Abstract
The respiratory epithelium lining the airway relies on mucociliary clearance and a complex network of inflammatory mediators to protect the lung. Alterations in the composition and volume of the periciliary liquid layer, as occur in cystic fibrosis (CF), lead to impaired mucociliary clearance and persistent airway infection. Moreover, the respiratory epithelium releases chemoattractants after infection, inciting airway inflammation. However, characterizing the inflammatory response of primary human airway epithelial cells to infection can be challenging because of genetic heterogeneity. Using well-characterized, differentiated, primary murine tracheal cells grown at an air–liquid interface, which provides an in vitro polarized epithelial model, we compared inflammatory gene expression and secretion in wild-type and ΔF508 CF airway cells after infection with Pseudomonas aeruginosa. The expression of several CXC-chemokines, including macrophage inflammatory protein–2, small inducible cytokine subfamily member 2, lipopolysaccharide-induced chemokine, and interferon-inducible cytokine–10, was markedly increased after infection, and these proinflammatory mediators were asymmetrically released from the airway epithelium, predominantly from the basolateral surface. Equal amounts of CXC-chemokines were released from wild-type and CF cells. Secreted mediators were concentrated in the thin, periciliary fluid layer, and the dehydrated apical microenvironment of CF airway epithelial cells amplified the inflammatory signal, potentially resulting in high chemokine concentration gradients across the epithelium. Consistent with this observation, the enhanced chemotaxis of wild-type neutrophils was detected in CF airway epithelial cultures, compared with wild-type cells. These data suggest that P. aeruginosa infection of the airway epithelium induces the expression and polarized secretion of CXC-chemokines, and the increased concentration gradient across the CF airway leads to an exaggerated inflammatory response.
Keywords: inflammation, chemotaxis, chemokine, airway, epithelium
The respiratory epithelium lining the airway relies on the mucociliary escalator and a complex network of inflammatory mediators to protect the lung. Bacterial clearance is also dependent on the thin, periciliary liquid layer lining the airway surfaces. This tightly regulated, low-viscosity solution for ciliary beating is rich in the mucins, defensins, and cytokines critical for host defense (1). Alterations in the composition and volume of the airway surface layer, as occur in cystic fibrosis (CF), can lead to impaired mucociliary clearance and persistent airway infection. Moreover, the inflammatory response of the CF airway to endobronchial infection is exaggerated, and accounts for progressive, suppurative pulmonary disease (2). Bronchoalveolar lavage (BAL) and airway biopsies from patients with CF demonstrate an intense endobronchial inflammatory process characterized by high concentrations of proinflammatory cytokines and large numbers of neutrophils, even in patients with clinically mild disease (3).
Bacterial infection with Pseudomonas aeruginosa is the most common insult associated with the decline of lung function in CF lung disease. However, studying infection in a native, intact lung is difficult, and examinations of the specific effects of infection on epithelial responses are confounded by the varied environmental and genetic backgrounds of host cells. The stimulation of airway epithelial cells (AECs) with various agents, including Pseudomonas aeruginosa and its exotoxins, was studied using transformed cell lines from individuals with and without CF, with variable results (4–14). The genetic manipulation of AECs to correct or induce CF transmembrane conductance regulator (CFTR) mutations or physiology can exert profound effects on cellular differentiation, morphology, function, and inflammatory gene expression (15). Primary cultures of human AECs pose additional drawbacks as in vitro models because of their genetic heterogeneity, making it difficult to clearly define the regulation of epithelial responses to inflammatory stimuli.
Several critical questions related to epithelial responses to bacteria in the normal and CF lung must be addressed to understand why chronic inflammation is central to the pathophysiology of many airway disorders, including the apparent dysregulation of inflammatory responses in the CF lung. Our previous work implicated the importance of dysregulated airway epithelial cell cytokine responses, not directly related to the CFTR function, in the pathophysiology of CF. As the first-line defender within the lung, the epithelial cell is polarized not only in structure but also in the secretion of biochemical signals. Basolateral secretion is required to signal subepithelial and vascular inflammatory cells, whereas apical signals may be important for communication with luminal inflammatory cell populations (16, 17). The identification of these vectorial responses to bacterial infection has not been defined, but may provide targets for controlling the inflammation that injures the CF lung.
Genomic assays have been useful for identifying differences in models of normal and CF AECs, and complementing these with proteomic approaches could determine specific inflammatory responses after bacterial stimulation. Pairing these approaches with genetically defined epithelial models is essential, and the system must also have the capacity to monitor vectorial responses. Accordingly, we examined syngeneic murine tracheal epithelial cells (mTECs) grown in primary cultures at an air–liquid interface (ALI), thus providing a reproducible, genetically homogeneous epithelium in vitro to evaluate inflammatory gene regulation and expression in airway cells. These well-characterized epithelial cell preparations approximate native respiratory epithelium, and provide a system in which genetic and environmental factors can be manipulated and polarized responses can be measured (18). Using this model, we examined the murine airway epithelial response to infection with P. aeruginosa, compared chemokine gene expression in CF and wild-type (WT) epithelial cells, and examined their effects on neutrophil chemoattraction. These studies confirmed the asymmetric release of CXC-chemokines (19). The secreted mediators were concentrated at the apical surface of AECs in a CFTR-dependent manner, and the increased concentration gradient across CF epithelia resulted in greater neutrophil chemotaxis.
MATERIALS AND METHODS
Mice
We studied ΔF508 CFTR mice backcrossed into a C57Bl/6 background and wild-type littermates, 6–8 weeks of age (20). The Animal Studies Committee at Washington University approved all the present experiments.
In Vitro Murine Primary Airway Epithelial Cell Preparations
Primary mTEC preparations were cultured as previously described (18) (see the online supplement for details).
Electrophysiology
Electrophysiological measurements of mTECs were performed using Ussing chambers adapted for Transwell filters, modified from previously described protocols (21).
Pseudomonas aeruginosa Culture and Cell Infection
Epithelial cell preparations were apically treated with 50 μl P. aeruginosa strain PAO1 (0, 104, 106, and 108 CFU/ml) for 1 hour at 37°C. Cells were washed, and serum-free media containing gentamicin were placed in both chambers of the Transwell (18). At each time point, conditioned basolateral media and apical washings (100 μl) were collected and assayed separately.
RNA Extraction and Oligonucleotide Array Analyses
Total cellular RNA was isolated from treated and control cell preparations using Trizol reagent (Invitrogen, Carlsbad, CA), and then repurified (Qiagen, Valencia, CA). Three replicate pools from each condition were hybridized to Illumina BeadChips (San Diego, CA), and the resulting images were imported into the Illumina BeadStudio application. Data were normalized and imported into the Partek Genomic Suite (St. Louis, MO) for ANOVA with a step-up false-discovery rate according to Benjamini and Hochberg, with multiple testing corrections (22).
TaqMan Real-Time Polymerase Chain Reaction
Total RNA from mTEC cultures was reverse-transcribed (Applied Biosystems, Foster City, CA), and standard quantitative real-time reverse transcription PCR (qRT-PCR) protocols were applied. Aliquots of cDNA (in triplicate) were subjected to qRT-PCR, using the Applied Biosystems 7500 Real Time PCR System and software, and were normalized to glyceraldehyde-3-phosphate dehydrogenase.
Enzyme-Linked Immunosorbent Assay for Secreted Murine Cytokines
CXC-chemokines were measured according to the manufacturer's instructions (R&D Systems, Minneapolis, MN) (23). Apical surface fluid volumes were calculated, based on the volume equation for a right circular cylinder (V = πr2h), using Transwell surface area (0.33 cm2) and previously measured fluid depths (WT, 7.9 μm; CF, 3.9 μm) (24, 25). Concentrations of chemokines were determined by dividing the amount of mediator secreted into the specific compartment by fluid volume.
In Vitro Neutrophil Chemotaxis Assays
Murine bone marrow cell suspensions were isolated from the femurs and tibia of WT mice by flushing with PBS (26). Cells were resuspended, and 6 hours after mTECs were stimulated with P. aeruginosa, 1.0 × 107 bone marrow cells were added to the basolateral compartment and incubated for 1 hour. Cells were then detached, using trypsin–EDTA and cell-dissociation solution (Sigma-Aldrich, St. Louis, MO), and collected. Released cells were washed, blocked with purified rat anti-mouse CD16/CD32 antibody (Mouse BD Fc Block; Becton Dickinson Biosciences, San Jose, CA), and stained with Gr1 antibody or rat IgG2b isotype control (eBioscience, San Diego, CA). Analyses were performed on a FACSCalibur flow cytometer (Franklin Lakes, NJ), using CELLquest software (Becton Dickinson Biosciences).
Statistical Analysis
Data are expressed as means ± standard error (SEM). Statistical comparisons between CF and non-CF groups were performed using unpaired two-tailed Student t tests or single-factor ANOVA.
RESULTS
Murine Airway Epithelial Cell Model
The mTEC preparations were established using ALI culture conditions, which create an airway model consisting of a highly differentiated epithelium, composed of ciliated and nonciliated cells. Immunostaining and scanning electron microscopic examination of non-CF and CF airway epithelial cultures revealed that cells of both genetic strains were similarly differentiated and exhibited features of the native tracheobronchial epithelium in vivo (data not shown).
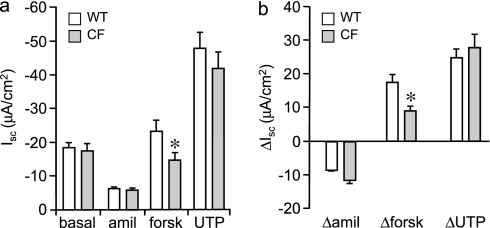
The ion transport function of mTEC preparations was measured for comparison to previous studies of WT and CF murine airways (Figure 1). WT and CF mTEC preparations demonstrated a similar short-circuit current (Isc) under basal conditions that was approximately 75% sensitive to 10 μM amiloride, indicating activity of the epithelial sodium channel. Subsequent treatment with 10 μM forskolin to stimulate intracellular cyclic adenosine monophosphate resulted in an increased Isc response in all preparations, but the magnitude of the response in CF mTECs was significantly lower than in WT mTECs, consistent with a loss of CFTR channel activity. The lower Isc response to forskolin in CF mTECs likely represents the mobilization of calcium and the secretion of anions via alternate chloride conductance, indicating that the Isc response of mTEC preparations is more aligned with ex vivo murine airway preparations than with primary murine airway epithelial cells placed in standard submersion culture (27). Treatment with 100 μM uridine 5′ triphosphate (UTP) to maximize the mobilization of calcium for the activation of calcium-dependent chloride channels resulted in a similar increase in Isc between the WT and CF mTEC preparations.
Figure 1.
Electrolyte transport in primary cultures of murine tracheal epithelial cells (mTECs). After 14 days of air–liquid interface (ALI) culture, wild-type (WT) and ΔF508 cystic fibrosis (CF) mTECs were evaluated for bioelectric properties using voltage-clamped Ussing chambers. In physiological Ringer's solution, all ALI monolayers exhibited electrical resistance (600–1,500 Ω/cm2) before studies. (a) Short-circuit current (Isc) of WT and CF mTECs under basal conditions and after sequential additions of amiloride (amil; 10 μM, apical bath), forskolin (forsk; 10 μM, apical and basolateral baths), and UTP (100 μM, apical bath). (b) Changes in Isc of WT and CF mTECs in response to sequential treatments with amiloride, forskolin, and UTP. Means ± SEM are shown (n = 5–6 Transwells). *Electrophysiology of CF mTECs was significantly different from that of WT cells, at P ≤ 0.05 (unpaired Student t tests).
Pseudomonas aeruginosa Infection of mTEC Preparations
After incubation with the P. aeruginosa strain PAO1 for 1 hour, cell junctions expressed zona occludence protein 1 (ZO-1) and E-cadherin, and maintained high transepithelial cell resistance, indicating that the model epithelium remained intact. Furthermore, exposure to P. aeruginosa did not alter the viability, transepithelial resistance, or cell number of WT or CF mTECs after 24 hours (data not shown).
Expression of Inflammatory Genes in Murine Tracheal Epithelial Cells
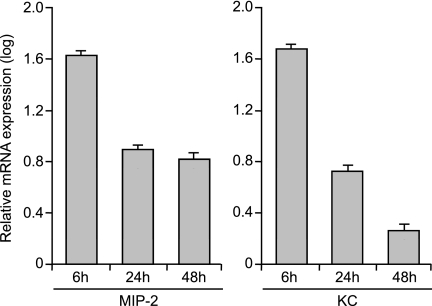
We examined inflammatory gene expression profiles in the in vitro epithelial model, focusing on CXC-chemokines. The expression of chemokines is important in the initiation and resolution of inflammation. RNA was isolated from polarized airway epithelial cells 6, 24, and 48 hours after infection, processed, and hybridized to microarrays. Specific samples were normalized to others and against the mean of control samples. Within the small set of transcripts that changed, several related CXC-chemokines were up-regulated in P. aeruginosa–infected airway cells, including small, inducible cytokine subfamily member 2 (KC, or Cxcl1), macrophage inflammatory protein–2 (MIP-2, or Cxcl2), lipopolysaccharide-induced chemokine (LIX, or Cxcl5), and interferon-inducible cytokine (IP-10, or Cxcl10) (Table 1). The expression of CXC-chemokines was validated using qRT-PCR. Peak chemokine mRNA expression occurred 6 hours after infection, and decreased over time (Figure 2). These changes paralleled the pattern of mRNA expression observed in the microarray analysis.
TABLE 1.
QUANTITATIVE ANALYSIS OF CXC-CHEMOKINE EXPRESSION IN WILD-TYPE MURINE TRACHEAL EPITHELIAL CELLS AFTER INFECTION WITH P. AERUGINOSA
| Chemokine | Aliases | 6 Hours | 24 Hours | 48 Hours |
|---|---|---|---|---|
| Cxcl1 | KC, GROα | 25.88† | 7.83† | 6.50† |
| Cxcl2 | MIP-2, GROβ | 32.74† | 8.79† | 7.64† |
| Cxcl4 | PF4 | 1.62 | 0.19 | 1.15 |
| Cxcl5 | LIX | 4.69† | 3.13† | 5.13† |
| Cxcl7 | PPBP | 1.93 | 5.02* | 5.34* |
| Cxcl9 | MIG | 0.46 | 1.56 | 4.80 |
| Cxcl10 | IP-10 | 6.87* | 7.44† | 3.49 |
| Cxcl11 | IP-9 | 0.39 | 0.99 | 0.46 |
| Cxcl12 | SDF1 | 1.17 | 1.08 | 1.08 |
| Cxcl13 | BLC | 0.87 | 0.57 | 0.12 |
| Cxcl14 | BRAK | 0.81 | 0.88 | 0.53 |
| Cxcl15 | Lungkine | 0.87 | 0.21 | 0.32 |
| Cxcl16 | SCYB16 | 1.22 | 1.02 | 0.93 |
Definition of abbreviations: BLC, B lymphocyte chemoattractant; BRAK, breast and kidney-expressed chemokine; Gro, growth-regulated oncogene; MIG, monokine induced by interferon-γ; PF4, platelet factor 4; PPBP, pro-platelet basic protein; SCYB16, small inducible cytokine subfamily B member 16; SDF1, stromal cell–derived factor-1.
Gene expression consistently up-regulated twofold was considered, and 114 genes were significantly increased after infection. Several related CXC-chemokines, including KC (Cxcl1), MIP-2 (Cxcl2), LIX (Cxcl5), and IP-10 (Cxcl10), were up-regulated at different times after 1 hour of stimulation with Pseudomonas aeruginosa PAO1.
*P ≤ 0.05 and †P ≤ 0.005, using single-factor ANOVA for comparisons.
Figure 2.
mRNA expression of representative CXC-chemokines, macrophage inflammatory protein–2 (MIP-2) and inducible cytokine subfamily member 2 (KC), at different times (6, 24, and 48 hours; n = 3–6 Transwells) after infection with Pseudomonas aeruginosa strain PAO1, using quantitative real-time reverse transcription PCR, thus confirming the microarray data. Peak CXC-chemokine expression was evident 6 hours after infection, and decreased with time. Data are presented relative to uninfected mRNA concentrations, normalized with the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as control. Means ± SEM are shown, and values differ significantly (ANOVA, P < 0.05).
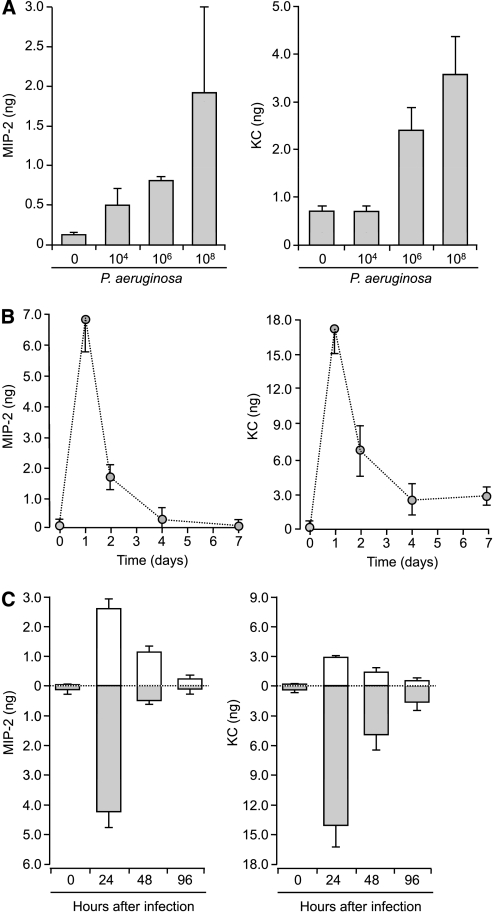
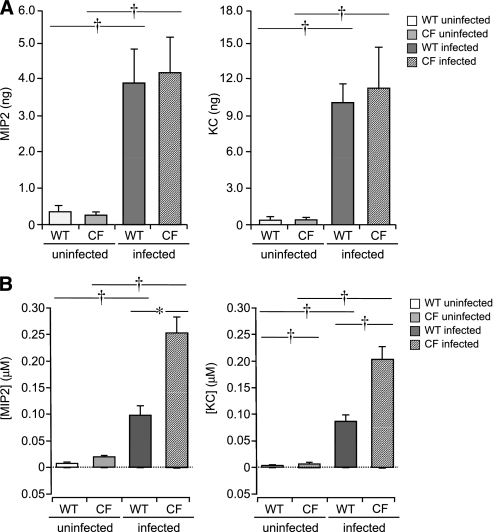
To characterize the dynamic range of inflammatory responses from the epithelium to infection, the apical surface of mTEC preparations was treated with increasing concentrations of P. aeruginosa PAO1 for 1 hour. Twenty-four hours after infection, apical surface fluid and conditioned basolateral media were collected and analyzed for the secretion of proinflammatory cytokines and chemokines, using specific ELISA (19). MIP-2 and KC were released from mTECs in a dose-dependent fashion (Figure 3a). The stimulation of epithelial cells with the highest concentration of bacteria resulted in the greatest release of CXC-chemokines, and was used in subsequent experiments. A time course revealed that the maximal total secretion of MIP-2 and KC occurred 24 hours after stimulation, and gradually decreased to prestimulation levels 7 days after infection (Figure 3b). Surprisingly, proinflammatory cytokines commonly found in CF BAL (IL-6, IL-1β, and TNF-α) were not detected after stimulation with P. aeruginosa (data not shown), suggesting that airway epithelial cells were not the major source of these cytokines after bacterial infection.
Figure 3.
Dose–response and time course for release of CXC-chemokines from WT mTECs after infection with P. aeruginosa. (a) Release of total MIP-2 and KC from mTECs 24 hours after infection with increasing doses of P. aeruginosa PAO1 (0, 104, 106, and 108 CFU/ml, n = 5–7 Transwells). (b) Total secretion of MIP-2 and KC at different times after infection (0, 1, 2, 4, and 7 days) with P. aeruginosa (108 CFU/ml, n = 6 Transwells). Peak release of CXC-chemokines occurred 24 hours after infection and decreased over time, which paralleled mRNA expression. (c) Vectorial secretion of chemokines from WT mTECs after infection with P. aeruginosa. After stimulation of WT mTECs with 108 CFU/ml P. aeruginosa PAO1 for 1 hour, apical washings or basolateral media were collected 0, 24, 48, or 96 hours after infection, and assayed for MIP-2 and KC (n = 5 Transwells). Means ± SEM are shown. Apical and basolateral CXC-chemokine concentrations before and after stimulation differed significantly (ANOVA, P < 0.005). Open columns indicate apical secretion; shaded columns indicate basolateral secretion. Both CXC-chemokines were predominately secreted from the basolateral surface, based on comparisons at different times after infection.
Polarity of Chemokine Release by Murine Airway Monolayers
Using this epithelial model, we examined the effects of stimulation with P. aeruginosa on the vectorial release of chemokines and cytokines, since the asymmetric release of these factors is critical for establishing gradients of immune cell movement. The patterns of chemokine secretion were examined by sampling the apical and basolateral chambers provided in the Transwell culture system following P. aeruginosa infection (19). Consistently greater amounts of MIP-2 and KC were released basolaterally (Figure 3c), and LIX and IP-10 had similar patterns of release (data not shown).
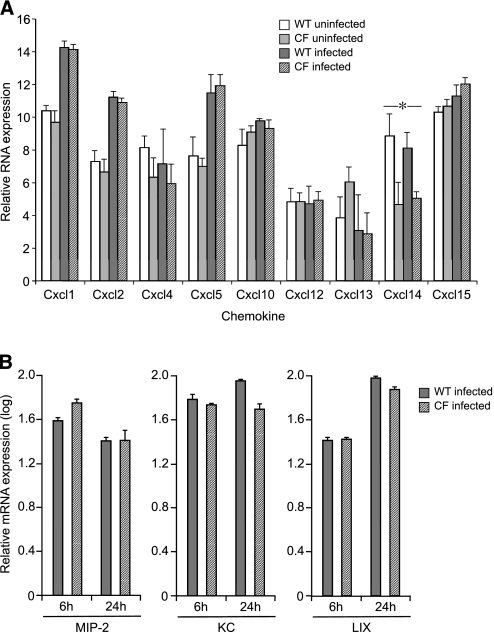
To assess the inflammatory responses in CF airway epithelia, chemokine mRNA expression and secretion in primary mTEC cultures of ΔF508 CF and WT littermates were compared. One chemokine, CXCL14 (BRAK), was expressed at a higher concentration in WT cells compared with CF cells (Figure 4a), although this difference was not confirmed using ELISA (data not shown). Other candidate CXC-chemokine gene expressions were similar before and 6 and 24 hours after infection (Figure 4b).
Figure 4.
CXC-chemokine mRNA expression of WT and ΔF508 CF mTECs, before and after stimulation with P. aeruginosa PAO1. (a) Microarray analyses of candidate chemokine expression compare responses of WT and ΔF508 CF mTECs (n = 3 Transwells). Means ± SEM are shown. *P ≤ 0.05. (b) Quantitative analysis of representative CXC-chemokine expression (MIP-2, KC, and LIX) in WT and ΔF508 CF mTECs, 6 and 24 hours after infection (n = 3 Transwells). Data are presented relative to uninfected mRNA concentrations, and are normalized to GAPDH mRNA. Means ± SEM are shown. No difference was detected between WT and ΔF508 mTEC chemokine expression before or after infection, using unpaired Student t tests for comparisons.
No differences were evident in total measured amounts of released MIP-2, KC, IP-10, or LIX in WT compared with ΔF508 CF mTECs (Figure 5a). These values represent the total amounts of chemokines released into the basolateral media and apical surface during the period after infection. Considering that the CF apical fluid volume is dehydrated because of increased water absorption across the CF airway epithelium (24, 28–30), the concentration of CXC-chemokines at the surface of CF epithelial cells could be significantly greater than measured for WT cells, based on airway surface fluid volumes (1.2 μL and 2.6 μl, respectively) 24 hours after infection (Figure 5b). Thus, the proinflammatory response would be heightened at the surface of CF cells compared with WT cells. Significant differences in surface (apical) concentrations of chemokines were also present before infection (Figure 5b). The basolateral concentrations of CXC-chemokines secreted from WT and CF mTECs were not different.
Figure 5.
Total chemokine secretion and concentrations of WT and ΔF508 CF mTECs, 24 hours after stimulation with P. aeruginosa PAO1. (a) Total MIP-2 and KC released from WT and ΔF508 CF mTECs, before and 24 hours after infection with 108 CFU/ml P. aeruginosa PAO1 (n = 5–7 Transwells). No differences were evident in patterns or quantities of chemokines released from WT and ΔF508 CF mTECs. (b) Apical and basolateral concentrations of MIP-2 and KC were derived, using calculated apical surface volumes for WT and ΔF508 CF mTECs before and 24 hours after infection with 108 CFU/ml P. aeruginosa PAO1 (n = 5–7 Transwells). Means ± SEM are shown. *P ≤ 0.05 and †P ≤ 0.005, using unpaired Student t tests for statistical comparisons. Although CXC-chemokines were primarily secreted basolaterally, greater concentrations were present at the apical surface because of the minute volume of surface-lining fluid. The apical microenvironment of airway epithelial cells amplifies the inflammatory signal, which is greater at the surface of CF epithelia.
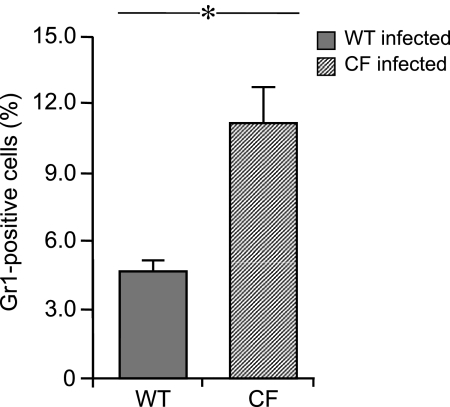
To test whether differences in apical chemokine concentrations were of functional significance, we examined the transit of murine WT neutrophils across polarized WT and CF murine epithelial cells grown on Transwell supports in response to stimulation with P. aeruginosa. Consistently greater numbers of murine neutrophils migrated through Transwells when co-cultured with CF mTECs compared with WT mTECs (Figure 6), which indicates a relative difference in the chemoattractant gradient across CF cells compared with WT cells.
Figure 6.
Neutrophil chemotaxis in WT and ΔF508 CF mTECs, 6 hours after apical stimulation with 108 CFU/ml P. aeruginosa PAO1 (n = 4–6 Transwells) in replicate experiments. Murine WT bone marrow cells were added to the basolateral compartment and incubated for 1 hour. Cells from the upper surface of Transwell supports were stained with Gr1 antibody. Greater numbers of murine neutrophils migrated through Transwells when co-cultured with infected CF airway epithelial cells. Means ± SEM are shown. *P ≤ 0.01, using unpaired Student t tests for statistical comparisons.
DISCUSSION
Chronic infection and neutrophilic inflammation are hallmarks of CF airway diseases. In this manuscript, we report our systematic and comprehensive study of chemokine responses in a genetically defined, polarized airway epithelial cell model, which demonstrated increased expression and asymmetric CXC-chemokine secretion after infection with P. aeruginosa. The bacteria-stimulated airway epithelium contributed to the inflammatory response by attracting immune cells through a vectorial chemokine signal. Furthermore, the desiccated microenvironment of the apical surface of CF epithelia concentrated the inflammatory signal, as evidenced by greater chemotaxis to murine CF epithelial cells compared with WT cells.
Because epithelial cells that line the airways express CFTR and contribute to the inflammatory response, several investigators postulated that the CF epithelial cell mediates a dysregulated inflammatory response. Different regulatory pathways have been implicated in the exaggerated inflammatory response described in CF airways (31–35). Potentially, CF AECs could amplify the recruitment of neutrophils through a constitutive and pathogen-induced release of inflammatory mediators disproportionate to the infectious threat. In vitro and in vivo CF epithelial cell models produce a greater release of CXC-chemokines and other mediators, either spontaneously or after exposure to inflammatory stimuli, compared with WT or complemented cells. Microarray expression profiles of immortalized cell lines after infection with Pseudomonas showed quantitative differences in the expression of genes in CF AECs controlled by the NF-κB transcription factor (32, 33). However, none of these studies examined functional effects of the exaggerated expression of chemokines.
The epithelial cell lines used in many of these investigations were derived from patients with CF, and CFTR-complemented control cell lines were created using plasmid transfection or viral vector infection for the transient or stable expression of wild-type CFTR. These lines are frequently derived from a single cell type, and their phenotype may be altered because of genetic manipulation or the unique genetic features of the individual from whom the cells were obtained. Other approaches reduced the expression or function of CFTR in epithelial cell lines from normal individuals by overexpressing the regulatory domain or antisense oligonucleotides. Such models are advantageous for “matched” comparisons of isogenic CF and non-CF cell lines when studying physiology (4–9). However, immortalization and complementation can affect cell morphology, function, and gene expression, thus limiting their usefulness in defining epithelial inflammatory responses. And not all reports have described a hyperinflammatory response in CF epithelial cells when compared with normal or complemented cells (10–14). We have previously shown that in vitro CF airway epithelial cell lines differ in their responses to inflammatory stimuli (15). The genetic heterogeneity of primary human-derived cells may affect inflammatory gene expression because of non-CFTR genetic modifiers, underscoring a limitation of these cells. Here, we used a murine tracheal epithelium model of polarized, well-differentiated CF and WT epithelia, consisting of syngeneic cells, with a cellular composition similar to that of the native murine airway. Ion transport in the mTEC model is consistent with reports of ex vivo tracheal preparations (25). Although this model may be limited by differences in murine strains, it provided a highly reproducible response to infection that can be directly compared among different CFTR mutations (36).
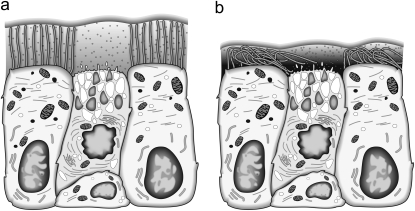
In response to inhaled bacteria, the airway epithelial cell is armed with cytokines and chemokines that can be vectorially secreted to control the inflammatory response. Within the airway lumen, concentrations of chemokines are controlled by the microenvironment of minute fluid volume covering the apical surface. The volume and composition of airway surface liquid are modified by ion transport across epithelial cells via the generation of osmotic gradients that regulate transepithelial fluid movement. The airway epithelium finely adjusts and regulates the volume of the thin airway-surface fluid layer, and the essential role of epithelial ion transport in this process is illustrated in the CF airway. In the absence of functioning CFTR, the impaired secretion of chloride and unregulated absorption of sodium lead to the depletion of airway-surface fluid. Using confocal microscopy to measure airway surface fluid, the depth of surface fluid covering human CF AECs grown in primary cultures was consistently half that measured in non-CF cells (24, 28–30). The depleted airway fluid layer causes impaired mucociliary clearance and chronic bacterial colonization (1), which are cardinal features of CF. Our observations extend this hypothesis, and suggest that the dehydrated airway surface fluid layer concentrates chemokines released at the apical surface after infection (Figure 7), amplifying the inflammatory response. This phenomenon may be present even in nonstimulated CF cells, possibly explaining the inflammatory phenotype described in uninfected CF airway models (37).
Figure 7.
Schematic diagram shows the concentration effect of secreted chemokines at the apical surface of WT (a) and CF (b) airway epithelia, which is greater in CF cells and is related to the desiccation of the periciliary fluid space.
The response of different epithelia to inflammatory stimuli is asymmetric, as described in other model systems. For example, polarized human bronchial epithelial cells release IL-8 and regulated on activation, normal T expressed and secreted chemokine (RANTES) from the basolateral side after stimulation with respiratory syncytial virus (RSV) (38). We observed similar responses in respiratory epithelial cells. Moreover, IL-8 is preferentially released at the basolateral surface of human colonic epithelial cells after treatment with enterotoxin (16), and in polarized renal tubular epithelial cells after stimulation with IL-1α (39). Although the chemokines we examined (MIP-2, KC, LIX, and IP-10) are released basolaterally, higher concentrations of chemokines were measured at the apical surface. Similar to our previous investigations using differentiated human airway epithelial cells (15), these results did not demonstrate a greater release of inflammatory mediators from CF mTECs.
Several inflammatory mediators, including CXC family members (MIP-2, KC, LIX, and IP-10), were up-regulated and preferentially released basolaterally. Interestingly, those CXC-chemokines cluster in the same region of chromosome 5 in the mouse, and are conserved in a similar structure on human chromosome 4, suggesting shared regulation. This region in the human genome is proximate to the IL-8 gene, the primary chemoattractant in the CF lung (40, 41), indicating a redundant role in the inflamed lung (42, 43). All four chemokines are involved in neutrophil chemotaxis mediated through CXC receptors 1 and 2 (44), consistent with the neutrophil-dominated pathology of the CF lung and corroborating the validity of responses in our cell model.
Although the concentration of basolaterally secreted chemokines did not differ between CF and WT cells, several factors may affect concentrations at the apical surface. The volume and composition of apical surface liquid are modified by ion transport across epithelial cells by generating osmotic gradients that regulate transepithelial fluid movement. The essential role of epithelial ion transport for this process is illustrated in the CF airway, where the impaired secretion of chloride and unregulated absorption of sodium lead to the depletion of airway surface fluid. Several studies showed that the depth of surface fluid covering human CF AECs grown in primary culture was consistently half that measured in non-CF cells (24, 28–30). The dehydrated airway surface fluid layer concentrates chemokines released at the apical surface after infection, amplifying the inflammatory response and thus promoting greater neutrophil chemotaxis. The effect of reduced apical fluid volume may even be greater during acute viral infections of the respiratory tract. Infections with RSV inhibit nucleotide-dependent periciliary fluid homeostasis in CF airway epithelial cells in vitro, further reducing the depth of periciliary fluid (28).
We present our findings with the caveat that airway epithelia from different species can express different electrophysiological properties (45), which could affect our assumptions about airway surface fluid volumes. Alternately, intrinsic differences in cell junctions may exist between CF and WT epithelial cells that enhance the ability of neutrophils to migrate pericellularly (46). Regardless, relative to the in vivo condition, this model may underestimate the inflammatory response, because it does not include the contributions of chemokine secretion from immune cells that reside at the airway surface (23).
In conclusion, our findings show that inflammatory mediators in the P. aeruginosa–infected airway model up-regulate CXC-chemokines, and activate a primarily basolateral release of chemokines from the airway epithelium. However, the minute surface fluid volume that covers the airway results in greater apical CXC concentrations, creating a concentration gradient across the epithelium. Moreover, because the apical microenvironment of the CF airway is different from that of normal epithelium with decreased airway surface fluid volume, the apical chemokine concentrations are even greater, thus signaling neutrophils to migrate into the CF airway lumen. Already a therapeutic target for CF lung disease (47), the restoration of apical surface fluid volume could confer the benefit of reducing the inflammatory response by lowering this gradient.
Supplementary Material
Acknowledgments
The authors thank Robert Senior and Diane Kelley for their assistance with neutrophil chemotaxis assays, and Lisa Hogue, Mary Baumann, Yingjian You, and Sara Pittman for expert technical support. The authors also thank Jingnan Mao for advice concerning the statistical analyses used in these experiments.
This research was supported by National Institutes of Health grant R01 HL08265 (T.W.F.), the March of Dimes, and Ruth L. Kirschstein National Research Service Award 2T32 HL007873 (M.M.F.) from the National Heart, Lung, and Blood Institute. Cell culture work was supported by the Respiratory Epithelial Cell Core of the Children's Discovery Institute of St. Louis Children's Hospital and Washington University.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0249OC on July 16, 2010
Author Disclosure: S.C. received an industry-sponsored grant from Pfizer ($1,001–$5,000), and holds stock in Isis Pharmaceuticals ($5,001–$10,000). M.M.F. received a sponsored grant from the National Institutes of Health ($10,001–$50,000). S.L.B. received a sponsored grant from the National Institutes of Health (more than $100,000) and payment for consultancies ($1,001–$5,000). T.W.F. received payment for lectures from Genentech ($5,001–$10,000), Healthmatters CME ($1,001–$5,000), and the France Foundation ($1,001–$5,000); received industry-sponsored grants from Gilead ($10,001–$50,000) and Boehringer-Ingelheim ($10,001–$50,000); has six United States patents with Case Western Reserve University (drug and gene delivery systems; no financial benefits); received grant support from the National Institutes of Health (more than $100,000), the Cystic Fibrosis Foundation (more than $100,000), and the Children's Discovery Institute (more than $100,000); and served on the American Board of Pediatrics ($1,001–$5,000). None of the other authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 2002;109:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elizur A, Cannon C, Ferkol T. Inflammation in the cystic fibrosis lung. Chest 2008;133:489–495. [DOI] [PubMed] [Google Scholar]
- 3.Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med 1994;150:448–454. [DOI] [PubMed] [Google Scholar]
- 4.Tabary O, Zahm JM, Hinnrasky J, Couetil JP, Cornillet P, Guenounou M, Gaillard D, Puchelle E, Jacquot J. Selective up-regulation of chemokine IL-8 expression cystic fibrosis bronchial gland cells in vivo and in vitro. Am J Pathol 1998;153:921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabary O, Escotte S, Couetil JP, Hubert D, Dusser D, Puchelle E, Jacquot J. Relationship between IkBα deficiency, NF-kB activity and interleukin-8 production in CF human airway epithelial cells. Eur J Phys 2001;443:S40–S44. [DOI] [PubMed] [Google Scholar]
- 6.Kube D, Sontich U, Fletcher D, Davis PB. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am J Physiol Lung Cell Mol Physiol 2001;280:L493–L502. [DOI] [PubMed] [Google Scholar]
- 7.Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol 1999;104:72–78. [DOI] [PubMed] [Google Scholar]
- 8.Blackwell TS, Sencenko AA, Christman JW. Dysregulated NF-κB activation in cystic fibrosis: evidence for a primary inflammatory disorder. Am J Physiol Lung Cell Mol Physiol 2001;281:L69–L70. [DOI] [PubMed] [Google Scholar]
- 9.Stecenko AA, King G, Torii K, Breyer RM, Dworski R, Blackwell TS, Christman JW, Brigham KL. Dysregulated cytokine production in human cystic fibrosis bronchial epithelial cells. Inflammation 2001;25:145–155. [DOI] [PubMed] [Google Scholar]
- 10.Becker MN, Sauer MS, Muhlebach MS, Hirsh AJ, Wu Q, Verghese MW, Randell SH. Cytokine secretion by cystic fibrosis airway epithelial cells. Am J Respir Crit Care Med 2004;169:645–653. [DOI] [PubMed] [Google Scholar]
- 11.Massengale ARD, Quinn F, Yankaskas J, Weissman D, McClellan WT, Cuff C, Aronoff SC. Reduced interleukin-8 production by cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol 1999;20:1073–1080. [DOI] [PubMed] [Google Scholar]
- 12.Pizurki L, Morris MA, Chanson M, Solomon M, Pavirani A, Bouchardy I, Suter S. Cystic fibrosis transmembrane conductance regulator does not affect neutrophil migration across cystic fibrosis airway epithelial monolayers. Am J Pathol 2000;156:1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryan R, Kube D, Perez A, Davis P, Prince A. Overproduction of the CFTR R domain leads to increased levels of asialoGM1 and increased Pseudomonas aeruginosa binding by epithelial cells. Am J Respir Cell Mol Biol 1998;19:269–277. [DOI] [PubMed] [Google Scholar]
- 14.Scheid P, Kempster L, Griesenbach U, Davies JC, Dewar A, Weber PP, Colledge WH, Evans MJ, Geddes DM, Alton EW. Inflammation in cystic fibrosis airways: relationship to increased bacterial adherence. Eur Respir J 2001;17:27–35. [DOI] [PubMed] [Google Scholar]
- 15.Aldallal N, McNaughton EE, Manzel LJ, Rabe AM, Karp PH, Zabner J, Jacquot J, Ferkol TW, Look DC. Inflammatory response in airway epithelial cells isolated from cystic fibrosis lungs. Am J Respir Crit Care Med 2002;166:1248–1256. [DOI] [PubMed] [Google Scholar]
- 16.Kim JM, Oh YK, Kim YJ, Oh HB, Cho YJ. Polarized secretion of CXC-chemokines by human intestinal epithelial cells in response to Bacteroides fragilis enterotoxin: NF-κB plays a major role in the regulation of IL-8 expression. Clin Exp Immunol 2001;123:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCormick BA, Parkos CA, Colgan SP, Carnes DK, Madara JL. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol 1998;160:455–466. [PubMed] [Google Scholar]
- 18.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 2002;283:L1315–L1321. [DOI] [PubMed] [Google Scholar]
- 19.Farberman MM, Akers KT, Crosby S, Brody S, Ferkol TW. Evaluation of inflammatory gene regulation and expression in Pseudomonas aeruginosa infected airway epithelial cells. Am J Respir Crit Care Med 2009;179:A1774. [Google Scholar]
- 20.Clarke LL, Gawenis LR, Hwang T-C, Walker NM, Gruis DB, Price EM. A domain mimic increases ΔF508 CFTR increases secretion in cystic fibrosis epithelia trafficking and restores cAMP-stimulated anion. Am J Physiol Cell Physiol 2004;287:192–199. [DOI] [PubMed] [Google Scholar]
- 21.Clarke LL, Burns KA, Bayle JY, Boucher RC, Van Scott MR. Sodium and chloride-conductive pathways in cultured mouse tracheal epithelium. Am J Physiol 1992;263:L519–L525. [DOI] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc, B 1995;57:289–300. [Google Scholar]
- 23.van Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest 1997;100:2810–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Button B, Picher M, Boucher RC. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J Physiol 2007;580:577–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godfried M, Roomans GM, Kozlova I, Nilsson H, Vanthanouvong V, Button B, Tarran R. Measurements of airway surface liquid height and mucus transport by fluorescence microscopy, and of ion composition by x-ray microanalysis. J Cyst Fibros 2004;3:135–139. [DOI] [PubMed] [Google Scholar]
- 26.Watt SM, Burgess AW, Metcalf D, Battye FL. Isolation of mouse bone marrow neutrophils by light scatter and autofluorescence. J Histochem Cytochem 1980;28:934–946. [DOI] [PubMed] [Google Scholar]
- 27.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev 1999;79:S193–S214. [DOI] [PubMed] [Google Scholar]
- 28.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, et al. Normal and cystic fibrosis airway surface liquid homeostasis: the effects of phasic shear stress and viral infections. J Biol Chem 2005;280:35751–35759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 1998;95:1005–1015. [DOI] [PubMed] [Google Scholar]
- 30.Blouquit S, Regnier A, Dannhoffer L, Fermanian C, Naline E, Boucher R, Chinet T. Ion and fluid transport properties of small airways in cystic fibrosis. Am J Respir Crit Care Med 2006;174:299–305. [DOI] [PubMed] [Google Scholar]
- 31.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol 2006;291:C218–C230. [DOI] [PubMed] [Google Scholar]
- 32.Perez A, Davis PB. Gene profile changes after Pseudomonas aeruginosa exposure in immortalized airway epithelial cells. J Struct Funct Genomics 2004;5:179–194. [DOI] [PubMed] [Google Scholar]
- 33.Virella-Lowell I, Herlihy JD, Liu B, Lopez C, Cruz P, Muller C, Baker HV, Flotte TR. Effects of CFTR, interleukin-10, and Pseudomonas aeruginosa on gene expression profiles in a CF bronchial epithelial cell line. Mol Ther 2004;10:562–573. [DOI] [PubMed] [Google Scholar]
- 34.Kreiselmeier NE, Kraynack NC, Corey DA, Kelley TJ. Statin-mediated correction of STAT1 signaling and inducible nitric oxide synthase expression in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol 2003;285:L1286–L1295. [DOI] [PubMed] [Google Scholar]
- 35.Lee JY, Elmer HL, Ross KR, Kelley TJ. Isoprenoid-mediated control of SMAD3 expression in a cultured model of cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol 2004;31:234–240. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y, Liu C, Clark JC, Whitsett JA. Functional genomic responses to cystic fibrosis transmembrane conductance regulator (CFTR) and CFTRΔ508 in the lung. J Biol Chem 2006;281:11279–11291. [DOI] [PubMed] [Google Scholar]
- 37.Tirouvanziam R, de Bentzmann S, Hubeau C, Hinnrasky J, Jacquot J, Péault B, Puchelle E. Inflammation and infection in naive human cystic fibrosis airway grafts. Am J Respir Cell Mol Biol 2000;23:121–127. [DOI] [PubMed] [Google Scholar]
- 38.Mellow TE, Murphy PC, Carson JL, Noah TL, Zhang L, Pickles RJ. The effect of respiratory synctial virus on chemokine release by differentiated airway epithelium. Exp Lung Res 2004;30:43–57. [DOI] [PubMed] [Google Scholar]
- 39.Krüger S, Brandt E, Klinger M, Kreft B. Interleukin-8 secretion of cortical tubular epithelial cells is directed to the basolateral environment and is not enhanced by apical exposure to Escherichia coli. Infect Immun 2000;68:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richman-Eisenstat JB, Jorens PG, Hébert CA, Ueki I, Nadel JA. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am J Physiol 1993;264:L413–L418. [DOI] [PubMed] [Google Scholar]
- 41.Ruef C, Jefferson DM, Schlegel-Haueter SE, Suter S. Regulation of cytokine secretion by cystic fibrosis airway epithelial cells. Eur Respir J 1993;6:1429–1436. [PubMed] [Google Scholar]
- 42.Huising MO, Stet RJ, Kruiswijk CP, Savelkoul HF, Lidy Verburg-van Kemenade BM. Molecular evolution of CXC-chemokines: extant CXC-chemokines originate from the CNS. Trends Immunol 2003;24:307–313. [DOI] [PubMed] [Google Scholar]
- 43.Colobran R, Pujol-Borrell R, Armengol MP, Juan M. The chemokine network: how the genomic organization of chemokines contains clues for deciphering their functional complexity. Clin Exp Immunol 2007;148:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bizzarri C, Beccari AR, Bertini R, Cavicchia MR, Giorgini S, Allegretti M. ELR+ CXC-chemokines and their receptors (CXC-chemokine receptor 1 and CXC-chemokine receptor 2) as new therapeutic targets. Pharmacol Ther 2006;112:139–149. [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Luo M, Zhang L, Ding W, Yan Z, Engelhardt JF. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am J Respir Cell Mol Biol 2007;36:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chanson M, Scerri I, Suter S. Defective regulation of gap junctional coupling in cystic fibrosis pancreatic duct cells. J Clin Invest 1999;103:1677–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med 2007;58:157–170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.