Abstract
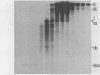
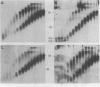
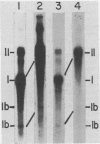
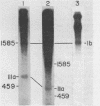
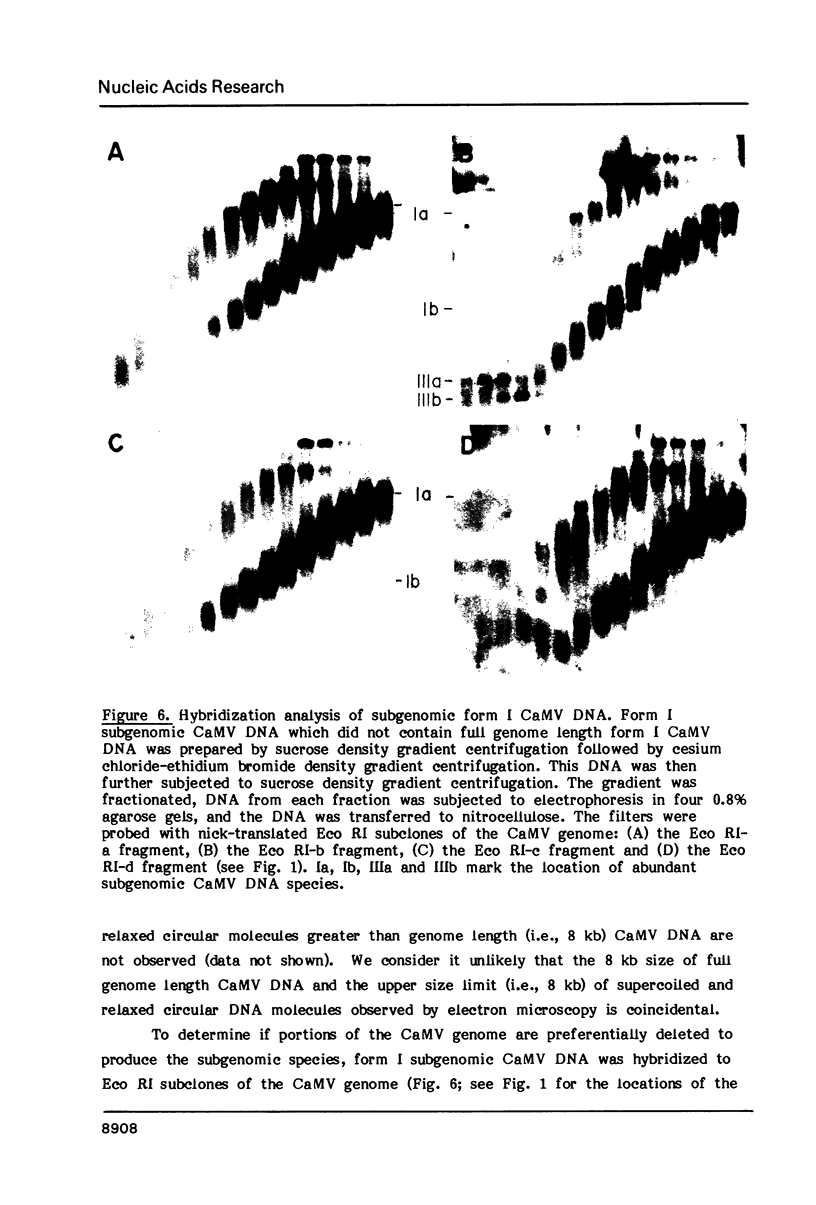
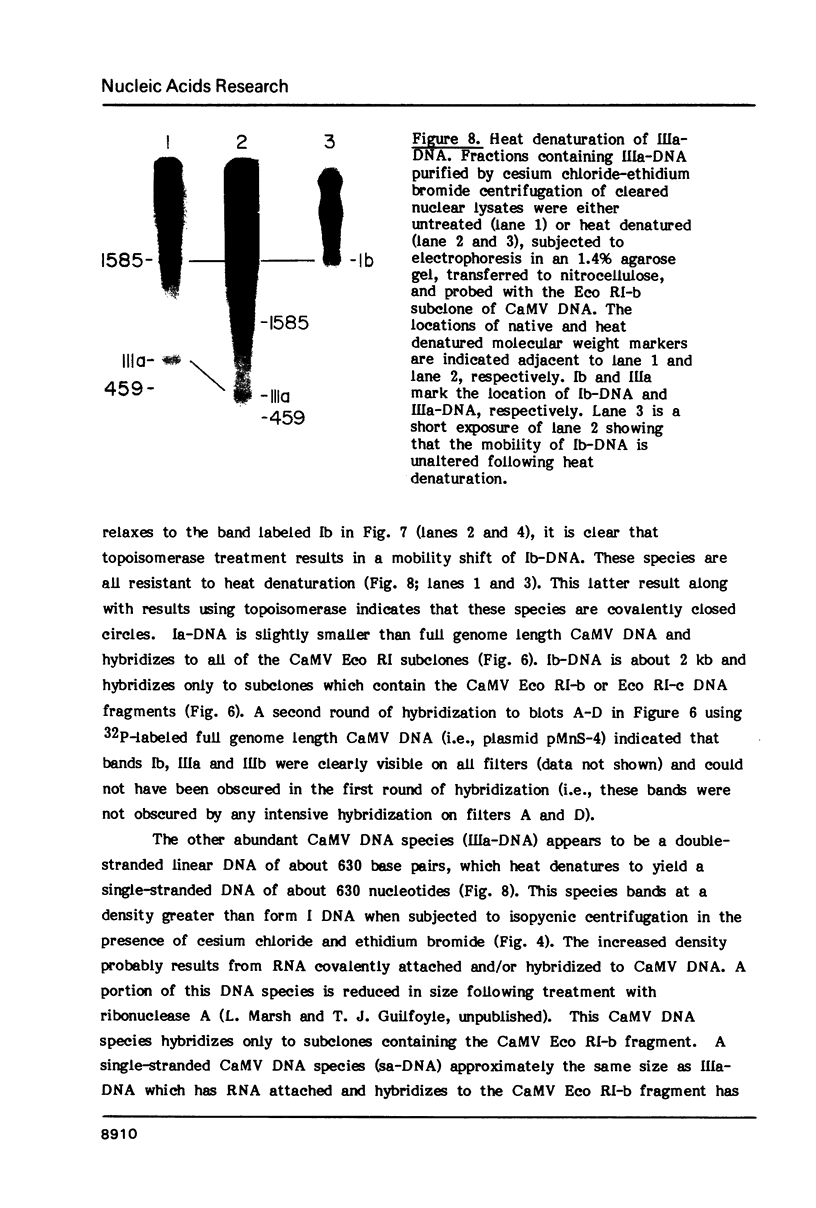
Nuclei isolated from cauliflower mosaic virus (CaMV) infected turnip leaves contain subgenomic CaMV DNA species in addition to the genome length CaMV DNA. These subgenomic CaMV DNA species are present as covalently closed circles (form I), relaxed circles (form II) and linear (form III) molecules. The subgenomic form I DNA species range in size from about 10% of genome length to nearly genome length. These subgenomic DNA species appear in tissue infected with cloned CaMV DNA, indicating that they arise rapidly and have not accumulated in the virus population from serial propagation of CaMV. No specific region of the CaMV genome appears to be preferentially deleted to form the subgenomic CaMV DNA species. At least three distinct subgenomic species appear to accumulate preferentially in nuclei isolated from infected tissue. Two of these abundant subgenomic CaMV DNA species are form I and the other one is form III. Some of the subgenomic CaMV DNA species appear to be minichromosomes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balàzs E., Guilley H., Jonard G., Richards K. Nucleotide sequence of DNA from an altered-virulence isolate D/H of the cauliflower mosaic virus. Gene. 1982 Oct;19(3):239–249. doi: 10.1016/0378-1119(82)90013-0. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Covey S. N., Lomonossoff G. P., Hull R. Characterisation of cauliflower mosaic virus DNA sequences which encode major polyadenylated transcripts. Nucleic Acids Res. 1981 Dec 21;9(24):6735–6747. doi: 10.1093/nar/9.24.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey S. N., Turner D., Mulder G. A small DNA molecule containing covalently-linked ribonucleotides originates from the large intergenic region of the cauliflower mosaic virus genome. Nucleic Acids Res. 1983 Jan 25;11(2):251–264. doi: 10.1093/nar/11.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck A., Guilley H., Jonard G., Richards K., Hirth L. Nucleotide sequence of cauliflower mosaic virus DNA. Cell. 1980 Aug;21(1):285–294. doi: 10.1016/0092-8674(80)90136-1. [DOI] [PubMed] [Google Scholar]
- Gardner R. C., Howarth A. J., Hahn P., Brown-Luedi M., Shepherd R. J., Messing J. The complete nucleotide sequence of an infectious clone of cauliflower mosaic virus by M13mp7 shotgun sequencing. Nucleic Acids Res. 1981 Jun 25;9(12):2871–2888. doi: 10.1093/nar/9.12.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Richards K. E., Jonard G. Observations concerning the discontinuous DNAs of cauliflower mosaic virus. EMBO J. 1983;2(2):277–282. doi: 10.1002/j.1460-2075.1983.tb01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntaka R. V., Richards O. C., Shank P. R., Kung H. J., Davidson N. Covalently closed circular DNA of avian sarcoma virus: purification from nuclei of infected quail tumor cells and measurement by electron microscopy and gel electrophoresis. J Mol Biol. 1976 Sep 15;106(2):337–357. doi: 10.1016/0022-2836(76)90090-5. [DOI] [PubMed] [Google Scholar]
- Hohn T., Richards K., Geneviève-Lebeurier Cauliflower mosaic virus on its way to becoming a useful plant vector. Curr Top Microbiol Immunol. 1982;96:194–236. [PubMed] [Google Scholar]
- Howell S. H., Hull R. Replication of cauliflower mosaic virus and transcription of its genome in turnip leaf protoplasts. Virology. 1978 May 15;86(2):468–481. doi: 10.1016/0042-6822(78)90086-7. [DOI] [PubMed] [Google Scholar]
- Howell S. H., Walker L. L., Dudley R. K. Cloned Cauliflower Mosaic Virus DNA Infects Turnips (Brassica rapa). Science. 1980 Jun 13;208(4449):1265–1267. doi: 10.1126/science.208.4449.1265. [DOI] [PubMed] [Google Scholar]
- Hull R., Covey S. N. Characterisation of cauliflower mosaic virus DNA forms isolated from infected turnip leaves. Nucleic Acids Res. 1983 Mar 25;11(6):1881–1895. doi: 10.1093/nar/11.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R., Howell S. H. Structure of the cauliflower mosaic virus genome. II. Variation in DNA structure and sequence between isolates. Virology. 1978 May 15;86(2):482–493. doi: 10.1016/0042-6822(78)90087-9. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Duesberg P. H. The a subunit in the RNA of transforming avian tumor viruses. I. Occurrence in different virus strains. II. Spontaneous loss resulting in nontransforming variants. Virology. 1972 Feb;47(2):494–497. doi: 10.1016/0042-6822(72)90287-5. [DOI] [PubMed] [Google Scholar]
- Ménissier J., de Murcia G., Lebeurier G., Hirth L. Electron microscopic studies of the different topological forms of the cauliflower mosaic virus DNA: knotted encapsidated DNA and nuclear minichromosome. EMBO J. 1983;2(7):1067–1071. doi: 10.1002/j.1460-2075.1983.tb01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N., Hagen G., Guilfoyle T. J. A transcriptionally active, covalently closed minichromosome of cauliflower mosaic virus DNA isolated from infected turnip leaves. Cell. 1982 Jun;29(2):395–402. doi: 10.1016/0092-8674(82)90156-8. [DOI] [PubMed] [Google Scholar]
- Pfeiffer P., Hohn T. Involvement of reverse transcription in the replication of cauliflower mosaic virus: a detailed model and test of some aspects. Cell. 1983 Jul;33(3):781–789. doi: 10.1016/0092-8674(83)90020-x. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards K. E., Guilley H., Jonard G. Further characterization of the discontinuities in cauliflower mosaic virus DNA. FEBS Lett. 1981 Nov 2;134(1):67–70. doi: 10.1016/0014-5793(81)80552-2. [DOI] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Spontaneous segregation of nontransforming viruses from cloned sarcoma viruses. Virology. 1971 Dec;46(3):939–946. doi: 10.1016/0042-6822(71)90092-4. [DOI] [PubMed] [Google Scholar]
- Volovitch M., Drugeon C., Yot P. Studies on the single-stranded discontinuities of the cauliflower mosaic virus genome. Nucleic Acids Res. 1978 Aug;5(8):2913–2925. doi: 10.1093/nar/5.8.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]