Abstract
Despite significant progress in our understanding of the cellular and molecular mechanisms underlying sensory transduction and nociception, clinical pain management remains a considerable challenge in health care and basic research. The identification of the superfamily of transient receptor potential (TRP) cation channels, particularly TRPV1 and TRPA1, has shed light on the molecular basis of pain signaling during inflammatory conditions. TRPV1 and TRPA1 are considered as potential targets in the treatment of inflammatory pain because of their ability to be activated by nociceptive signals and sensitized by pro-inflammatory mediators. Notably, TRPA1 is expressed in visceral afferent neurons and is known to participate in inflammatory responses and the establishment of hypersensitivity. This review summarizes the current knowledge of the role of TRPA1 in sensory transduction, particularly in the context of visceral inflammation and pain in the gastrointestinal and urinary tracts.
Key words: transient receptor potential ankyrin 1, inflammation, dorsal root ganglia, hypersensitivity, visceral pain, gastrointestinal tract, therapeutics
According to the World Health Organization, over 20% of the world population has experienced some degree of chronic pain.1 Pain is the most important symptom in terms of prevalence and potential personal/economic consequences; in the USA only, the cost of chronic pain exceeds US$210 billion annually.2 Despite significant advances in our understanding of the mechanisms underlying sensory transduction and nociception, the efficacy of therapeutic approaches remains variable and clinical improvements are modest. The discovery and characterization of ion channels expressed in primary afferent neurons and their involvement in nociception has provided new potential targets for the management of clinical pain syndromes.
Nicolas Jancsó was the first to report that the vanilloid capsaicin, the chemical responsible for the piquancy of hot pepper, acted on nociceptive afferent neurons (nociceptors) to induce pain.3 Almost 40 years after these preliminary observations, the ion channel transient receptor potential (TRP) vanilloid 1 was identified as the molecular sensor for capsaicin.4 TRPV1 is highly expressed in nociceptors and can be activated by a wide range of noxious stimuli; while protons (H+), heat, pressure or lipids directly activate TRPV1,5,6 pro-inflammatory mediators such as serotonin, bradykinin, histamine, proteases, chemokines or nerve growth factors indirectly sensitize the channel by lowering its activation threshold.7,8 Since the discovery of TRPV1, 28 different TRP subunit genes have been identified, and their products classified into three TRP subfamilies: vanilloid TRPs (TRPVs), melastatin TRPs (TRPMs) and ankyrin TRPs (TRPAs) (reviewed in ref. 9). Over the last decade, a growing body of evidence has suggested that TRP channels, particularly TRPV1, could play a role in the establishment of inflammation and pain. This is supported by the observation that TRPV1 knockout mice show impaired response to heat and reduced thermal hyperalgesia during inflammation.10,11 More recently, another member of the TRP channel family, TRPA1, has also emerged as an important player in these neurological processes. The focus of this review is to provide a better understanding of the role of TRPA1 in sensory transduction, particularly in the context of visceral inflammation and pain.
TRPA1: Structure, Distribution and Regulation
TRPA1, formerly referred to as ANKTM1, was originally identified and cloned by Jaquemar and colleagues in 1999.12 In their study, the authors described a transformation-sensitive mRNA present in fibroblasts, which encoded a transmembranous TRP-like protein supporting several ankyrin-like domains.12 It was later established that the mammalian TRPA1 gene is orthologous to the nociception gene painless in Drosophila melanogaster, thus suggesting a conserved role for TRPA1 in sensory functions in humans.13,14
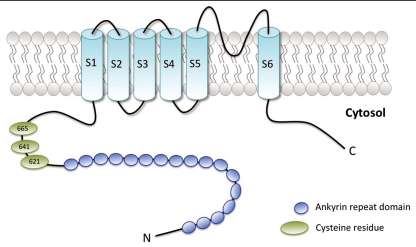
TRPA1, is composed of six putative transmembrane regions (S1–S6), flanked by cytosolic C- and N-terminal tails with several amino-terminal ankyrin repeats (Fig. 1). In its functional configuration, TRPA1 forms tetramers via the interaction of the cation-permeable pore regions located between the fifth and sixth transmembrane domains of the channel subunits.
Figure 1.
Schematic representation of human TRPA1. Each subunit is composed of six membrane-spanning domains (S1–S6) flanked by cytosolic C- and N-terminal domains. The amino-terminal tail supports several ankyrin repeat domains (blue circles). The pore region is located between the fifth and sixth transmembrane domains of each channel subunit. Green ovals represent cysteine residues identified as essential for covalent activation of TRPA1.
TRPA1 is mainly expressed in small-diameter peptidergic nociceptors of the dorsal root (DRG), nodose and trigeminal ganglia, along with TRPV1.15,16 Recently, TRPA1 expression was also observed in colonic myenteric neurons, where it is believed to modulate spontaneous colonic functions.17 Although TRPA1 has been characterized and studied mainly in the nervous system, its expression was also reported in non-neuronal tissues such as skeletal muscle, lung, small intestine, colon and pancreas.18
TRPA1 is activated by a wide spectrum of chemical and mechanical stimuli; TRPA1 is known to respond to dietary irritants such as isothyocyanates (mustard oil, wasabi, horse-radish) and allycin (garlic), to name only a few.19–22 Several endogenous pro-inflammatory mediators, including cyclopentane prostaglandins and byproducts of oxidative stress (4-hydroxynonenal [4-HNE], 4-oxononenal), have been shown to directly activate TRPA1 by covalent modification of cysteine residues on the channel.23–27 Subsequent to TRPA1 activation, increases in intracellular Ca2+ induce the peripheral release of neuropeptides (substance P and calcitonin gene-related peptide (CGRP)), purines, and other transmitters from sensitized nerve fiber endings, which ultimately results in neurogenic inflammation and hypersensitivity.28–31 It is interesting to note that Ca2+ does not only represent an intermediate player in TRPA1-mediated events, but also acts as a direct modulator of TRPA1 activity. Indeed, electrophysiological recordings have demonstrated an increase in TRPA1 activity during Ca2+ perfusion in vitro.21,32–34 Furthermore, extracellular Ca2+ is also known to induce TRPA1 desensitization, either by direct binding to the extracellular portion of the channel, or by mediating increases in intracellular Ca2+, also recognized to desensitize other TRP channels.21,32,34,35
During inflammatory conditions, TRPA1 activity is regulated by several different mechanisms, including the modulation of its trafficking to the membrane (Fig. 2). Indeed, in vitro experiments on HEK293T cells and mouse DRGs have demonstrated that mustard oil induces TRPA1 activation and translocation to the cell membrane, in a protein kinase A (PKA) and phospholipase C (PLC)-dependent manner.36 This increase in TRPA1 trafficking to the cell surface is believed to be mediated by induction of vesicle fusion with the plasma membrane.37–40
Figure 2.
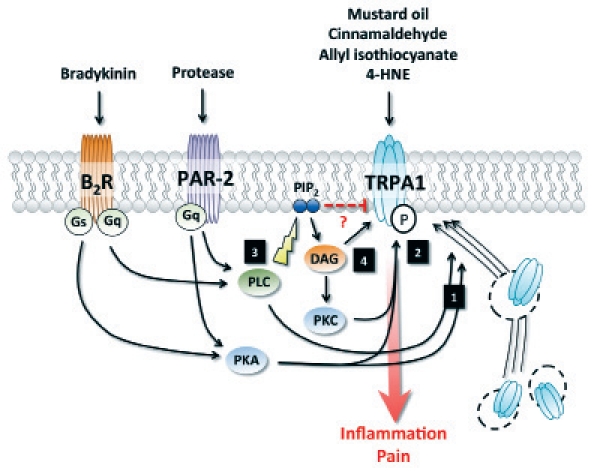
Modulation of TRPA1 activity and trafficking. (1) Activation of PKA and PLC by PAR-2 or B2R induces trafficking of TRPA1 to the plasma membrane. (2) PKA and PKC-dependent phosphorylation potentiate TRPA1 activation. (3) Activation of PLC downstream of PAR-2 or B2R enhances TRPA1 activity by releasing the channel from PIP2-mediated inhibition. (4) DAG modulates TRPA1 activity either directly, by activation of the channel itself, or in a PKC-dependent manner. 4-hydroxynonenal, 4-HNE; B2 Bradykine receptor, B2R; Diacylglycerol, DAG; protease-activated receptor-2, PAR-2; protein kinase A, PKA; protein kinase C, PKC; phosphatidylinositide, PIP2; phospholipase C, PLC.
Finally, TRP channels can also be regulated downstream of G protein-coupled receptor activation. TRPV1 activation has been reported following stimulation of sensory neurons with the pro-inflammatory mediator bradykinin, which results in thermal hyperalgesia and acute pain.41 Interestingly, this effect was maintained in TRPV1-deficient mice, suggesting the involvement of another channel in the cellular events evoked by bradykinin.41 Using TRPA1-deficient animals, independent studies have demonstrated that TRPA1 is required for bradykinin-evoked sensory neuron excitation ex vivo and hyperalgesia in vivo.19,42 These observations indicate that TRPV1 and TRPA1 are interdependently regulated downstream of B2 bradykinin receptor (B2R) activation, and act in concert to induce hyperalgesia. Further research is warranted in order to determine if this crosslink requires physical association of the two channels, or is dependent on a regulatory partnership. In addition to its effect on TRPA1/TRPV1 interaction, bradykinin has also been shown to sensitize TRPA1 via activation of PLC (Fig. 2).43 PIP2 has been suggested as an endogenous inhibitor of TRPA1;44 by breaking down phosphatidylinositides (PIP2), PLC would block its inhibitory effect on TRPA1, and thus sensitize the channel.43 Diacylglycerol (DAG), a byproduct of PIP2 hydrolysis by PLC, could also be involved in this process, either by directly activating TRPA1 or by mediating PKC-dependent phosphorylation of TRPA1.19,45 Finally, PKA has also been suggested as a signaling intermediate molecule in bradykinin-mediated TRPA1 sensitization, by directly phosphorylating the channel (Fig. 2).43,45
TRPA1 and Visceral Pain
Gastrointestinal tract.
Abdominal pain is a hallmark of several inflammatory diseases of the gastrointestinal (GI) tract, including irritable bowel syndrome, inflammatory bowel disease and functional dyspepsia.46,47 The GI tract is certainly the most studied among the internal organs with regards to the role of TRPA1 in visceral inflammation and nociception. Indeed, several experimental models of colitis have demonstrated the efficacy of mustard oil in inducing colitis.28–31 Mustard oil-induced colitis is characterized by the upregulation of several pro inflammatory mediators including interleukin (IL)-1β, IL-6, granulocyte macrophage colony stimulating factor (GM-CSF), macrophage chemotactic protein 1 (MCP-1) and macrophage inflammatory protein 1 (MIP-1α), all of which are implicated in leukocyte recruitment and activation.28 This inflammatory response, along with the release of substance P and CGRP from sensitized nerve fiber endings, contributes to the development of visceral hypersensitivity.28–31
A few years ago, Yang et al. demonstrated that reduction of TRPA1 expression by antisense oligodeoxynucleotide significantly reduced colonic hypersensitivity induced by trinitrobenzene-sulphonic acid (TNBS)-mediated colitis in mice.48 These results were further supported and expanded by a recent study showing that TRPA1 agonists allyl isothiocyanate and trans-cinnamaldehyde induced mechanosensory responses in vagal and pelvic serosal afferents of TRPA1+/+ mice, but not in TRPA1-deficient animals.49 These observations were corroborated by in vivo recording of visceromotor responses to colorectal distention, which showed a significant reduction in mechanical hyperalgesia in TRPA−/− mice, therefore directly implicating TRPA1 in colonic pain.49 Similar results were obtained in the rat stomach using intrathecal injection of TRPA1 antisense.50 In the colon, it is interesting to note that mechanical hypersensitivity evoked by TRPA1 agonists was further amplified in afferents from mice with chemically induced colitis, suggesting a role for TRPA1 in mechanosensory function and sensitization during inflammatory conditions.49
Activation of the G protein-coupled receptor protease-activated receptor-2 (PAR-2) can induce colitis and visceral hypersensitivity. Interestingly, PAR-2 is also involved in the modulation of TRPA1 activity (Fig. 2). TRPA1 and PAR-2 have been observed in a co-localization pattern in rat DRG neurons.43 The same study also demonstrated TRPA1 sensitization downstream of PAR-2 activation. Similar to what has been observed upon bradykinin treatment, this mechanism appears to be dependent on the cleavage of PIP2 by PLC, which releases the inhibition of TRPA1.43 PAR-2 has also been shown to modulate TRPA1 in a PKA-dependent manner, either via TRPA1 phosphorylation or induction of its trafficking to the membrane (Fig. 2).36,45 The interaction between PAR-2 and TRPA1 is suggested to play a role in different experimental models of GI disorders. Notably, PAR-2-mediated sensitization of TRPA1 represent a key mechanism in mast cell-mediated mechanical hyperalgesia in the guinea pig esophagus.45,51 Furthermore, Cattaruza and colleagues have reported that TRPA1 deletion significantly reduced mechanical colonic hyperalgesia induced by PAR-2 activating peptide.52
The expression of TRPA1 has also been demonstrated in DRG neurons innervating the pancreas, and is believed to participate in inflammation and pain related to acute pancreatitis in mice.53,54 TRPA1 agonists have been shown to induce pancreatic inflammation and hyperexcitability of spinal nociceptors.53 Furthermore, cerulein-evoked pancreatic inflammation induced a significant increase in the expression and activation of both TRPA1 and TRPV1, as well as overall excitability of pancreatic sensory neurons.54 Interestingly, while the inhibition of both TRPA1 and TRPV1 individually reduced the severity of pancreatitis, the combined treatment appeared to be more effective. Importantly, these observations were corroborated in pain-related behavior experiments; combined treatment with TRPA1 and TRPV1 antagonists prevented the reduction of exploratory behaviors observed in mice treated with cerulean alone.54 These observations, similar to what was found in thermal hyperalgesia-induced by bradykinin, suggest a functional crosstalk between TRPA1 and TRPV1 in the modulation of pain signaling during pancreatitis.
Urinary tract.
Alteration in afferent activity is believed to be a key factor in urinary tract dysfunction; hyperexcitablity of afferent neurons innervating the urinary tract has been proposed as a possible mechanism behind idiopathic detrusor over activity and painful bladder symptoms (reviewed in ref. 55 and 56).
In mice, TRPA1 expression has been reported in small afferent fibers innervating the trigone of the bladder,16 where it appears to modulate bladder contraction under physiological conditions.33 Subsequent studies also demonstrated that the majority of DRG neurons innervating the mouse bladder expressed TRPA1, often in a co-localization pattern with TRPV1.57
The urothelium, the epithelial layer lining the bladder, interacts closely with underlying afferent nerve fibers and is believed to play an important role in sensory transduction in the urinary tract.58 TRPA1 expression has been reported in the human and rat urothelium, and is believed to modulate bladder function in pathological conditions.59,60 Notably, TRPA1 upregulation has been observed in the urothelium of patients with bladder outlet obstruction, when compared to healthy controls.59 Furthermore, activation of TRPA1 by intravesical administration of trans-cinnamaldehyde has been shown to induce hyperreflexia through C-fiber-mediated afferent pathway in rats.60 These results are supported by another study demonstrating altered urodynamic functions, including increase micturition frequency and reduced voiding volume, in response to other TRPA1 agonists such as allyl isothiocyanate and hydrogen sulfide.61 Taken together, these observations suggest that TRPA1 is involved in the regulation of bladder sensory function and micturition reflex.
TRPA1 is also implicated in the pathology of overactive bladder, a chronic condition often linked to spinal cord injury and associated with spontaneous and involuntary bladder contractions.62 Andrade and colleagues reported an upregulation of TRPA1 channels in the bladder and bladder-innervating DRG neurons of rats subjected to spinal cord injury.62 Importantly, the inhibition of TRPA1 by either selective antagonists or targeted gene deletion was shown to normalize bladder contractions in this model, thus identifying TRPA1 as an important player in overactive bladder syndrome.62
Conclusion
Although we have gained significant insights into the mechanisms underlying sensory transduction and nociception, efficient pain management remains a considerable challenge in health care and basic research. One of the most important breakthroughs with regards to inflammatory pain has been the discovery of the sensory and nociceptive role of TRP channels. After TRPV1, TRPA1 has recently emerged as another potential therapeutic target in the treatment of chronic visceral pain. Indeed, the role of TRPA1 in GI inflammatory disorders is becoming increasingly clear and, although causal implication remains to be established, TRPA1 upregulation has been observed in several disease model systems. In-depth research is warranted to determine the exact role of TRPA1 in visceral pain and neurogenic inflammation, but our new understanding of its homeostatic and pathophysiological functions certainly offers bright perspectives for the development of novel therapeutic approaches in the treatment of chronic pain associated with visceral inflammation.
Acknowledgments
We gratefully acknowledge Kirsten Marshall for critical reading of the manuscript.
Abbreviations
- 4-HNE
4-hydroxynonenal
- B2R
B2 bradykinin receptor
- CGRP
calcitonin gene-related peptide
- DAG
diacylglycerol
- DRG
dorsal root ganglia
- GM-CSF
granulocyte macrophage colony stimulating factor
- IL
interleukin
- MIP-1α
macrophage inflammatory protein 1
- MCP-1
macrophage chemotactic protein 1
- PAR-2
protease-activated receptor-2
- PKA
protein kinase A
- PKC
protein kinase C
- PIP2
phosphatidylinositide biphosphate
- PLC
phospholipase C
- TNBS
trinitrobenzene-sulphonic acid
- TRP
transient receptor potential
- TRPA
ankyrin TRP
- TRPM
melastatin TRP
- TRPV
vanilloid TRP
References
- 1.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: A World Health Organization study in primary care. JAMA. 1998;280:147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 2.National Research Council, author. Musculoskeletal Disorders and the Workplace. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 3.Jancsó N. Role of the nerve terminals in the mechanism of inflammatory reactions. Bull Millard Fillmore Hosp. 1960;7:53–77. [Google Scholar]
- 4.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Julius D. Sense and specificity: a molecular identity for nociceptors. Curr Opin Neurobiol. 1999;9:525–530. doi: 10.1016/S0959-4388(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 6.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 7.Holzer P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br J Pharmacol. 2008;155:1145–1162. doi: 10.1038/bjp.2008.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Planells-Cases R, Valente P, Ferrer-Montiel A, Qin F, Szallasi A. Complex regulation of TRPV1 and related thermo-TRPs: implications for therapeutic intervention. Adv Exp Med Biol. 2011;704:491–515. doi: 10.1007/978-94-007-0265-3_27. [DOI] [PubMed] [Google Scholar]
- 9.Liedtke WB, Heller S. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton, FL: CRC Press; 2007. [PubMed] [Google Scholar]
- 10.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 11.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 12.Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem. 1999;274:7325–7333. doi: 10.1074/jbc.274.11.7325. [DOI] [PubMed] [Google Scholar]
- 13.Corey DP. New TRP channels in hearing and mechanosensation. Neuron. 2003;39:585–588. doi: 10.1016/s0896-6273(03)00505-1. [DOI] [PubMed] [Google Scholar]
- 14.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. Painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 15.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 16.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole DP, Pelayo JC, Cattaruzza F, Kuo YM, Gai G, Chiu JV, et al. Transient receptor potential ankyrin 1 is expressed by inhibitory motoneurons of the mouse intestine. Gastroenterology. 2011;141:565–575. doi: 10.1053/j.gastro.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 18.Stokes A, Wakano C, Koblan-Huberson M, Adra CN, Fleig A, Turner H. TRPA1 is a substrate for deubiquitination by the tumor suppressor CYLD. Cell Signal. 2006;18:1584–1594. doi: 10.1016/j.cellsig.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 20.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 22.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz-Orengo L, Dhaka A, Heuermann RJ, Young TJ, Montana MC, Cavanaugh EJ, et al. Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1. Mol Pain. 2008;4:30. doi: 10.1186/1744-8069-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Materazzi S, Nassini R, Andre E, Campi B, Amadesi S, Trevisani M, et al. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2008;105:12045–12050. doi: 10.1073/pnas.0802354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi N, Mizuno Y, Kozai D, Yamamoto S, Kiyonaka S, Shibata T, et al. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels (Austin) 2008;2:287–298. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- 28.Kimball ES, Prouty SP, Pavlick KP, Wallace NH, Schneider CR, Hornby PJ. Stimulation of neuronal receptors, neuropeptides and cytokines during experimental oil of mustard colitis. Neurogastroenterol Motil. 2007;19:390–400. doi: 10.1111/j.1365-2982.2007.00939.x. [DOI] [PubMed] [Google Scholar]
- 29.Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 30.Palecek J, Willis WD. The dorsal column pathway facilitates visceromotor responses to colorectal distention after colon inflammation in rats. Pain. 2003;104:501–507. doi: 10.1016/S0304-3959(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 31.Engel MA, Leffler A, Niedermirtl F, Babes A, Zimmermann K, Filipovic MR, et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology. 2011;141:1346–1358. doi: 10.1053/j.gastro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 33.Andrade EL, Ferreira J, Andre E, Calixto JB. Contractile mechanisms coupled to TRPA1 receptor activation in rat urinary bladder. Biochem Pharmacol. 2006;72:104–114. doi: 10.1016/j.bcp.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283:32691–32703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007;583:175–193. doi: 10.1113/jphysiol.2007.133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009;64:498–509. doi: 10.1016/j.neuron.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seino S, Shibasaki T. PKA-dependent and PKAindependent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 40.Holz RW, Axelrod D. Localization of phosphatidylinositol 4,5-P(2) important in exocytosis and a quantitative analysis of chromaffin granule motion adjacent to the plasma membrane. Ann NY Acad Sci. 2002;971:232–243. doi: 10.1111/j.1749-6632.2002.tb04467.x. [DOI] [PubMed] [Google Scholar]
- 41.Katanosaka K, Banik RK, Giron R, Higashi T, Tominaga M, Mizumura K. Contribution of TRPV1 to the bradykinin-evoked nociceptive behavior and excitation of cutaneous sensory neurons. Neurosci Res. 2008;62:168–175. doi: 10.1016/j.neures.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, et al. Phospholipase C and protein kinase A mediate Bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain. 2008;131:1241–1251. doi: 10.1093/brain/awn060. [DOI] [PubMed] [Google Scholar]
- 44.Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, et al. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Yang C, Wang ZJ. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4 and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience. 2011;193:440–451. doi: 10.1016/j.neuroscience.2011.06.085. [DOI] [PubMed] [Google Scholar]
- 46.Blackshaw LA, Brierley SM, Hughes PA. TRP channels: new targets for visceral pain. Gut. 2010;59:126–135. doi: 10.1136/gut.2009.179523. [DOI] [PubMed] [Google Scholar]
- 47.Christianson JA, Bielefeldt K, Altier C, Cenac N, Davis BM, Gebhart GF, et al. Development, plasticity and modulation of visceral afferents. Brain Res Rev. 2009;60:171–186. doi: 10.1016/j.brainresrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang J, Li Y, Zuo X, Zhen Y, Yu Y, Gao L. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci Lett. 2008;440:237–241. doi: 10.1016/j.neulet.2008.05.093. [DOI] [PubMed] [Google Scholar]
- 49.Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O'Donnell TA, et al. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137:2084–2095. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kondo T, Obata K, Miyoshi K, Sakurai J, Tanaka J, Miwa H, et al. Transient receptor potential A1 mediates gastric distention-induced visceral pain in rats. Gut. 2009;58:1342–1352. doi: 10.1136/gut.2008.175901. [DOI] [PubMed] [Google Scholar]
- 51.Yu S, Gao G, Peterson BZ, Ouyang A. TRPA1 in mast cell activation-induced long-lasting mechanical hypersensitivity of vagal afferent C-fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol. 2009;297:34–42. doi: 10.1152/ajpgi.00068.2009. [DOI] [PubMed] [Google Scholar]
- 52.Cattaruzza F, Spreadbury I, Miranda-Morales M, Grady EF, Vanner S, Bunnett NW. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:81–91. doi: 10.1152/ajpgi.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ceppa E, Cattaruzza F, Lyo V, Amadesi S, Pelayo JC, Poole DP, et al. Transient receptor potential ion channels V4 and A1 contribute to pancreatitis pain in mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:556–571. doi: 10.1152/ajpgi.00433.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz ES, Christianson JA, Chen X, La JH, Davis BM, Albers KM, et al. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology. 2011;140:1283–1291. doi: 10.1053/j.gastro.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shea VK, Cai R, Crepps B, Mason JL, Perl ER. Sensory fibers of the pelvic nerve innervating the Rat's urinary bladder. J Neurophysiol. 2000;84:1924–1933. doi: 10.1152/jn.2000.84.4.1924. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimura N, Seki S, Chancellor MB, de Groat WC, Ueda T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology. 2002;59:61–67. doi: 10.1016/s0090-4295(01)01639-9. [DOI] [PubMed] [Google Scholar]
- 57.La JH, Schwartz ES, Gebhart GF. Differences in the expression of transient receptor potential channel V1, transient receptor potential channel A1 and mechanosensitive two pore-domain K+ channels between the lumbar splanchnic and pelvic nerve innervations of mouse urinary bladder and colon. Neuroscience. 2011;186:179–187. doi: 10.1016/j.neuroscience.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Everaerts W, Vriens J, Owsianik G, Appendino G, Voets T, De Ridder D, et al. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol. 2010;298:692–701. doi: 10.1152/ajprenal.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du S, Araki I, Kobayashi H, Zakoji H, Sawada N, Takeda M. Differential expression profile of cold (TRPA1) and cool (TRPM8) receptors in human urogenital organs. Urology. 2008;72:450–455. doi: 10.1016/j.urology.2007.11.127. [DOI] [PubMed] [Google Scholar]
- 60.Du S, Araki I, Yoshiyama M, Nomura T, Takeda M. Transient receptor potential channel A1 involved in sensory transduction of rat urinary bladder through C-fiber pathway. Urology. 2007;70:826–831. doi: 10.1016/j.urology.2007.06.1110. [DOI] [PubMed] [Google Scholar]
- 61.Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, et al. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol. 2008;53:391–399. doi: 10.1016/j.eururo.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 62.Andrade EL, Forner S, Bento AF, Leite DF, Dias MA, Leal PC, et al. TRPA1 receptor modulation attenuates bladder overactivity induced by spinal cord injury. Am J Physiol Renal Physiol. 2011;300:1223–1234. doi: 10.1152/ajprenal.00535.2010. [DOI] [PubMed] [Google Scholar]