temperature insensitive K2p channel, abolish thermosensitivity.75 This argues that the C-ter of TREK-1 is a major regulatory domain for mechano- and thermo-sensitivities. However, unlike mecha-nosensitivity, thermosensivity of TREK and TRAAK channels requires cell integrity. Indeed, thermo- and mechanosensitivity are conserved in cell-attached patch-clamp configuration (when a small patch of membrane is isolated under a glass patch-pipette and the cell integrity is conserved), but, unlike mechano-sensitivity, thermosensitivity is lost when the patch of membrane is excised in inside-out patch-clamp configuration.39,75 Therefore, it is not clear if thermosensitivity is an inherent property of TREK and TRAAK channels or if it requires a partner protein or such a membrane configuration that is lost in excised patches.
Fatty acid regulation of TREK and TRAAK channels.
TREK and TRAAK channels are gradually and reversibly activated by polyunsaturated fatty acids (PUFA) including arachidonic acid (AA).17–19,64,70 These lipids potentiate TREK and TRAAK background currents 5- to 20-fold. A number of evidences argue for structural specificity and direct effect of PUFA on TREK and TRAAK channels. Indeed, saturated fatty acids and derivatives of PUFA with substitutions of the carboxyl group by hydroxyl or methyl ester groups (AA-OH, AA-ME and DOHAME) have no effect on these channels.19,76 The potency of AA on TREK and TRAAK channels is conserved in the presence of cyclo-oxygenase and lipoxygenase inhibitors indicating that the effect is independent of AA metabolism. Also, AA derivative anandamide does not influence TREK and TRAAK channels activity.76 Finally, activation of TREK and TRAAK by PUFA is conserved in the excised patch-clamp configuration.17,19,64 The regulatory domain in the initial segment of TREK and TRAAK channels cytoplasmic C-ter confers sensitivity to PUFA.64,73 The effect of PUFA on TREK channels has profound physiological implication since TREK-1 was shown to mediate the neuroprotection induced by PUFA.14,77
TREK and TRAAK channels are also activated by lysophospholipids with long hydrophobic acyl chains and large polar heads, such as lypophosphatidylcholine and platelet-activating factor.18,78 However, these lipids do not have a direct effect on TREK and TRAAK channels since they require cell integrity. They are not effective in the excised patch-clamp configuration or when they are applied on the intracellular side of the membrane.
Membrane phospholipids, including phosphatidylinositol-4,5-bisphosphate (PIP2) and phosphatidylserine, are also potent openers of TREK-1 channel.74,79,80 Recently, it has been shown that interaction between the C-ter regulatory domain of TREK-1 and the plasma membrane controls channel activity.66 This association involves interactions between PIP2 which is the most abundant phosphoinositol in the inner leaflet of the membrane and a polybasic motif in the TREK-1 C-ter66,74 (Fig. 1). Conversely, hydrolysis of PIP2 to diacylglycerol by Gq-protein-activated phospholipase C, inhibits TREK-1.74,79–81 This inhibition has been proposed to be due to PIP2 depletion81 and unbinding of the TREK-1 C-ter from the plasma membrane.66 It has also been reported that Gq coupled receptors activation inhibits TREK channels by phosphorylation of a serine located in the proximal part of the C-ter tail via protein kinase C (PKC) activation6,82 (the regulation of TREK channels by G protein coupled receptors is detailed in the next paragraph). Phosphorylation by PKC adds negative charges to the C-ter domain of TREK, which may reduce binding to the plasma membrane and therefore channel activity.
pH regulation of TREK and TRAAK channels.
Internal acidification converts low-activity TREK channels into robust leak conductances that are insensitive to AA, stretch and phosphorylation.71 Alanine scanning of the proximal C-terminal region of TREK-1 led to the identification of the glutamine 306 (E306) as the intracellular proton sensor. Mutation of this glutamine to alanine converts TREK-1 into a constitutively active K+ channel. E306 is located in the region containing five basic residues that have been found to interact with membrane phospholipids (see above) (Fig. 1).
TREK and TRAAK channels are sensitive to variations of the extracellular pH.83,84 Acidification strongly inhibits TREK-1 with an apparent pK of 7.4. The all or none effect of pH variation is steep, and is observed within one pH unit. TREK-2 is not inhibited but activated by acidification within the same range of pH. A single conserved residue, H126 in TREK-1 and H151 in TREK-2, is involved in proton sensing. This histidine is located in the TMD1P1 extracellular loop preceding the first P domain. The differential effect of acidification, i.e., activation for TREK-2 and inhibition for TREK-1, involves other residues located in the P2M4 loop linking the second P domain and the fourth membrane-spanning segment. Attraction or repulsion between the protonated side chain of histidine and closely located negatively or positively charged residues in P2M4 may control outer gating of these channels.83
Regulation of TREK and TRAAK channels by neurotransmitters and hormone receptors.
TREK and TRAAK channels have multiple residues phosphorylated by protein kinase A (PKA) and PKC on their cytoplasmic C-ter.17,19,64 But only TREK-1 and TREK-2 are inhibited by PKA and/or PKC phosphorylation following stimulation of Gs or Gq coupled receptors.5,18,19,64,81,82,85–87 On the contrary, TRAAK is not regulated by these kinases.19,81 For TREK-1, phosphorylation of serine S333 by PKA was shown to be required for subsequent phosphorylation of serine S300 by PKA or PKC,82 which suggests a possible interaction between the two phosphorylation sites with a prominent role for PKA in the regulation of TREK-1 activity. The inhibitory coupling with TREK channels has been demonstrated for Gs or Gq coupled receptors serotonin 5HT4sR, 5HT2bR, PGE2 receptor, muscarinic M3R, OrexinR, angiotensin IIR and glutamate mGluR1 and 5. On the contrary, stimulation of Gi coupled receptors MGluR2 and MGluR4 that diminish cAMP formation and PKA activity enhances TREK current,18,88 which suggests a tonic partial inhibition of TREK channels activity by PKA phosphorylation. Stimulation of TREK-2 channel activity by GABAB and noradrenergic receptor activation has recently been shown to underlie inhibition of neuronal excitability in the entorhinal cortex.89,90
Functional interaction of TREK-1 with actin cytoskeleton.
The actin cytoskeleton inhibits TREK-1 mechano-sensitivity.91 On the other hand, TREK-1 expression has a profound effect on the assembly of the actin network and the formation of filopodia-like structures. The molecular mechanism of the cross talk between TREK-1 and actin is still unknown. However, it has been clearly demonstrated that remodeling of the actin cytoskeleton is independent of channel activity but requires both the PKA phosphorylation site S333 and proton sensor E306 in the intracellular C-ter regulatory domain of TREK-1.91 The reciprocal regulation between TREK-1 and the actin cytoskeleton may associate cell morphology and electrogenesis.
Regulatory Partners of TREK and TRAAK Channels
A proteomic approach based on immunoprecipitation and mass spectrometry analysis of native complexes established the A-kinase-anchoring protein AKAP150 as a constituent of brain TREK-1 channels.92,93 AKAP150 is a scaffolding protein known to organize signaling complexes in neurons.94 AKAP150 brings in close interaction PKA, PKC, protein phosphatase 2B, PDZ-containing synaptic proteins PSD95 and SAP97 as well as synaptic receptors and ion channels (Fig. 1). AKAP150 binds between V298 and R311 in the key regulatory domain of TREK-1.93 AKAP150 also interacts with the highly homologous regulatory domain of TREK-2, but not with TRAAK. Once associated with AKAP150 TREK-1 is fully activated and cannot be further stimulated by AA, acidic pHi or mechanical stress but it is still regulated by protein kinases. AKAP150 binding to TREK-1 increases the kinetic of TREK-1 inactivation by Gs coupled receptors and decreases inhibition by Gq coupled receptors. In neurons, both AKAP150 and TREK-1 have been detected in postsynaptic densities. The large leak K+ current properties of TREK-1 when associated with AKAP150 is expected to affect electrogenesis in the corresponding cell compartments, possibly affecting synaptic transmission and dendritic integration. This interaction may be involved in spatial learning.89
The same proteomic approach identified Microtubule-associated protein 2 (Mtap2, also called MAP2) as another partner of TREK-1 and TREK-2.27 Mtap2 is a scaffolding protein for the localization of signaling complexes in dendrites, particularly near spines. Mtap2 is also present in growth cones. Mtap2 binding site encompass residues 335 to 360 in TREK-1, within the intracellular C-ter. This region includes an eight amino acid segment (residues 342–349) that contains four positively charged residues (K342, K347, R348, K349) that are essential for Mtap2 binding. These residues are conserved in TREK-2 but not TRAAK channels, and accordingly Mtap2 binds TREK-2 but not TRAAK. Therefore, Mtap2 and AKAP150 interacting sites in TREK channels are distinct and both proteins can dock simultaneously.27 Mtap2 does not change TREK-1 channel gating properties but increases channel density at the plasma membrane. The simultaneous binding of Mtap2 to TREK-1 and tubulin is required for this effect. The effects of Mtap2 on TREK-1 trafficking and AKAP150 on TREK-1 gating are additive. Altogether, Mtap2 and AKAP150 place TREK-1 at the center of dynamic protein complexes that comprise cytoskeleton elements, regulatory proteins, protein kinases, ion channels and neurotransmitters receptors.
A subunit of coatomer protein complex 1 (COP1), β-COP was identified as a direct interacting partner of TREK-1 in a yeast two-hybrid screen on human brain cDNA library using the N-ter region of TREK-1 as bait.95 It is not known if β-COP interacts with TREK-2 and TRAAK, although this would not be predicted by the low conservation of the N-ter sequences. The COP1 complex is involved in the formation of coated vesicles from the Golgi that mediate the anterograde and retrograde transport of membrane proteins, including K2P channels TASK-1 and TASK-3.96–98 The binding of β-COP to TASK channels occurs in the Golgi and leads to accumulation of channels in the endoplasmic reticulum (ER) and decreased surface expression.97 The interaction between TREK-1 and β-COP differs from that of TASK channels. Indeed, TREK-1 and β-COP are colocalized at the membrane. Expression of β-COP increases TREK-1 surface expression and current density.95 This argues that β-COP enhances anterograde transport of TREK-1 to the membrane. How TREK-1 trafficking to the membrane by β-COP and Mtap2 that binds to the C-ter interplay is not clear. Masking of an ER retention signal by β-COP or Mtap2, as was shown between 14-3-3 and β-COP for TASK channels,96,97 is unlikely because both partners increase TREK-1 channel trafficking to the membrane. However, β-COP interaction with TREK-1 may have major functional implication because of the important functional diversity of the recently discovered TREK-1 N-ter isoforms.26,28
Another partner involved in TREK-1 trafficking is the neurotensin receptor 3 (NTSR3), also called gp95/sortilin.99 NTSR3/sortilin is essentially localized in the Golgi apparatus where it is involved in intracellular protein trafficking. NTSR3 is also found at the plasma membrane where it is a receptor or a co-receptor. For example, NTSR3 associates with p75NTR to form a proNGF receptor that is involved in neuronal cell apoptosis.100 NTSR3/sortilin directly binds TREK-1 in the Trans Golgi Network. This association enhances TREK-1 trafficking to the plasma membrane and TREK-1 current density.99 However, regulation of TREK-1 membrane expression by the NTSR3/sortilin signaling path is bidirectional. Indeed, post-translational maturation process on the extracellular N-ter domain of NTSR3 releases a 44-amino-acid polypeptide in the circulation. This polypeptide directly binds to TREK-1-NTSR3/sortilin complex to trigger TREK-1 endocytosis.99 A polypeptide derived from a short variant of NTSR3, called Spadin, injected in mice was shown to reproduce the depression-resistant phenotype of TREK-1 knockout mice.13,99 For this reason spadin has a very interesting therapeutic potential.
Conclusion
Because of a lack of specific pharmacology, gene inactivation in the mouse is the principal approach to study the physiological roles of TREK channels. Phenotyping of TREK knockout mice is revealing the implication of these channels in functions as diverse as vasodilatation, general anesthesia, neuroprotection, depression and pain perception. As described in this review, TREK channels activity is under the control of many different mechanisms acting either on channel trafficking and surface density or directly on gating properties. Another level of TREK channel regulation is linked to the generation of numerous variants produced by alternative splicing and alternative translation initiation. All these mechanisms are expected to contribute to the functional diversity of background TREK currents and functions.
Acknowledgments
We thank Drs. Eric LINGUEGLIA, Emmanuel DEVAL and Pr. Michel LAZDUNSKI for helpful discussion. We thank the Agence Nationale de la Recherche, the Société Française d'Etude et de Traitement de la Douleur, the Fondation pour la Recherche sur le Cerveau, the Fondation pour la Recherche Médicale and the Philippe Fondation for financial support.
References
- 1.Kubo Y, Adelman JP, Clapham DE, Jan LY, Karschin A, Kurachi Y, et al. International Union of Pharmacology LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol Rev. 2005;57:509–526. doi: 10.1124/pr.57.4.11. [DOI] [PubMed] [Google Scholar]
- 2.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein SA, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International Union of Pharmacology LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol Rev. 2005;57:527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- 4.Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- 5.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 6.Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- 7.Lesage F, Reyes R, Fink M, Duprat F, Guillemare E, Lazdunski M. Dimerization of TWIK-1 K+ channel subunits via a disulfide bridge. EMBO J. 1996;15:6400–6407. [PMC free article] [PubMed] [Google Scholar]
- 8.Lesage F, Reyes R, Lazdunski M, Barhanin J. Leak K+ channels with two pore domains. Kidney and Blood Pressure Research. 2001;24:402. [Google Scholar]
- 9.Treptow W, Klein ML. The membrane-bound state of K2P potassium channels. J Am Chem Soc. 2010;132:8145–8151. doi: 10.1021/ja102191s. [DOI] [PubMed] [Google Scholar]
- 10.Kollewe A, Lau AY, Sullivan A, Roux B, Goldstein SA. A structural model for K2P potassium channels based on 23 pairs of interacting sites and continuum electrostatics. J Gen Physiol. 2009;134:53–68. doi: 10.1085/jgp.200910235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milac A, Anishkin A, Fatakia SN, Chow CC, Sukharev S, Guy HR. Structural models of TREK channels and their gating mechanism. Channels (Austin) 2011;5:23–33. doi: 10.4161/chan.5.1.13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garry A, Fromy B, Blondeau N, Henrion D, Brau F, Gounon P, et al. Altered acetylcholine, bradykinin and cutaneous pressure-induced vasodilation in mice lacking the TREK1 potassium channel: the endothelial link. EMBO Rep. 2007;8:354–359. doi: 10.1038/sj.embor.7400916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- 14.Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alloui A, Zimmermann K, Mamet J, Duprat F, Noel J, Chemin J, et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006;25:2368–2376. doi: 10.1038/sj.emboj.7601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009;28:1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- 18.Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi and Gq protein-coupled receptors. J Biol Chem. 2000;275:28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- 19.Fink M, Lesage F, Duprat F, Heurteaux C, Reyes R, Fosset M, Lazdunski M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bockenhauer D, Zilberberg N, Goldstein SA. KCNK2: reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat Neurosci. 2001;4:486–491. doi: 10.1038/87434. [DOI] [PubMed] [Google Scholar]
- 21.Xian Tao L, Dyachenko V, Zuzarte M, Putzke C, Preisig-Muller R, Isenberg G, Daut J. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc Res. 2006;69:86–97. doi: 10.1016/j.cardiores.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Gu W, Schlichthorl G, Hirsch JR, Engels H, Karschin C, Karschin A, et al. Expression pattern and functional characteristics of two novel splice variants of the two-pore-domain potassium channel TREK-2. J Physiol. 2002;539:657–668. doi: 10.1113/jphysiol.2001.013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesage F, Maingret F, Lazdunski M. Cloning and expression of human TRAAK, a polyunsaturated fatty acids-activated and mechano-sensitive channel. K+ FEBS Lett. 2000;471:137–140. doi: 10.1016/s0014-5793(00)01388-0. [DOI] [PubMed] [Google Scholar]
- 25.Ozaita A, Vega-Saenz d, Miera E. Cloning of two transcripts, HKT4.1a and HKT4.1b, from the human two-pore K+ channel gene KCNK4. Chromosomal localization, tissue distribution and functional expression. Brain Res Mol Brain Res. 2002;102:18–27. doi: 10.1016/s0169-328x(02)00157-2. [DOI] [PubMed] [Google Scholar]
- 26.Veale EL, Rees KA, Mathie A, Trapp S. Dominant negative effects of a non-conducting TREK1 splice variant expressed in brain. J Biol Chem. 2010;285:29295–29304. doi: 10.1074/jbc.M110.108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandoz G, Tardy MP, Thummler S, Feliciangeli S, Lazdunski M, Lesage F. Mtap2 is a constituent of the protein network that regulates twik-related K+ channel expression and trafficking. J Neurosci. 2008;28:8545–8552. doi: 10.1523/JNEUROSCI.1962-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas D, Plant LD, Wilkens CM, McCrossan ZA, Goldstein SA. Alternative translation initiation in rat brain yields K2P2.1 potassium channels permeable to sodium. Neuron. 2008;58:859–870. doi: 10.1016/j.neuron.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simkin D, Cavanaugh EJ, Kim D. Control of the single channel conductance of K2P10.1 (TREK-2) by the amino-terminus: role of alternative translation initiation. J Physiol. 2008;586:5651–5663. doi: 10.1113/jphysiol.2008.161927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hervieu GJ, Cluderay JE, Gray CW, Green PJ, Ranson JL, Randall AD, Meadows HJ. Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience. 2001;103:899–919. doi: 10.1016/s0306-4522(01)00030-6. [DOI] [PubMed] [Google Scholar]
- 31.Aller MI, Wisden W. Changes in expression of some two-pore domain potassium channel genes (KCNK) in selected brain regions of developing mice. Neuroscience. 2008;151:1154–1172. doi: 10.1016/j.neuroscience.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, et al. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res Mol Brain Res. 2001;86:101–114. doi: 10.1016/s0169-328x(00)00263-1. [DOI] [PubMed] [Google Scholar]
- 33.Meadows HJ, Benham CD, Cairns W, Gloger I, Jennings C, Medhurst AD, et al. Cloning, localisation and functional expression of the human orthologue of the TREK-1 potassium channel. Pflugers Arch. 2000;439:714–722. doi: 10.1007/s004249900235. [DOI] [PubMed] [Google Scholar]
- 34.Han J, Truell J, Gnatenco C, Kim D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol. 2002;542:431–444. doi: 10.1113/jphysiol.2002.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gnatenco C, Han J, Snyder AK, Kim D. Functional expression of TREK-2 K+ channel in cultured rat brain astrocytes. Brain Res. 2002;931:56–67. doi: 10.1016/s0006-8993(02)02261-8. [DOI] [PubMed] [Google Scholar]
- 36.Zhou M, Xu G, Xie M, Zhang X, Schools GP, Ma L, et al. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J Neurosci. 2009;29:8551–8564. doi: 10.1523/JNEUROSCI.5784-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Descoeur J, Pereira V, Pizzoccaro A, Francois A, Ling B, Maffre V, et al. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med. 2011;3:266–278. doi: 10.1002/emmm.201100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang D, Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol. 2006;291:138–146. doi: 10.1152/ajpcell.00629.2005. [DOI] [PubMed] [Google Scholar]
- 39.Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 2005;564:103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao H, Sprunger LK, Simasko SM. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am J Physiol Gastrointest Liver Physiol. 2010;298:212–221. doi: 10.1152/ajpgi.00396.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cadaveira-Mosquera A, Ribeiro SJ, Reboreda A, Perez M, Lamas JA. Activation of TREK currents by the neuroprotective agent riluzole in mouse sympathetic neurons. J Neurosci. 2011;31:1375–1385. doi: 10.1523/JNEUROSCI.2791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanders KM, Koh SD. Two-pore-domain potassium channels in smooth muscles: new components of myogenic regulation. J Physiol. 2006;570:37–43. doi: 10.1113/jphysiol.2005.098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker SA, Hennig GW, Han J, Britton FC, Smith TK, Koh SD. Methionine and its derivatives increase bladder excitability by inhibiting stretch-dependent K+ channels. Br J Pharmacol. 2008;153:1259–1271. doi: 10.1038/sj.bjp.0707690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tichenor JN, Hansen ET, Buxton IL. Expression of stretch-activated potassium channels in human myometrium. Proc West Pharmacol Soc. 2005;48:44–48. [PubMed] [Google Scholar]
- 45.Buxton IL, Singer CA, Tichenor JN. Expression of stretch-activated two-pore potassium channels in human myometrium in pregnancy and labor. PLoS One. 2010;5:12372. doi: 10.1371/journal.pone.0012372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blondeau N, Petrault O, Manta S, Giordanengo V, Gounon P, Bordet R, et al. Polyunsaturated fatty acids are cerebral vasodilators via the TREK-1 potassium channel. Circ Res. 2007;101:176–184. doi: 10.1161/CIRCRESAHA.107.154443. [DOI] [PubMed] [Google Scholar]
- 47.Gardener MJ, Johnson IT, Burnham MP, Edwards G, Heagerty AM, Weston AH. Functional evidence of a role for two-pore domain potassium channels in rat mesenteric and pulmonary arteries. Br J Pharmacol. 2004;142:192–202. doi: 10.1038/sj.bjp.0705691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terrenoire C, Lauritzen I, Lesage F, Romey G, Lazdunski M. A TREK-1-like potassium channel in atrial cells inhibited by beta-adrenergic stimulation and activated by volatile anesthetics. Circ Res. 2001;89:336–342. doi: 10.1161/hh1601.094979. [DOI] [PubMed] [Google Scholar]
- 49.Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. Eur Biophys J. 2009;38:305–318. doi: 10.1007/s00249-008-0326-8. [DOI] [PubMed] [Google Scholar]
- 50.Kelly D, Mackenzie L, Hunter P, Smaill B, Saint DA. Gene expression of stretch-activated channels and mechanoelectric feedback in the heart. Clin Exp Pharmacol Physiol. 2006;33:642–648. doi: 10.1111/j.1440-1681.2006.04392.x. [DOI] [PubMed] [Google Scholar]
- 51.Inglis SK, Brown SG, Constable MJ, McTavish N, Olver RE, Wilson SM. A Ba2+-resistant, acid-sensitive K+ conductance in Na+-absorbing H441 human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:1304–1312. doi: 10.1152/ajplung.00424.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enyeart JJ, Xu L, Danthi S, Enyeart JA. An ACTH- and ATP-regulated background K+ channel in adrenocortical cells is TREK-1. J Biol Chem. 2002;277:49186–49199. doi: 10.1074/jbc.M207233200. [DOI] [PubMed] [Google Scholar]
- 53.Enyeart JA, Danthi SJ, Enyeart JJ. TREK-1 K+ channels couple angiotensin II receptors to membrane depolarization and aldosterone secretion in bovine adrenal glomerulosa cells. Am J Physiol Endocrinol Metab. 2004;287:1154–1165. doi: 10.1152/ajpendo.00223.2004. [DOI] [PubMed] [Google Scholar]
- 54.Brenner T, O'Shaughnessy KM. Both TASK-3 and TREK-1 two-pore loop K channels are expressed in H295R cells and modulate their membrane potential and aldosterone secretion. Am J Physiol Endocrinol Metab. 2008;295:1480–1486. doi: 10.1152/ajpendo.90652.2008. [DOI] [PubMed] [Google Scholar]
- 55.Voloshyna I, Besana A, Castillo M, Matos T, Weinstein IB, Mansukhani M, et al. TREK-1 is a novel molecular target in prostate cancer. Cancer Res. 2008;68:1197–1203. doi: 10.1158/0008-5472.CAN-07-5163. [DOI] [PubMed] [Google Scholar]
- 56.Zhang H, Shepherd N, Creazzo TL. Temperature-sensitive TREK currents contribute to setting the resting membrane potential in embryonic atrial myocytes. J Physiol. 2008;586:3645–3656. doi: 10.1113/jphysiol.2008.153395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mongahan K, Baker SA, Dwyer L, Hatton WC, Park KS, Sanders KM, Koh SD. The stretch-dependent potassium conductance (TREK-1)and its function in murine myometrium. J Physiol. 2011;589:1221–1233. doi: 10.1113/jphysiol.2010.203869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- 59.Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide and cyclopropane. Mol Pharmacol. 2004;65:443–452. doi: 10.1124/mol.65.2.443. [DOI] [PubMed] [Google Scholar]
- 60.Punke MA, Licher T, Pongs O, Friederich P. Inhibition of human TREK-1 channels by bupivacaine. Anesth Analg. 2003;96:1665–1673. doi: 10.1213/01.ANE.0000062524.90936.1F. [DOI] [PubMed] [Google Scholar]
- 61.Nayak TK, Harinath S, Nama S, Somasundaram K, Sikdar SK. Inhibition of human two-pore domain K+ channel TREK1 by local anesthetic lidocaine: negative cooperativity and half-of-sites saturation kinetics. Mol Pharmacol. 2009;76:903–917. doi: 10.1124/mol.109.056838. [DOI] [PubMed] [Google Scholar]
- 62.Duprat F, Lesage F, Patel AJ, Fink M, Romey G, Lazdunski M. The neuroprotective agent riluzole activates the two P domain K+ channels TREK-1 and TRAAK. Mol Pharmacol. 2000;57:906–912. [PubMed] [Google Scholar]
- 63.Kennard LE, Chumbley JR, Ranatunga KM, Armstrong SJ, Veale EL, Mathie A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br J Pharmacol. 2005;144:821–829. doi: 10.1038/sj.bjp.0706068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thummler S, Duprat F, Lazdunski M. Antipsychotics inhibit TREK but not TRAAK channels. Biochem Biophys Res Commun. 2007;354:284–289. doi: 10.1016/j.bbrc.2006.12.199. [DOI] [PubMed] [Google Scholar]
- 66.Sandoz G, Bell SC, Isacoff EY. Optical probing of a dynamic membrane interaction that regulates the TREK1 channel. Proc Natl Acad Sci USA. 2011;108:2605–2610. doi: 10.1073/pnas.1015788108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 68.Honore E, Patel AJ, Chemin J, Suchyna T, Sachs F. Desensitization of mechano-gated K2P channels. Proc Natl Acad Sci USA. 2006;103:6859–6864. doi: 10.1073/pnas.0600463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maingret F, Fosset M, Lesage F, Lazdunski M, Honore E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem. 1999;274:1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- 70.Patel AJ, Lazdunski M, Honore E. Lipid and mechanogated 2P domain K+ channels. Curr Opin Cell Biol. 2001;13:422–428. doi: 10.1016/s0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 71.Honore E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim Y, Bang H, Gnatenco C, Kim D. Synergistic interaction and the role of C-terminus in the activation of TRAAK K+ channels by pressure, free fatty acids and alkali. Pflugers Arch. 2001;442:64–72. doi: 10.1007/s004240000496. [DOI] [PubMed] [Google Scholar]
- 73.Kim Y, Gnatenco C, Bang H, Kim D. Localization of TREK-2 K+ channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pHi. Pflugers Arch. 2001;442:952–960. doi: 10.1007/s004240100626. [DOI] [PubMed] [Google Scholar]
- 74.Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honore E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 2005;24:44–53. doi: 10.1038/sj.emboj.7600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, et al. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J. 2000;19:1784–1793. doi: 10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J Biol Chem. 2000;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- 79.Chemin J, Patel AJ, Duprat F, Sachs F, Lazdunski M, Honore E. Up and downregulation of the mechano-gated K(2P) channel TREK-1 by PIP (2) and other membrane phospholipids. Pflugers Arch. 2007;455:97–103. doi: 10.1007/s00424-007-0250-2. [DOI] [PubMed] [Google Scholar]
- 80.Lopes CM, Rohacs T, Czirjak G, Balla T, Enyedi P, Logothetis DE. PIP2 hydrolysis underlies agonist-induced inhibition and regulates voltage gating of two-pore domain K+ channels. J Physiol. 2005;564:117–129. doi: 10.1113/jphysiol.2004.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chemin J, Girard C, Duprat F, Lesage F, Romey G, Lazdunski M. Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. EMBO J. 2003;22:5403–5411. doi: 10.1093/emboj/cdg528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murbartian J, Lei Q, Sando JJ, Bayliss DA. Sequential phosphorylation mediates receptor- and kinase-induced inhibition of TREK-1 background potassium channels. J Biol Chem. 2005;280:30175–30184. doi: 10.1074/jbc.M503862200. [DOI] [PubMed] [Google Scholar]
- 83.Sandoz G, Douguet D, Chatelain F, Lazdunski M, Lesage F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc Natl Acad Sci USA. 2009;106:14628–14633. doi: 10.1073/pnas.0906267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cohen A, Ben-Abu Y, Hen S, Zilberberg N. A novel mechanism for human K2P2.1 channel gating. Facilitation of C-type gating by protonation of extracellular histidine residues. J Biol Chem. 2008;283:19448–19455. doi: 10.1074/jbc.M801273200. [DOI] [PubMed] [Google Scholar]
- 85.Enyeart JJ, Danthi SJ, Liu H, Enyeart JA. Angiotensin II inhibits bTREK-1 K+ channels in adrenocortical cells by separate Ca2+- and ATP hydrolysis-dependent mechanisms. J Biol Chem. 2005;280:30814–30828. doi: 10.1074/jbc.M504283200. [DOI] [PubMed] [Google Scholar]
- 86.Kang D, Han J, Kim D. Mechanism of inhibition of TREK-2 (K2P10.1) by the Gq-coupled M3 muscarinic receptor. Am J Physiol Cell Physiol. 2006;291:649–656. doi: 10.1152/ajpcell.00047.2006. [DOI] [PubMed] [Google Scholar]
- 87.Mathie A. Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol. 2007;578:377–385. doi: 10.1113/jphysiol.2006.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cain SM, Meadows HJ, Dunlop J, Bushell TJ. mGlu4 potentiation of K(2P)2.1 is dependant on C-terminal dephosphorylation. Mol Cell Neurosci. 2008;37:32–39. doi: 10.1016/j.mcn.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 89.Deng PY, Xiao Z, Yang C, Rojanathammanee L, Grisanti L, Watt J, et al. GABA(B) receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK-2 K+ channels. Neuron. 2009;63:230–243. doi: 10.1016/j.neuron.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xiao Z, Deng PY, Rojanathammanee L, Yang C, Grisanti L, Permpoonputtana K, et al. Noradrenergic depression of neuronal excitability in the entorhinal cortex via activation of TREK-2 K+ channels. J Biol Chem. 2009;284:10980–10991. doi: 10.1074/jbc.M806760200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lauritzen I, Chemin J, Honore E, Jodar M, Guy N, Lazdunski M, Jane Patel A. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005;6:642–648. doi: 10.1038/sj.embor.7400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sandoz G, Lesage F. Protein complex analysis of native brain potassium channels by proteomics. Methods Mol Biol. 2008;491:113–123. doi: 10.1007/978-1-59745-526-8_9. [DOI] [PubMed] [Google Scholar]
- 93.Sandoz G, Thummler S, Duprat F, Feliciangeli S, Vinh J, Escoubas P, et al. AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K+ channels into open leak channels. EMBO J. 2006;25:5864–5872. doi: 10.1038/sj.emboj.7601437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Welch EJ, Jones BW, Scott JD. Networking with AKAPs: context-dependent regulation of anchored enzymes. Mol Interv. 2010;10:86–97. doi: 10.1124/mi.10.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim E, Hwang EM, Yarishkin O, Yoo JC, Kim D, Park N, et al. Enhancement of TREK1 channel surface expression by protein-protein interaction with beta-COP. Biochem Biophys Res Commun. 2010;395:244–250. doi: 10.1016/j.bbrc.2010.03.171. [DOI] [PubMed] [Google Scholar]
- 96.Zuzarte M, Heusser K, Renigunta V, Schlichthorl G, Rinne S, Wischmeyer E, et al. Intracellular traffic of the K+ channels TASK-1 and TASK-3: role of N- and C-terminal sorting signals and interaction with 14-3-3 proteins. J Physiol. 2009;587:929–952. doi: 10.1113/jphysiol.2008.164756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O'Kelly I, Butler MH, Zilberberg N, Goldstein SA. Forward transport. 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell. 2002;111:577–588. doi: 10.1016/s0092-8674(02)01040-1. [DOI] [PubMed] [Google Scholar]
- 98.Mathie A, Rees KA, El Hachmane MF, Veale EL. Trafficking of neuronal two pore domain potassium channels. Curr Neuropharmacol. 2010;8:276–286. doi: 10.2174/157015910792246146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mazella J, Petrault O, Lucas G, Deval E, Beraud-Dufour S, Gandin C, et al. Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: a new concept in the antidepressant drug design. PLoS Biol. 2010;8:1000355. doi: 10.1371/journal.pbio.1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]