Abstract
All animals endowed with the ability to detect light through visual pigments must have evolved pathways in which dietary precursors for the involved chromophore are absorbed, transported, and metabolized. Knowledge about this metabolism has exponentially increased over the past decade. Genetic manipulation of animal models provided insights into the metabolic flow of these compounds through the body and in the eyes, unraveling their regulatory aspects and aberrant side reactions. The scheme that emerges reveals a common origin of key components for chromophore metabolism that have been adapted to the specific requirements of retinoid biology in different animal classes.
Keywords: Carotenoid, Retinoid, Rhodopsin, Vision, Vitamin A, Visual Cycle
Introduction
The visual process is a paramount example for the complex interactions of our body with the environment. It acquires most of the brain's sensory input and strictly depends on a dietary chromophore. To establish and sustain vision, animals have evolved pathways by which dietary chromophore precursors such as vitamin A (all-trans-retinol (ROL)2) and provitamin A (β,β-carotene) are absorbed in the intestine, transported in the body, taken up by cells, and metabolized to the chromophore.
Night blindness, a condition that can be caused by an inadequate vitamin A supply to the eyes, is the oldest described eye disease known since ancient times (1). This deficiency is still a major health problem that causes blindness of children in developing countries (2). Although this problem was prevalent in Western societies about 100 years ago, an improved dietary intake largely defeated this ailment (1). However, even disease-preventing micronutrients have a defined window of benefit; too much vitamin A or carotenoids can cause adverse health effects. Moreover, mutations in genes encoding key players involved in chromophore metabolism can cause inherited retinal diseases such as retinitis pigmentosa and Leber congenital amaurosis (3). Aberrant side products of chromophore metabolism also can trigger pathology such as age-related macular degeneration (4). In vertebrates, vitamin A is critical not only for vision but also for gene regulation in the form of all-trans-retinoic acid (RA), which binds ligand-activated transcription factors such as retinoic acid receptors (RARs) and retinoid X receptors (5). Therefore, the metabolic flow of dietary precursors into pathways for the production of different biologically active retinoids must be regulated to avoid a deficiency or an excess of these compounds.
Monophyletic Origin of Carotenoid/Retinoid Metabolism
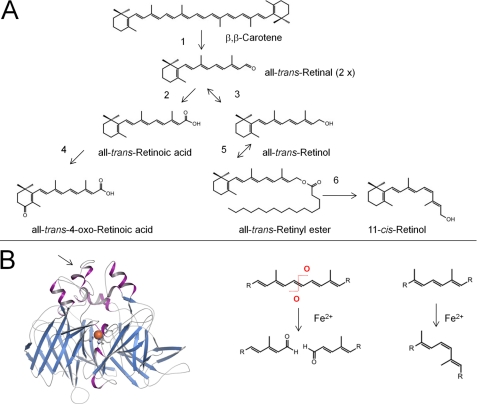
Carotenoids and their retinoid metabolites are isoprenoids that can undergo only a limited number of chemical transformations, and just a few of these occur naturally (Fig. 1A). The formal first step in chromophore metabolism is the conversion of the parent C40 carotenoid precursor into a C20 retinaldehyde by symmetric oxidative cleavage at C15/C15′ in the carbon backbone. Enzymatic oxidative cleavage of carotenoids at a specific position of the polyene chain has been proposed for all existing kingdoms of nature as a method for the synthesis of apocarotenoids, including retinoids. The first carotenoid-cleaving enzyme (CCE) was molecularly identified by analysis of a maize mutant deficient in the apocarotenoid abscisic acid (6). This breakthrough was followed by the molecular cloning and biochemical characterization of structurally related enzymes in different living kingdoms of nature (7).
FIGURE 1.
Carotenoids and retinoids undergo limited number of chemical reactions. A, enzymatic steps in vertebrate carotenoid/retinoid metabolism. Note that similar enzymatic modifications of carotenoids and apocarotenoids occur in plants, fungi, and bacteria, and these are catalyzed by related enzymes. Step 1, oxidative cleavage of double bonds; steps 2 and 3, oxidation and reduction of oxygen end groups; step 4, introduction of oxygen into the ionone ring; step 5, esterification of hydroxyl groups; step 6, trans-to-cis isomerization of double bonds of the polyene chain. B, left, the crystal structure of RPE65 (retinoid isomerase) from Bos taurus is shown. The structural fold is well conserved in plant and bacterial CCEs. The arrow points to a region of RPE65 that associates with the lipid membrane. The ferrous iron that demarks the reaction center of this type of protein is highlighted as an orange sphere. Right, the enzymatic reactions catalyzed by this class of enzymes include oxidative cleavage in and trans-to-cis isomerization of double bonds of the polyene chain of carotenoids and apocarotenoids.
Insect genomes encode only one and vertebrate genomes encode three distinct CCE family members. The β,β-carotene 15,15′-monooxygenase BCMO1 converts a limited number of provitamin A carotenoids such as β,β-carotene to retinaldehyde by symmetric cleavage at C15/C15′ (8). The role of BCMO1 as the key enzyme for retinoid production has been well established (9). The β,β-carotene 9,10-dioxygenase BCDO2 catalyzes cleavage of carotenoids at the C9′/C10′ double bond (10) and displays broad substrate specificity (11–13). BCDO2 can further metabolize its primary cleavage product by oxidative tailoring at C9/C10, indicating that the enzyme also plays a role in apocarotenoid metabolism (13). There also is a marked difference in the subcellular localization of the two vertebrate carotenoid oxygenases. BCMO1 is a cytoplasmic protein (8), whereas BCDO2 localizes to mitochondria (13). Analysis of a knock-out mouse model for Bcdo2 demonstrated a critical role of the second CCE for carotenoid homeostasis in tissues (13, 14).
The third family member, RPE65 (retinal pigment epithelium 65-kDa protein), was the first animal CCE molecularly identified (15), but RPE65 was regarded for a long time as a retinoid-binding protein (16, 17). Mutations in RPE65 can cause Leber congenital amaurosis in humans (18). Analysis of mouse models revealed that this enzyme's dysfunction disrupts chromophore synthesis and leads to the accumulation of retinyl esters (REs) in the retinal pigment epithelium (RPE) (19). It was later shown that RPE65 is the retinoid isomerase in the vertebrate visual cycle that catalyzes the conversion of all-trans-REs to 11-cis-retinol (20–22).
The insect CCE, encoded by ninaB (neither inactivation or after potential gene B), catalyzes a combined oxidative cleavage at C15/C15′ and isomerization at C10/C11, yielding one molecule each of the cis- and trans-chromophores (23). Like RPE65 for vertebrates, NinaB is critical for insect vision (24, 25). Thus, both oxidative cleavage and trans-to-cis double bond conversion of carotenoids and retinoids are intrinsic catalytic activities of animal CCE family members (Fig. 1B).
The structural scaffold is well conserved between CCE family members of different kingdoms, the basic motif being a seven-bladed β-propeller (Fig. 1B) (26–28). The iron cofactor is coordinated by four conserved histidine residues and three second-shell glutamate residues and is accessible through a long non-polar tunnel. The essential role of ferrous iron for enzymatic catalysis was demonstrated for BCMO1 and RPE65 (29, 30). However, the precise mechanism of the isomerization and oxidative cleavage reaction remained unproven (31).
Upon oxidative cleavage, the aldehyde end group of all-trans-retinal (RAL) can undergo catalytic reduction/oxidation to form either ROL or RA. Comparative analysis of apocarotenoid metabolism revealed that these steps are catalyzed by related dehydrogenases in plants and animals (7). Additionally, the turnover of the plant hormone abscisic acid is catalyzed by the same type of oxygenase used by vertebrates for RA catabolism (32). Thus, key players for carotenoid and apocarotenoid metabolism are evolutionarily well conserved in different kingdoms. Animals took advantage of this ancestral gene pool to evolve enzymes specific for chromophore metabolism.
Absorption and Transport of Carotenoids
In contrast to carotenogenic organisms, animals must acquire carotenoids from the diet. Initially, it was proposed that absorption of such lipids took place by passive non-ionic diffusion. However, increasing evidence indicates that this absorption is a protein-facilitated process (Fig. 2) (33). The protein dependence of carotenoid absorption was demonstrated by the chromophore deficiency and blindness of the Drosophila mutant ninaD (34). The ninaD gene has been molecularly identified and encodes a cytoplasmic transmembrane protein expressed in the gut (35, 36). Carotenoids are then transported to neuronal and glial cells adjacent to the eyes. Uptake of circulating carotenoids by these cells is facilitated by the NinaD-related protein SANTA MARIA (36). Upon absorption by SANTA MARIA, carotenoids are metabolized by NinaB to yield the chromophore (supplemental Fig. S1).
FIGURE 2.
Carotenoid uptake is protein-facilitated process. A, in animals, class B scavenger receptors such as NinaB, SANTA MARIA, and SR-BI facilitate the cellular uptake of carotenoids from micelles in the gut and/or circulating lipoproteins in the blood. Structural prediction based on the primary amino acid sequence of this class of proteins indicates that these receptors possess two membrane-spanning domains at the N and C termini as well as a large extracellular loop. B, upon cellular absorption, carotenoids can be sequestered by binding proteins and lipid droplets. Additionally, carotenoids can be converted to apocarotenoids, including retinoids, by carotenoid oxygenases. Genetic disruption of these processes alters carotenoid homeostasis. Left panel, mutations in the Yellow Cocoon gene encoding a class B scavenger receptor or mutations in the Yellow Blood gene encoding a carotenoid-binding protein alter silk color in B. mori. Genetic disruption of Bcmo1 results in β,β-carotene accumulation in the fat pads of mice. Mutations in the ninaB gene lead to carotenoid accumulation in the eye. Genetic disruption of the Bcdo2 gene results in a yellow color of isolated liver mitochondria due to carotenoid accumulation.
Mutant analyses of the silk worm Bombyx mori identified molecular players in a pathway for tissue-specific accumulation of carotenoids in the silk gland. In this pathway, carotenoid absorption is mediated by a NinaD-related protein encoded by the Yellow Cocoon gene (37). For cellular accumulation, Bombyx expresses a specific carotenoid-binding protein encoded by the Yellow Blood gene that is a member of the family of StAR (steroidogenic acute regulatory) proteins (37).
NinaD and related insect proteins belong to the family of class B scavenger receptors. The role of mammalian scavenger receptor class B type I (SR-BI) in cholesterol metabolism has been well established and reviewed previously (38). However, SR-BI also facilitates the absorption of other isoprenoids such carotenoids and tocopherols from circulating lipoproteins (39, 40). Similar to insects, specific binding proteins help sequester these compounds in tissues such as the macula lutea of primate retina. The StARD3 protein and a GST isoform have been identified as lutein- and zeaxanthin-binding proteins of the human retina, respectively (41, 42). CCEs also control the cellular fate of these isoprenoid compounds (Fig. 2). BCDO2 is critical for the catabolism of carotenoids in cells. In Bcdo2−/− mice, carotenoids accumulate in several tissues and cause mitochondrial oxidative stress (13).
Studies in knock-out mouse models also demonstrated an important role of SR-BI in the intestinal absorption of carotenoids (43). This is highlighted by the unique transcriptional regulation of SR-BI expression in enterocytes by the homeodomain transcription factor Isx (44). Isx also controls the intestinal expression of the vitamin A-forming enzyme BCMO1 (45). This regulation depends on the vitamin A status of the diet. When preformed vitamin A is present, Isx expression is induced, and both SR-BI and Bcmo1 expression are decreased. However, in the absence of dietary retinoids, the expression patterns of these genes are reversed (45). The molecular basis of this dietary responsiveness was found in the RAR-binding site in the Isx promoter (46). Thus, RA via RARs induces Isx expression and controls vitamin A production by negative feedback regulation.
Mammals Possess Transport and Storage Systems Specific for Retinoids
Insects absorb carotenoids intact, whereas vertebrates metabolize most of the absorbed provitamin A in enterocytes of the intestine. RAL is then converted into ROL and REs in a stepwise fashion (47). The resulting REs are packaged into chylomicrons that are secreted into the lymph (48). A smaller fraction of these circulating REs are taken up by peripheral tissue in a process that likely involves lipoprotein lipase (49). The remainder (∼70%) are cleared by hepatocytes and hydrolyzed back to ROL (50), which is then transferred into hepatic stellate cells and esterified by lecithin:retinol acyltransferase (LRAT) for storage (supplemental Fig. S2) (51).
During fasting, ROL bound to the 21-kDa serum retinol-binding protein (RBP; holo-RBP) is the major retinoid found in the circulation. The liver expresses RBP, which is secreted from hepatocytes into the circulation in an ROL-dependent manner. Once in the blood, ROL-RBP forms a protein-protein complex with 55-kDa transthyretin (supplemental Fig. S2) (52). Transthyretin is required for normal blood ROL homeostasis and prevents excessive loss of the relatively small RBP molecule by glomerular filtration (52). RBP−/− mice develop normally on retinoid sufficient diets but suffer from visual chromophore deficiency early in life (53). Later in life, this deficiency is corrected when animals are kept on vitamin A-sufficient diets, demonstrating that, analogous to RA-dependent processes, other blood retinoid transport systems can substitute for RBP deficiency. Similarly, patients with RBP deficiency display only mild ocular defects (54).
A receptor for the holo-RBP complex has recently been identified as being encoded by the Stra6 (stimulated by retinoic acid 6) gene (55). Cell culture studies showed that ROL uptake via this transmembrane-spanning protein is driven by metabolic conversion of ROL to RE by LRAT. These studies also provided biochemical evidence that the STRA6-dependent flux of ROL between RBP and cells is bidirectional, indicating that STRA6 is a retinoid transporter (supplemental Fig. S2) (56, 57).
Stra6 is expressed in several but not all retinoid-metabolizing tissues, including the eyes (55). Interestingly, the liver as the major organ for retinoid storage does not express STRA6, indicating that this receptor is required mainly for the delivery of ROL from the liver to peripheral tissues.
A critical physiological role for STRA6 in retinoid metabolism is supported by genetic analyses in humans. Postulated loss-of-function mutations were found in individuals with Matthew-Wood syndrome, characterized by anophthalmia/microphthalmia in association with variable malformations of the heart, lungs, and diaphragm (58, 59). Similar developmental malformations can result from the maternal retinoid deficiency syndrome (60). Thus, it has been proposed that STRA6 is required for ROL uptake for subsequent RA production. However, the consequences of STRA6 deficiency are surprising because genetic disruption of its ligand RBP results only in a mild ocular defect (54). Furthermore, the RA-induced expression of Stra6 is problematic if we would consider this transporter as being required for the production of this hormone in tissues. Thus, the role of STRA6 in retinoid homeostasis needs to be further defined.
“Canonical” Visual Cycle
Once absorbed by vertebrate eyes, ROL must be converted to the chromophore to establish and sustain vision. Individual steps in the canonical visual cycle have been delineated in biochemical detail, and the function of key enzymes has been confirmed in mouse models (Fig. 3). Mutations in genes encoding these proteins are associated with various blinding diseases in humans (supplemental Fig. S3) (3). In the disc membranes of rod outer segments (ROS), rhodopsin exists as an integral membrane protein, and the chromophore is covalently bound via a Schiff base linkage. Light induces a cis-to-trans isomerization of the protein-bound chromophore to initiate phototransduction (61). Hydrolysis of the Schiff base linkage by bulk water entering from the cytoplasmic side liberates the RAL photoproduct (62). Part of RAL is released into the disc lumen and must be transferred to the cytosol by ABCA4 (ATP-binding cassette transporter 4) (63). The first step in the visual cycle involves reduction of RAL to ROL catalyzed by retinol dehydrogenases (RDHs) (64, 65). Two enzymes, RDH8 in photoreceptor outer segments and RDH12 in photoreceptor inner segments, that belong to the short-chain dehydrogenase/reductase family and employ NADPH as a cofactor are mainly responsible for catalyzing this reaction in mouse photoreceptors (66). However, the redundancy of retinal reductase activity shown in mice suggests that photoreceptors contain additional functional RDHs besides RDH12 and RDH8 (67). This redundancy could be due to the need for a large enzymatic capacity to convert the chemically reactive aldehyde group of the photoproduct to the corresponding alcohol under bright light conditions. After bright light bleaching of rhodopsin, the photoproduct can exist in millimolar concentrations within cells. The aldehyde group of the photoproduct can form adducts with primary amino groups that exist in many cellular molecules, including lipids, proteins, and ribonucleotides. The natural occurrence of such an aberrant side reaction is documented by the presence of the bisretinoid A2E, formed by a condensation reaction of two molecules of RAL with the membrane lipid phosphatidylethanolamine. Ocular accumulation of A2E and A2E-mediated redox reactions have been implicated in the pathology of eye diseases such as age-related macular degeneration (68). The importance for rapid clearance of the photoproduct is also demonstrated by the consequences of mutations in RDH12 and ABCA4 in humans (69, 70). Mouse models with impaired retinal clearance have been established to characterize the underlying pathology (71). However, the mechanisms by which the photoproduct induces photoreceptor cell death remain controversial (72).
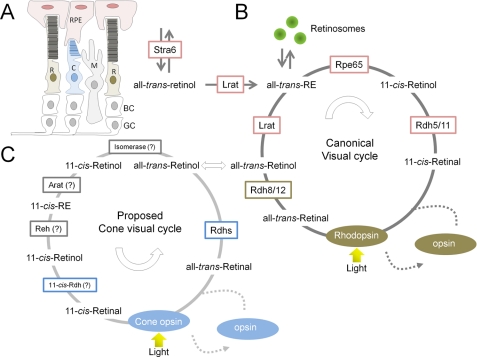
FIGURE 3.
Retinoid metabolism in mammalian eyes. A, simplified schematic overview of the mammalian retina. R, rod photoreceptors; C, cone photoreceptors; M, Müller glial cells. Second-order bipolar cells (BC) and third-order ganglion cells (GC) are shown but not discussed. The drawing is adapted from Ref. 86. B, biochemical key steps of the canonical visual cycle. C, proposed biochemical steps of the alternative visual cycle for rods. Different enzymes involved in the canonical and cone-specific visual cycles are indicated. Enzymes that have not been molecularly identified are denoted by question marks. The colored boxes enclosing the enzyme names indicate their cellular localization: pink, RPE; green, ROS; blue, cone outer segments; gray, Müller glial cells. Note that transport of retinoids in and between cells requires binding proteins such as intracellular and extracellular RBPs as well as the putative retinoid transporter ABCA4. Reh, RE hydrolase; Arat, acetyl-CoA:retinol acyltransferase.
ROL formed in ROS is transported to the RPE, where it is esterified. This process is facilitated by two retinoid-binding proteins: interphotoreceptor retinoid-binding protein, which binds retinoids in the extracellular space, and CRBP1 (cellular retinol-binding protein-1), located within RPE cells (73, 74). The major ester synthase in RPE is LRAT (75, 76). The LRAT reaction constitutes an important intersection in ocular retinoid metabolism. This enzyme is required for the clearance of ROL from ROS and for the uptake of ROL from the blood. Because of their high hydrophobicity, all-trans-REs constitute a stable storage form of vitamin A within internal membranes and oil droplet-like structures called retinosomes (77). Additionally, all-trans-RE serves as a substrate for RPE65, which catalyzes the endothermic transformation of all-trans-retinoid to its 11-cis conformation. The product of this isomerization reaction is 11-cis-retinol, which is subsequently oxidized in the final catalytic step of the visual cycle to 11-cis-retinal. The enzymatic activities of short-chain dehydrogenases/reductases such as RDH5, RDH10, and RDH11 are mainly responsible for this reaction (78), but additional 11-cis-RDHs may participate within the RPE (67). Newly synthesized 11-cis-retinal is protected by binding to cellular retinaldehyde-binding protein (CRALBP), which mediates its transport back to photoreceptor ROS, where the chromophore couples to opsin, thereby completing the cycle (80). Disrupting the enzymatic steps of chromophore regeneration in the RPE, especially those involving LRAT and/or RPE65, has severe consequences for retinal health. The resulting chromophore deficiency causes slow progressive death of rods that is attributed to continuous activation of visual phototransduction by unliganded opsin (81). Moreover, disordered vectorial transport of cone visual pigments lacking bound chromophore leads to very rapid cone degeneration (82).
Alternative Visual Cycle for Cones?
Although outnumbered by more than 20:1 by rod photoreceptors, cone photoreceptors in the human eye mediate daylight vision and are critical for visual acuity and color discrimination (83). Cones operate under bright light that saturates rods, but rods still consume 11-cis-retinal. This scenario might require an additional cone-specific chromophore regeneration pathway to avoid competition for 11-cis-retinal. Such competition has been demonstrated in the eyes of R91W Rpe65 mutant mice, which produce only minute amounts of chromophore (84).
Older studies in lower vertebrates indicate that cone (but not rod) visual pigment regenerates in isolated neuronal retinas independent of the RPE (reviewed in Ref. (85), but recent work provides evidence that an intraretinal pathway for cone visual pigment regeneration also exists in mice and humans (86). Biochemical analysis of cone-dominant ground squirrels and chickens led to the identification of retinoid-metabolizing enzymes in the neuronal retina and the proposal of a cone-specific pathway (Fig. 3) (87). In this alternative cone-specific visual cycle, ROL released from cone outer segments is taken up by Müller cells, where, in contrast to the RPE, it is directly isomerized back to the 11-cis configuration and subsequently esterified to 11-cis-REs by acyl-CoA:retinol acyltransferase. 11-cis-REs can be mobilized by 11-cis-RE hydrolase to yield 11-cis-retinol, which then binds to CRALBP and is transported back to cone photoreceptors. Finally, NADP+/NADPH-dependent 11-cis-RDH activity found exclusively in cone photoreceptors expedites regeneration of visual chromophore from 11-cis-retinol (87). Studies of the cone-dominated retinas of zebrafish larvae provided in vivo evidence for this alternative cycle. Disruption of the function of RPE65, the canonical visual cycle isomerase, did not completely abolish chromophore regeneration and adversely affected rod rather than cone photoreceptor function (88). Additionally, genetic disruption of Müller glial cell-specific CRALBP did affect cone visual pigment regeneration in fish larvae (89).
In contrast, genetic disruption of RPE65 abolishes both cone and rod vision in mice. In Rpe65−/− mice, some residual light sensitivity has been attributed to rods (90) and is mediated by 9-cis-retinal and isorhodopsin (91). Furthermore, RPE65 is critical for chromophore production and vision in Nrl−/− mice, which possess a cone-only retina due to developmental defects (92). However, this dependence of chromophore production on RPE65 does not contradict an additional cone-specific pathway if we consider that ROL uptake in the eyes occurs via the RPE and is driven by esterification by LRAT (76). This all-trans-RE must be metabolized by RPE65 to the cis-chromophore as noted by the tremendous accumulation of REs in Rpe65−/− mice (19). This bottleneck would explain why both rods and cones are affected in RPE65 deficiency. Additionally, a previous study proposed that cone-specific RPE65 expression contributes to chromophore regeneration (93). The ultimate description of the alternative visual cycle requires identification of genes that encode proteins responsible for the key enzymatic steps.
Visual Cycle in Invertebrate Eyes
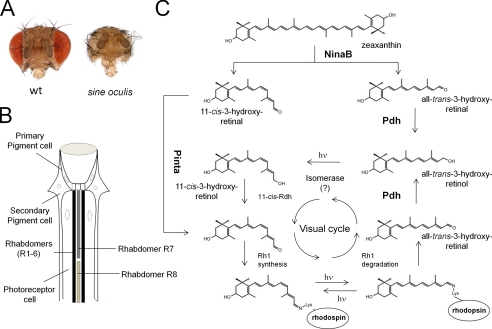
In contrast to vertebrates, retinoid production and function are restricted to the eyes in Drosophila (94). This feature is demonstrable by the absence of retinoids in the sine oculis mutant, which lacks compound eyes (Fig. 4A) (24). Genome-wide microarray analysis of this mutant also revealed eye-enriched expression of genes that have homologs in the vertebrate visual cycle, including ninaB (Rpe65), pinta (CRALBP), and pigment cell-enriched dehydrogenase (PDH; RDH12) (95). NinaB catalyzes the conversion of carotenoids such as zeaxanthin into 11-cis- and all-trans-3-hydroxyretinal in a 1:1 molar ratio (Fig. 4C) (24). The all-trans-3-hydroxyretinal cleavage product is then converted to the chromophore in a light-dependent pathway that lacks molecular description (96). As in vertebrates, a retinaldehyde-binding protein, encoded by the pinta gene, protects the newly synthesized chromophore. PINTA mutants display significantly reduced light sensitivity and evidence a strong reduction of rhodopsin levels (97). Because pinta flies can produce the chromophore, PINTA is likely required for transport of the chromophore from pigment cells to the photoreceptor cells to promote rhodopsin production (24).
FIGURE 4.
Ocular carotenoid/retinoid metabolism in Drosophila. A, images of the heads of wild-type flies and sine oculis flies, which lack compound eyes. B, schematic drawing of a longitudinal section through a fly omatidium. The visual cycle of the fly operates between the photoreceptor cells and the secondary pigment cells. Visual pigments are sequestered in rhabdomeres R1–R6 of photoreceptor cells, which express ninaE, and rhabdomers R7 and R8, which express other opsin genes. C, zeaxanthin is converted to one molecule of 11-cis-3-hydroxyretinal and one molecule of all-trans-3-hydroxyretinal by NinaB expressed in neuronal and glial cells. Transport of 11-cis-3-hydroxyretinal to photoreceptors depends on the PINTA protein. The all-trans-3-hydroxyretinal cleavage product is converted to all-trans-3-hydroxyretinol by PDH. A blue light-dependent isomerase reaction mediates 11-cis-3-hydroxyretinol production. 11-cis-3-Hydroxyretinol is then converted to 11-cis-3-hydroxyretinal. The 11-cis-chromophore binds to different fly opsins to form functional visual pigments. Insect visual pigments are bistable, so the opsin-bound chromophore can be isomerized back to the 11-cis geometric state by light. However, a regeneration pathway for the chromophore is required for the released chromophore from internalized rhodopsin. This all-trans-3-hydroxyretinal is converted by PDH into all-trans-3-hydroxyretinol, which can be recycled back to the chromophore by the enzymatic steps described above. hν, one photon.
Once bound to opsin, the chromophore can be regenerated by invertebrate photoreceptors after bleaching by absorption of another photon (5). Therefore, it was believed that invertebrate eyes do not employ enzymes for chromophore regeneration. Identification of an RDH12 homolog in insects and generation of the corresponding mutant provided evidence that a chromophore regeneration pathway also exists in the fly (98). In PDH mutants, de novo synthesis of the chromophore is not affected. However, illumination leads to a progressive loss of rhodopsin, which is accompanied by degeneration of photoreceptors. Biochemical evidence indicates that PDH catalyzes RAL to ROL conversion. Furthermore, retinal degeneration can be prevented by expressing human RDH12 in the PDH mutant (98).
Flies likely require this pathway to recycle RAL from internalized rhodopsin (98), and the pathway may also prevent RAL toxicity. Such toxicity has been demonstrated in ninaE flies, which lack the major opsin Rh1. Retinal degeneration in this mutant can be prevented by chromophore deficiency, indicating that, as in vertebrates, the aldehyde group of this compound can undergo aberrant side reactions (24). Furthermore, recycling of the chromophore allows adult flies to maintain normal rhodopsin levels when they are exposed to continuous dietary vitamin A deficiency (98). Thus, the pigment cells of the omatidium, expressing PINTA and PDH, display an analogous function to the RPE in the vertebrate eyes by regenerating and supplying the photoreceptor cells with the chromophore (Fig. 4B).
Conclusions
There has been substantial progress in elucidating the metabolism of retinoids and carotenoids related to vision. These studies have revealed an intriguing evolutionary conservation of key components involved in chromophore production and recycling in animals. However, it is also evident that the same genes have adapted to the specific requirements of retinoid biology in invertebrates and vertebrates. Vertebrates have evolved a unique transport, uptake, and storage system for retinoids. Disruption of this system can cause a broad spectrum of pathologies ranging from mild ocular defects to fatal outcomes. The canonical visual cycle of the vertebrate eyes has been described in functional detail. Increasing evidence for an additional cone-specific pathway exists, but this cycle still lacks detailed molecular description. Although insects possess bistable visual pigments, a pathway for chromophore regeneration is still required, indicating that such pathways are intrinsic to vision. Studies in mouse models have helped to understand the pathogenesis of human blinding diseases caused by mutations of genes encoding key components of the canonical retinoid cycle. This knowledge can expedite the development of pharmacological therapies for the prevention and cure of such diseases (79). Only advances in knowledge about the basic chemistry of vision can guarantee that drug discovery and development will progress in parallel.
Supplementary Material
Acknowledgments
I am grateful to Dr. Leslie Webster for critical comments and important suggestions on the manuscript. I extend a special thanks to my present and former coworkers for their great enthusiasm and research contributions.
This work was supported, in whole or in part, by National Institutes of Health Grants EY019641 and EY020551. This is the third article in the Thematic Minireview Series on Focus on Vision.

This article contains supplemental Figs. S1–S3.
- ROL
- all-trans-retinol
- RA
- all-trans-retinoic acid
- RAR
- retinoic acid receptor
- CCE
- carotenoid-cleaving enzyme
- RE
- retinyl ester
- RPE
- retinal pigment epithelium
- RAL
- all-trans-retinal
- SR-BI
- scavenger receptor class B type I
- LRAT
- lecithin:retinol acyltransferase
- RBP
- retinol-binding protein
- ROS
- rod outer segment(s)
- RDH
- retinol dehydrogenase
- CRALBP
- cellular retinaldehyde-binding protein
- PDH
- pigment cell-enriched dehydrogenase.
REFERENCES
- 1. Wolf G. (2001) J. Nutr. 131, 1647–1650 [DOI] [PubMed] [Google Scholar]
- 2. Underwood B. A. (2004) J. Nutr. 134, 231S–236S [DOI] [PubMed] [Google Scholar]
- 3. Travis G. H., Golczak M., Moise A. R., Palczewski K. (2007) Annu. Rev. Pharmacol. Toxicol. 47, 469–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sparrow J. R. (2010) Adv. Exp. Med. Biol. 703, 63–74 [DOI] [PubMed] [Google Scholar]
- 5. Mark M., Ghyselinck N. B., Chambon P. (2006) Annu. Rev. Pharmacol. Toxicol. 46, 451–480 [DOI] [PubMed] [Google Scholar]
- 6. Schwartz S. H., Tan B. C., Gage D. A., Zeevaart J. A., McCarty D. R. (1997) Science 276, 1872–1874 [DOI] [PubMed] [Google Scholar]
- 7. Moise A. R., von Lintig J., Palczewski K. (2005) Trends Plant Sci. 10, 178–186 [DOI] [PubMed] [Google Scholar]
- 8. Lindqvist A., Andersson S. (2002) J. Biol. Chem. 277, 23942–23948 [DOI] [PubMed] [Google Scholar]
- 9. Hessel S., Eichinger A., Isken A., Amengual J., Hunzelmann S., Hoeller U., Elste V., Hunziker W., Goralczyk R., Oberhauser V., von Lintig J., Wyss A. (2007) J. Biol. Chem. 282, 33553–33561 [DOI] [PubMed] [Google Scholar]
- 10. Kiefer C., Hessel S., Lampert J. M., Vogt K., Lederer M. O., Breithaupt D. E., von Lintig J. (2001) J. Biol. Chem. 276, 14110–14116 [DOI] [PubMed] [Google Scholar]
- 11. Hu K. Q., Liu C., Ernst H., Krinsky N. I., Russell R. M., Wang X. D. (2006) J. Biol. Chem. 281, 19327–19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mein J. R., Dolnikowski G. G., Ernst H., Russell R. M., Wang X. D. (2011) Arch. Biochem. Biophys. 506, 109–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amengual J., Lobo G. P., Golczak M., Li H. N., Klimova T., Hoppel C. L., Wyss A., Palczewski K., von Lintig J. (2011) FASEB J. 25, 948–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ford N. A., Clinton S. K., von Lintig J., Wyss A., Erdman J. W., Jr. (2010) J. Nutr. 140, 2134–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamel C. P., Tsilou E., Pfeffer B. A., Hooks J. J., Detrick B., Redmond T. M. (1993) J. Biol. Chem. 268, 15751–15757 [PubMed] [Google Scholar]
- 16. Mata N. L., Moghrabi W. N., Lee J. S., Bui T. V., Radu R. A., Horwitz J., Travis G. H. (2004) J. Biol. Chem. 279, 635–643 [DOI] [PubMed] [Google Scholar]
- 17. Xue L., Gollapalli D. R., Maiti P., Jahng W. J., Rando R. R. (2004) Cell 117, 761–771 [DOI] [PubMed] [Google Scholar]
- 18. Marlhens F., Bareil C., Griffoin J. M., Zrenner E., Amalric P., Eliaou C., Liu S. Y., Harris E., Redmond T. M., Arnaud B., Claustres M., Hamel C. P. (1997) Nat. Genet. 17, 139–141 [DOI] [PubMed] [Google Scholar]
- 19. Redmond T. M., Yu S., Lee E., Bok D., Hamasaki D., Chen N., Goletz P., Ma J. X., Crouch R. K., Pfeifer K. (1998) Nat. Genet. 20, 344–351 [DOI] [PubMed] [Google Scholar]
- 20. Moiseyev G., Chen Y., Takahashi Y., Wu B. X., Ma J. X. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 12413–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Redmond T. M., Poliakov E., Yu S., Tsai J. Y., Lu Z., Gentleman S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 13658–13663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin M., Li S., Moghrabi W. N., Sun H., Travis G. H. (2005) Cell 122, 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oberhauser V., Voolstra O., Bangert A., von Lintig J., Vogt K. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 19000–19005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Voolstra O., Oberhauser V., Sumser E., Meyer N. E., Maguire M. E., Huber A., von Lintig J. (2010) J. Biol. Chem. 285, 2130–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von Lintig J., Dreher A., Kiefer C., Wernet M. F., Vogt K. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 1130–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kloer D. P., Ruch S., Al-Babili S., Beyer P., Schulz G. E. (2005) Science 308, 267–269 [DOI] [PubMed] [Google Scholar]
- 27. Kiser P. D., Golczak M., Lodowski D. T., Chance M. R., Palczewski K. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 17325–17330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Messing S. A., Gabelli S. B., Echeverria I., Vogel J. T., Guan J. C., Tan B. C., Klee H. J., McCarty D. R., Amzel L. M. (2010) Plant Cell 22, 2970–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poliakov E., Gentleman S., Cunningham F. X., Jr., Miller-Ihli N. J., Redmond T. M. (2005) J. Biol. Chem. 280, 29217–29223 [DOI] [PubMed] [Google Scholar]
- 30. Moiseyev G., Takahashi Y., Chen Y., Gentleman S., Redmond T. M., Crouch R. K., Ma J. X. (2006) J. Biol. Chem. 281, 2835–2840 [DOI] [PubMed] [Google Scholar]
- 31. von Lintig J., Kiser P. D., Golczak M., Palczewski K. (2010) Trends Biochem. Sci. 35, 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kushiro T., Okamoto M., Nakabayashi K., Yamagishi K., Kitamura S., Asami T., Hirai N., Koshiba T., Kamiya Y., Nambara E. (2004) EMBO J. 23, 1647–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reboul E., Borel P. (2011) Prog. Lipid Res. 50, 388–402 [DOI] [PubMed] [Google Scholar]
- 34. Kiefer C., Sumser E., Wernet M. F., Von Lintig J. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 10581–10586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Voolstra O., Kiefer C., Hoehne M., Welsch R., Vogt K., von Lintig J. (2006) Biochemistry 45, 13429–13437 [DOI] [PubMed] [Google Scholar]
- 36. Wang T., Jiao Y., Montell C. (2007) J. Cell Biol. 177, 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sakudoh T., Iizuka T., Narukawa J., Sezutsu H., Kobayashi I., Kuwazaki S., Banno Y., Kitamura A., Sugiyama H., Takada N., Fujimoto H., Kadono-Okuda K., Mita K., Tamura T., Yamamoto K., Tsuchida K. (2010) J. Biol. Chem. 285, 7739–7751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rigotti A., Miettinen H. E., Krieger M. (2003) Endocr. Rev. 24, 357–387 [DOI] [PubMed] [Google Scholar]
- 39. Mardones P., Strobel P., Miranda S., Leighton F., Quiñones V., Amigo L., Rozowski J., Krieger M., Rigotti A. (2002) J. Nutr. 132, 443–449 [DOI] [PubMed] [Google Scholar]
- 40. During A., Doraiswamy S., Harrison E. H. (2008) J. Lipid Res. 49, 1715–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bhosale P., Larson A. J., Frederick J. M., Southwick K., Thulin C. D., Bernstein P. S. (2004) J. Biol. Chem. 279, 49447–49454 [DOI] [PubMed] [Google Scholar]
- 42. Li B., Vachali P., Frederick J. M., Bernstein P. S. (2011) Biochemistry 50, 2541–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Bennekum A., Werder M., Thuahnai S. T., Han C. H., Duong P., Williams D. L., Wettstein P., Schulthess G., Phillips M. C., Hauser H. (2005) Biochemistry 44, 4517–4525 [DOI] [PubMed] [Google Scholar]
- 44. Choi M. Y., Romer A. I., Hu M., Lepourcelet M., Mechoor A., Yesilaltay A., Krieger M., Gray P. A., Shivdasani R. A. (2006) Development 133, 4119–4129 [DOI] [PubMed] [Google Scholar]
- 45. Seino Y., Miki T., Kiyonari H., Abe T., Fujimoto W., Kimura K., Takeuchi A., Takahashi Y., Oiso Y., Iwanaga T., Seino S. (2008) J. Biol. Chem. 283, 4905–4911 [DOI] [PubMed] [Google Scholar]
- 46. Lobo G. P., Hessel S., Eichinger A., Noy N., Moise A. R., Wyss A., Palczewski K., von Lintig J. (2010) FASEB J. 24, 1656–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wongsiriroj N., Piantedosi R., Palczewski K., Goldberg I. J., Johnston T. P., Li E., Blaner W. S. (2008) J. Biol. Chem. 283, 13510–13519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Paik J., Vogel S., Quadro L., Piantedosi R., Gottesman M., Lai K., Hamberger L., Vieira Mde M., Blaner W. S. (2004) J. Nutr. 134, 276S–280S [DOI] [PubMed] [Google Scholar]
- 49. van Bennekum A. M., Kako Y., Weinstock P. H., Harrison E. H., Deckelbaum R. J., Goldberg I. J., Blaner W. S. (1999) J. Lipid Res. 40, 565–574 [PubMed] [Google Scholar]
- 50. Goodman D. W., Huang H. S., Shiratori T. (1965) J. Lipid Res. 6, 390–396 [PubMed] [Google Scholar]
- 51. O'Byrne S. M., Wongsiriroj N., Libien J., Vogel S., Goldberg I. J., Baehr W., Palczewski K., Blaner W. S. (2005) J. Biol. Chem. 280, 35647–35657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Episkopou V., Maeda S., Nishiguchi S., Shimada K., Gaitanaris G. A., Gottesman M. E., Robertson E. J. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Quadro L., Blaner W. S., Salchow D. J., Vogel S., Piantedosi R., Gouras P., Freeman S., Cosma M. P., Colantuoni V., Gottesman M. E. (1999) EMBO J. 18, 4633–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Seeliger M. W., Biesalski H. K., Wissinger B., Gollnick H., Gielen S., Frank J., Beck S., Zrenner E. (1999) Invest. Ophthalmol. Vis. Sci. 40, 3–11 [PubMed] [Google Scholar]
- 55. Kawaguchi R., Yu J., Honda J., Hu J., Whitelegge J., Ping P., Wiita P., Bok D., Sun H. (2007) Science 315, 820–825 [DOI] [PubMed] [Google Scholar]
- 56. Isken A., Golczak M., Oberhauser V., Hunzelmann S., Driever W., Imanishi Y., Palczewski K., von Lintig J. (2008) Cell Metab. 7, 258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kawaguchi R., Yu J., Ter-Stepanian M., Zhong M., Cheng G., Yuan Q., Jin M., Travis G. H., Ong D., Sun H. (2011) ACS Chem. Biol. 6, 1041–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pasutto F., Sticht H., Hammersen G., Gillessen-Kaesbach G., Fitzpatrick D. R., Nürnberg G., Brasch F., Schirmer-Zimmermann H., Tolmie J. L., Chitayat D., Houge G., Fernández-Martínez L., Keating S., Mortier G., Hennekam R. C., von der Wense A., Slavotinek A., Meinecke P., Bitoun P., Becker C., Nürnberg P., Reis A., Rauch A. (2007) Am. J. Hum. Genet. 80, 550–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Golzio C., Martinovic-Bouriel J., Thomas S., Mougou-Zrelli S., Grattagliano-Bessieres B., Bonniere M., Delahaye S., Munnich A., Encha-Razavi F., Lyonnet S., Vekemans M., Attie-Bitach T., Etchevers H. C. (2007) Am. J. Hum. Genet. 80, 1179–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clagett-Dame M., DeLuca H. F. (2002) Annu. Rev. Nutr. 22, 347–381 [DOI] [PubMed] [Google Scholar]
- 61. Arshavsky V. Y., Lamb T. D., Pugh E. N., Jr. (2002) Annu. Rev. Physiol. 64, 153–187 [DOI] [PubMed] [Google Scholar]
- 62. Jastrzebska B., Palczewski K., Golczak M. (2011) J. Biol. Chem. 286, 18930–18937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tsybovsky Y., Molday R. S., Palczewski K. (2010) Adv. Exp. Med. Biol. 703, 105–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Haeseleer F., Huang J., Lebioda L., Saari J. C., Palczewski K. (1998) J. Biol. Chem. 273, 21790–21799 [DOI] [PubMed] [Google Scholar]
- 65. Rattner A., Smallwood P. M., Nathans J. (2000) J. Biol. Chem. 275, 11034–11043 [DOI] [PubMed] [Google Scholar]
- 66. Maeda A., Maeda T., Imanishi Y., Kuksa V., Alekseev A., Bronson J. D., Zhang H., Zhu L., Sun W., Saperstein D. A., Rieke F., Baehr W., Palczewski K. (2005) J. Biol. Chem. 280, 18822–18832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Maeda A., Maeda T., Sun W., Zhang H., Baehr W., Palczewski K. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19565–19570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhou J., Jang Y. P., Kim S. R., Sparrow J. R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16182–16187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Janecke A. R., Thompson D. A., Utermann G., Becker C., Hübner C. A., Schmid E., McHenry C. L., Nair A. R., Rüschendorf F., Heckenlively J., Wissinger B., Nürnberg P., Gal A. (2004) Nat. Genet. 36, 850–854 [DOI] [PubMed] [Google Scholar]
- 70. Allikmets R., Shroyer N. F., Singh N., Seddon J. M., Lewis R. A., Bernstein P. S., Peiffer A., Zabriskie N. A., Li Y., Hutchinson A., Dean M., Lupski J. R., Leppert M. (1997) Science 277, 1805–1807 [DOI] [PubMed] [Google Scholar]
- 71. Maeda A., Maeda T., Golczak M., Palczewski K. (2008) J. Biol. Chem. 283, 26684–26693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maeda A., Maeda T., Golczak M., Chou S., Desai A., Hoppel C. L., Matsuyama S., Palczewski K. (2009) J. Biol. Chem. 284, 15173–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Redmond T. M., Wiggert B., Robey F. A., Nguyen N. Y., Lewis M. S., Lee L., Chader G. J. (1985) Biochemistry 24, 787–793 [DOI] [PubMed] [Google Scholar]
- 74. Edwards R. B., Adler A. J. (1994) Exp. Eye Res. 59, 161–170 [DOI] [PubMed] [Google Scholar]
- 75. Saari J. C., Bredberg D. L. (1988) J. Biol. Chem. 263, 8084–8090 [PubMed] [Google Scholar]
- 76. Batten M. L., Imanishi Y., Maeda T., Tu D. C., Moise A. R., Bronson D., Possin D., Van Gelder R. N., Baehr W., Palczewski K. (2004) J. Biol. Chem. 279, 10422–10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Imanishi Y., Gerke V., Palczewski K. (2004) J. Cell Biol. 166, 447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Haeseleer F., Jang G. F., Imanishi Y., Driessen C. A., Matsumura M., Nelson P. S., Palczewski K. (2002) J. Biol. Chem. 277, 45537–45546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Palczewski K. (2010) Trends Pharmacol. Sci. 31, 284–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Saari J. C., Bredberg D. L. (1987) J. Biol. Chem. 262, 7618–7622 [PubMed] [Google Scholar]
- 81. Woodruff M. L., Wang Z., Chung H. Y., Redmond T. M., Fain G. L., Lem J. (2003) Nat. Genet. 35, 158–164 [DOI] [PubMed] [Google Scholar]
- 82. Zhang H., Fan J., Li S., Karan S., Rohrer B., Palczewski K., Frederick J. M., Crouch R. K., Baehr W. (2008) J. Neurosci. 28, 4008–4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mustafi D., Engel A. H., Palczewski K. (2009) Prog. Retin. Eye Res. 28, 289–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Samardzija M., Tanimoto N., Kostic C., Beck S., Oberhauser V., Joly S., Thiersch M., Fahl E., Arsenijevic Y., von Lintig J., Wenzel A., Seeliger M. W., Grimm C. (2009) Hum. Mol. Genet. 18, 1266–1275 [DOI] [PubMed] [Google Scholar]
- 85. Fleisch V. C., Neuhauss S. C. (2010) Prog. Retin. Eye Res. 29, 476–486 [DOI] [PubMed] [Google Scholar]
- 86. Wang J. S., Kefalov V. J. (2009) Curr. Biol. 19, 1665–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mata N. L., Radu R. A., Clemmons R. C., Travis G. H. (2002) Neuron 36, 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schonthaler H. B., Lampert J. M., Isken A., Rinner O., Mader A., Gesemann M., Oberhauser V., Golczak M., Biehlmaier O., Palczewski K., Neuhauss S. C., von Lintig J. (2007) Eur. J. Neurosci. 26, 1940–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fleisch V. C., Schonthaler H. B., von Lintig J., Neuhauss S. C. (2008) J. Neurosci. 28, 8208–8216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Seeliger M. W., Grimm C., Ståhlberg F., Friedburg C., Jaissle G., Zrenner E., Guo H., Remé C. E., Humphries P., Hofmann F., Biel M., Fariss R. N., Redmond T. M., Wenzel A. (2001) Nat. Genet. 29, 70–74 [DOI] [PubMed] [Google Scholar]
- 91. Fan J., Rohrer B., Moiseyev G., Ma J. X., Crouch R. K. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13662–13667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wenzel A., von Lintig J., Oberhauser V., Tanimoto N., Grimm C., Seeliger M. W. (2007) Invest. Ophthalmol. Vis. Sci. 48, 534–542 [DOI] [PubMed] [Google Scholar]
- 93. Tang P. H., Wheless L., Crouch R. K. (2011) J. Neurosci. 31, 10403–10411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Harris W. A., Ready D. F., Lipson E. D., Hudspeth A. J., Stark W. S. (1977) Nature 266, 648–650 [DOI] [PubMed] [Google Scholar]
- 95. Xu H., Lee S. J., Suzuki E., Dugan K. D., Stoddard A., Li H. S., Chodosh L. A., Montell C. (2004) EMBO J. 23, 811–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ozaki K., Nagatani H., Ozaki M., Tokunaga F. (1993) Neuron 10, 1113–1119 [DOI] [PubMed] [Google Scholar]
- 97. Wang T., Montell C. (2005) J. Neurosci. 25, 5187–5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang X., Wang T., Jiao Y., von Lintig J., Montell C. (2010) Curr. Biol. 20, 93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.