Summary
Extracellular Hsp90 proteins, including “membrane-bound”, “released” and “secreted”, were first reported more than two decades ago. Only studies of the past seven years have begun to reveal a picture for when, how and why Hsp90 get exported by both normal and tumor cells. Normal cells secrete Hsp90 in response to tissue injury. Tumor cells have managed to constitutively secrete Hsp90 for tissue invasion. In either case, sufficient supply of the extracellular Hsp90 can be guaranteed by its unusually abundant storage inside the cells. A well-characterized function of secreted Hsp90α is to promote cell motility, a crucial event for both wound healing and cancer. The reported targets for extracellular Hsp90α include MMP2, LRP-1, tyrosine kinase receptors and possibly more. The pro-motility activity of secreted Hsp90α resides within a fragment at the boundary between linker region and middle domain. Inhibition of its secretion, neutralization of its extracellular action or interruption of its signaling through LRP-1 block wound healing and tumor invasion in vitro and in vivo. In normal tissue, topical application of F-5 promotes acute and diabetic wound healing far more effectively than US FDA-approved conventional growth factor therapy in mice. In cancer, drugs that selectively target the F-5 region of secreted Hsp90 by cancer cells may be more effective and less toxic than those that target the ATPase of the intracellular Hsp90.
Introduction
Since the discovery of the first “growth factor” in the 70s, it has become widely believed that local growth factors are the driving force for wound healing, i.e. the lateral migration and proliferation of epidermal keratincoytes over the wound bed to close the wound and the inward migration and growth of dermal fibroblasts and microvascular endothelial cells into the wound bed to remodel the damaged tissue and to build a new vascularized neodermis [1, 2, 3]. However, after two decades of extensive studies and clinical trials on a handful of growth factors alone or in combination [3, 4], only recombinant human platelet-derived growth factor-BB (PDGF-BB) has received US FDA approval for topical treatment of diabetic ulcers (Regranex/becaplermin gel, 0.01%, Ortho-McNeil Pharmaceutical, Raritan, NJ) [5, 6, 7]. Since then, however, its modest efficacy, high cost and risk of causing cancer in patients (who receive three tubes or more of the treatment) have limited the use of PDGF-BB in clinical practice [8, 9]. While these rather unexpected outcomes have clearly implicated that conventional growth factors are not the critical force of wound closure as they were hoped for, this disappointing reality has been continuously overlooked or simply ignored. In 2007, Li and colleagues proposed that the factor that is primarily responsible for promoting the initial wound closure comes from secretion of the stressed skin cells at the wound edge. From the secreted proteins of primary human keratinocytes and human dermal fibroblasts, these authors found that secreted Hsp90α promotes wound closure in mice far more strongly than the becaplermin gel [10, 11, 12]. Why is secreted Hsp90α chosen? Is secreted Hsp90α, instead of growth factors, responsible for the initial wound closure? No one would have imagined and believed this.
Going back to a decade ago, Csermely and colleagues raised a question of “Why do we need constitutively so much of Hsp90 (in a cell)”? They argued that the major cellular function of Hsp90 is not entirely as an intracellular chaperone. Instead, it is due to some other unrecognized functions that would require such a large amount of Hsp90 protein storage in the cell [13]. In as early as the late 70's, a number of laboratories have repeatedly reported expression of Hsp90 on the cell surface, as either a tumor antigen or a protein that assists antigen presentation to antigen-presenting cell [13, 14, 15]. Then, secreted form of Hsp90 was reported to cause activation of ERK1/2 in rat vascular smooth muscle cells [16] and stimulation of growth in lymphoid cells [17]. However, results of these earlier studies on extracellular Hsp90 were largely viewed as experimental artifacts for good reasons (see later sections). Since 2004, new evidence has started to accumulate and begun to shed light on the Csermely's prediction. These new studies argue that the purpose for the steady-state storage of such a large amount of Hsp90 in cells by Mother Nature is for normal cells to launch a rapid protective response to environmental insults, including heat, hypoxia, UV, gamma-irradiation, reactive oxygen species (ROS), injury-released growth factors [14]. Tumor cells also recognize the usefulness of secreted Hsp90 in tissue invasion and metastasis [15, 18]. In this review, we provide an updated analysis of extracellular Hsp90, focusing on its role in skin wound healing and cancer progression. We are not going to discuss the antigen-presenting role of cell-released Hsp90, which has been well covered in recent reviews [19, 20].
Suggestions for nomenclature of extracellular Hsp90
Cell surface-bound, cell-released and cell-secreted Hsp90 can be collectively referred as “extracellular Hsp90”. It has become clear that there are fundamental distinctions between intracellular and extracellular Hsp90. First, one locates inside and the other outside the cell. Second, one acts as a chaperone and the other as a pro-motility factor (and possibly more). Third, one depends upon its N-terminal ATPase, the middle domain for client molecule binding and C-terminal dimerization/co-factor binding domains for effectiveness, whereas the other uses a short peptide epitope at the boundary between the highly charged linker region (LR) and the middle domain (M) for promotion of cell motility. Therefore, it has become necessary to distinguish these two pools of Hsp90 by nomenclature. There have been several intuitive usages of nomenclatures, including “surface-bound”, “released”, “secreted” and “extracellular”. Among them, only the last nomenclature can include both “cell surface-bound”, “released” and “secreted” Hsp90. Extracellular Hsp90 was referred as “eHsp90” [21], similar to the nomenclature of eDNA (extracellular DNA). Learning from other nomenclatures that include the word “extracellular”, such as extracellular matrix (ECM) and extracellular fluid (ECF), would extracellular Hsp90 go for “EC-Hsp90”? These are suggestions for discussion. For the time being, we use “eHsp90” throughout this article.
How much Hsp90 does a normal vs. a cancer cell have?
Hsp90 is widely reported as one of the most abundant proteins in all types of cells, frequently being referred as “1–2% of the total cellular proteins”. Three original reports, which were repeatedly cited for this statement before the 90's, included a published abstract and two research articles [22, 23, 24]. However, none of these publications contained any direct supporting data for that statement or even made that statement. Since then, a number of studies actually tried to quantitate the content of Hsp90 protein either in mouse or human issues (25, 26, 27) or in various normal or cancer cell lines (28, 29, 30, 31). Some of these studies indeed provided numbers for how much Hsp90 was found in their experiments. However, regardless the accuracy of those reported numbers, none of these studies followed the minimum requirements of the principles for protein quantitation in biochemistry. At the least, the following steps should have been included for estimation of a protein amount: 1) using a series of known amounts of a protein standard (such as bovine serum albumin, MW ~55 kDa) to establish a standard curve of O.D. readings versus the actual amounts (μg) of the protein; 2) a serious titrations of the total post-nuclear extract of a given cell type, O.D. reading and conversion of the O.D. readings into μg of proteins according to the Standard Curve; 3) subjecting the cell extracts with known μg of proteins together with a series of known μg of recombinant Hsp90α protein side-by-side to SDS-PAGE and Western blot with a common monoclonal anti-Hsp90α antibody, provided that the entire procedure of SDS-PAGE electrophoresis, transfer onto nitrocellulose membranes, blotting, primary antibody, secondary antibody, washing and ECL reactions of the two sets of samples are operated all in common apparatus, containers and the same exposure cassette for the exactly same period of time; 4) subjecting Hsp90α protein bands of the cell lysates and the recombinant Hsp90α to densitometry scanning with identical settings of parameters (i.e. in Alpha Innotech Fluorchem SP); 5) using the scanning readings of the recombinant Hsp90α bands to establish a second Standard Curve that measures densitometry readings versus the actual amounts (μg) of the recombinant Hsp90α protein; 6) Using this standard curve to convert the scanning readings of the Hsp90α bands from the total cell extracts to actual amount (μg) of proteins; 7) dividing the amount of Hsp90α (μg) in a given volume of cell extract by the total proteins (μg) from the same extract × 100 to obtain the approximate % of Hsp90α in the total proteins of the given cell type; and 8) taking the means of the % from the multiple sets of the Hsp90α versus cell extract pairs as the final estimated % of Hsp90α for a given cell type. When Sahu et al followed these procedures to compare multiple normal and cancer cell lines, they found that Hsp90α protein accounts for 2–3% of the total cellular proteins in the normal cells tested and up to 7% in certain tumor cell lines. However, they also found that it was not always true that cancer cells have higher levels of Hsp90 than its normal counterparts (32). These numbers would translate up to several hundred folds of Hsp90α proteins over other cellular proteins in both normal and cancer cells, and these ratios could become even larger if the copy number of Hsp90 is compared to any of its chaperoned clients, which are often in much lower abundance than other house keeping gene products.
eHsp90, secreted by living cells or leaked by dead cells?
Although the first observation was made two decades ago, the notion of “surface-bound” and “secreted” Hsp90 by living cells has only recently gained traction in the field with specific attributions of a number of review articles by experts then and now [13, 14, 15, 18]. Even though, skepticisms remain among many others as whether these extracellular Hsp90 proteins are results of pathophysiological processes or of a leak from a small number of dead cells in culture. Multhoff and Hightower tried to address this critical issue 15 years ago with a number of Hsp and non-Hsp examples in the studies that used various pharmacological approaches and argued that eHap90 was no artifact [33].
A primary reason for the skepticism is that Hsp90 does not fit into any of the conventional categories of the actively secreted proteins, such as growth factors, extracellular matrices (ECMs) and matrix metaloproteinases (MMPs). First, Hsp90 has neither any signal peptide (SP) for secretion via the endoplasmic reticulum (ER)/Golgi protein secretory pathway nor a recognizable transmembrane sequence for membrane anchoring. Second, there had already been reports that Hsp90 could be released to extracellular environment following cell necrosis [14]. In the latter case, the released Hsp90 binds and helps antigen recognition and triggers innate immune responses. Therefore, it was arguable that eHsp90 found in conditioned media of cultured cells may come from a small portion of dead cells [13]. Recent studies by independent groups have provided stronger arguments that the cells secrete Hsp90 for purpose. First, quiescent normal cells do not secrete Hsp90 [10, 11, 16]. However, many pathological and stress cues trigger normal cells to secrete Hsp90, including reactive oxygen species (ROS) [15], heat [34, 35], hypoxia [10], gamma-irradiation [36] and tissue injury-released cytokins, such as TGFα [11]. Fir instance, TGFα is low or undetectable in intact skin and only appears in the wound following skin injury. TGFα is known to increase cell survival and cell number rather than causing cell death in human keratinocytes. Interestingly, Cheng et al showed that TGFα stimulation causes rapid membrane translocation and secretion of both endogenous and an exogenously expressed GFP-tagged Hsp90α with defect in ATPase [11]. More specifically, Tsutsumi and colleagues reported that a conserved hydrophobic motif in a beta-strand at the boundary between the N-terminal domain and charged linker of Hsp90 is required for Hsp90 secretion (37). Wang et al showed that a C-terminal EEVD motif and the Thr-90 phosphorylation both play a regulatory role in Hsp90 secretion (38). On one hand, these findings strongly support the notion that Hsp90 secretion is a regulated process. On the other hand, it is hard to imagine why Hsp90 needs multiple distinct sequence motifs across the entire protein for regulation of its secretion, not to mention how these motifs relate to the most reported exosome trafficking pathway for secretion of Hsp90 and other Hsp proteins (see later section). More on regulation of Hsp90 secretion, Li and colleagues identified a key upstream regulator of Hsp90α secretion, the hypoxia-inducible factor-1alpha (HIF-1α) in human dermal fibroblasts and keratinocytes [10, 39]. Dominant negative mutant of HIF-1α (DN-HIF-1α) blocks the secretion, whereas a constitutively active mutant of HIF-1α (CA-HIF-1α) makes the cells to secrete Hsp90α even under normoxia [39]. The same mechanism appears to take place in tumor cells. Depletion of the constitutively expressed HIF-1α or HIF-1β from breast cancer cells, MDA-MB-23, completely blocked secretion of Hsp90α, which could be rescued by exogenously re-introduced the CA-HIF-1α, but not DN-HIF-1α (32). Despite of the above remarkable findings, direct support for releasing Hsp90 on purpose by living cells would require additional studies on the detailed routes of its membrane translocation and secretion in response to environmental cues.
Induced secretion for normal cells and constitutive secretion for tumor cells
Normal cells do not secrete Hsp90 unless being triggered by environmental insults. Hightower and Guidon reported first that heat-shocked rat embryonic cells secrete Hsp90 and Hsp70. This secretion could not be blocked by monensin or colchicine, two inhibitors of the conventional ER/Golgi protein secretory pathway [34]. Clayton and colleagues used proteomic approach to analyze the peptide contents of B cell-secreted proteins under either physiological temperature (37 °C) or heat shock (44 °C for 3 hours). They found that heat shock induced Hsp90α to go into the nano-vesicles called exosomes and then secreted to outside the cells [35]. Liao et al reported that treatment of rat vascular smooth muscle cells with LY83583, an oxidative stress generator, caused secretion of Hsp90α. The eHsp90α in turn induced a late phase activation of the ERK1/2 pathway [16]. Yu and colleagues found that γ irradiation induced secretion of Hsp90β, but not Hsp90α, in a p53-dependent fashion via exosomes, proposing a “DNA damage > p53 > Hsp90β secretion” pathway [34]. Cheng et al. showed that TGFα-induced Hsp90α membrane translocation and secretion to culture medium in primary human keratinocytes were sensitive to inhibitors of the exosome protein trafficking, but not the conventional ER/Golgi protein trafficking, pathway [11]. Finally, Li and colleagues showed that hypoxia (1% O2) induced Hsp90α secretion via HIF-1α. Blockade of eHsp90α function by neutralizing antibodies completely inhibited hypoxia-induced cell motility [10].
In particular, the identification of HIF-1α as a key upstream regulator of Hsp90α secretion has an important implication in cancer. Hypoxia is a known micro-environmental stress that is connected to the growth, invasion, and metastasis of many solid tumors [40]. Under a constant hypoxia, cancer cells are forced to adapt, via HIF-1α, alternative and self-supporting mechanisms for continued survival and expansion. Overexpression of HIF-1α has been estimated to occur in approximately 40% of the tumors in humans [41]. Therefore, surface expression and/or secretion of Hsp90α should become constitutive in those HIF-1α-overexpressing tumors. While this prediction remains to be formally tested, many tumor cell lines have been reported to secrete Hsp90. Kuroita and colleagues reported purification of Hsp90α from conditioned media of human hybridoma SH-76 cells [17]. Eustace et al reported Hsp90α, but not Hsp90β, in conditioned media of HT-1080 tumor cells [42]. Wang et al reported secretion of Hsp90α by MCF-7 human breast cells [38]. Suzuki and Kulkarni found Hsp90β secreted by MG63 osteosarcoma cells [45]. Chen and colleagues reported secretion of Hsp90α by colorectal cancer cell line, HCT-8 [44]. Work by Trutrumi and colleagues implied secretion of Hsp90α by a variety of tumor cell lines (43). Finally, recent study from our laboratory demonstrated that breast cancer cells, MDA-MB-231 and MDA-MB-468, overexpress HIF-1α that causes constitutive secretion of Hsp90α in a HIF-1-depemndent fashion (32). Figure 1 summarizes what triggers Hsp90α secretion in normal cells versus tumor cells, in which HIF-1α is a central regulator. However, the signaling steps between HIF-1α and Hsp90α secretion machinery (such as exosomes) remain entirely unknown.
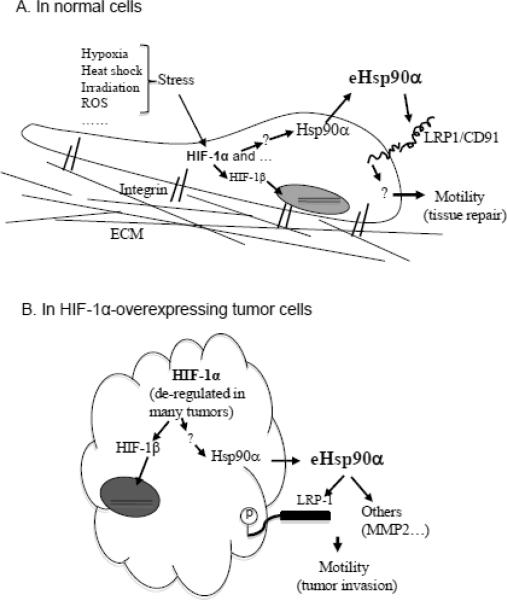
Figure 1. A schematic presentation of the “Hypoxia > Hsp90α secretion > LRP-1 > Cell migration” signaling in normal vs. tumor cells.
(A) In normal cells, secretion of Hsp90 does not occur unless cells are “hit” with environmental stress cues, as listed. HIF-1α is a central regulator of Hsp90 secretion. Little is known about how HIF-1 “pushes” Hsp90 out of the cell. The eHsp90 may act as an autocrine and paracrine factor to promote cell motility via LRP-1. The main duty for eHsp90 is to help tissue repair.
(B) In many types of tumors, HIF-1α is constitutively accumulated, which triggers secretion of Hsp90 even in the absence of any environmental stress cues. Tumor-secreted eHsp90α may promote tumor cell migration via binding to LRP1 receptor, MMP2 and/or other targets. The tumor cells use eHsp90 mainly for tissue invasion. Therefore, eHsp90α and its extracellular targets present new lines of targets for wound healing and anti-tumor drugs.
Secretion of Hsp90α via non-classical exosomal protein secretory pathway
How does Hsp90 travel through the cell membrane? There are several cellular protein trafficking machineries. First, the classical ER/Golgi protein secretory pathway requires the to-be-secreted protein to have a 15–30 amino acid signal peptide (SP) at its amino terminus and to use it as the “permit” for going out of the cell. The second protein secretory pathway is mediated by secreted nano-vesicles, called exosomes, which are used for secreting proteins that do not have any SP sequences. Exosomes, also called,, intraluminal vesicle' (ILVs), are non-plasma-membrane-derived vesicles that are 30–90 nm in diameter and initially contained within the multivesicular bodies (MVB). A well-known function of MVB is to serve as an intermediate station during degradation of the proteins internalized from the cell surface or sorted from the trans Golgi organelle [46, 47]. However, the MVB-derived exosomes can also fuse with the plasma membrane to release their cargo proteins into the extracellular space. This release process was reported to include 1) sorting into smaller vesicles; 2) fusing with the cell membrane; and 3) release of the vesicles to the extracellular space. All the proteins that have been identified in exosomes are located in the cell cytosol or endosomal compartments, but never in the ER, Golgi apparatus, mitochondria or nucleus. Making use of two chemical inhibitors, brefeldin A (BFA) that selectively blocks the classical ER/Golgi protein secretory pathway and dimethyl amiloride (DMA) that blocks the exosome protein secretory pathway, several groups showed that DMA selectively inhibits the membrane translocation and secretion of Hsp90α or Hsp90β by various types of cells [11, 48, 49, 50, 51]. In the same experiments, BFA had little inhibition of Hsp90 secretion [11]. There have been no reports that BFA or DMA causes more or less cell death. An unanswered question, however, is how stress signals, such as HIF-1α, are connected to this novel protein secretory pathway and what the relationships between the reported secretion motifs in Hsp90 and exosomes. However, once Hsp90α proteins are secreted to outside of the cells, they appear to be “naked”, instead of continued being wrapped in the exosomes, since neutralizing anti-Hsp90α antibodies were able to completely block their actions (10, 11, 32, 39).
eHsp90 does not need ATPase for function
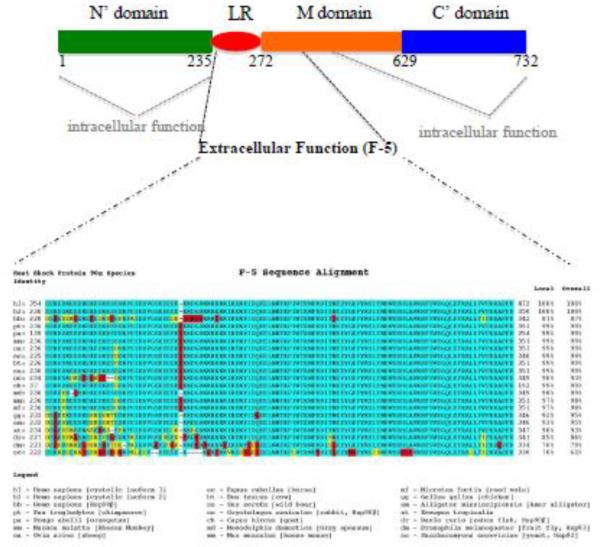
Eustace and colleagues studied the role of eHsp90α found in conditioned media of HT-1080 fibrosarcoma cells by using two approaches. First, they used the fluorophore-assisted light inactivation (FALI) technique, in which fluorophore fluorescein induces antibody-coupled FITC to generate short-lived hydroxyl radicals that cause damage of proteins within 40 Å. Therefore, the damage is often specific on the antibody-bound protein, i.e. Hsp90α, but not surrounding proteins. This technique damages the whole molecule. Second, they used DMAG-N-oxide, a geldanamycin/17-AAG-derived but cell-impermeable Hsp90 inhibitor that targets the Hsp90's ATPase activity. They reported an importance of eHsp90α in the tumor cell invasion [42]. Tsutsumi and colleagues also tested the effect of DMAG-N-oxide on invasion of several cancer cell lines in vitro and lung colonization by melanoma cells in mice and reported inhibition of cell invasion in vitro and/or tumor formation in vivo by DMAG-N-oxide [45]. The results of these studies suggested that the N'-terminal ATP-binding and ATPase of Hsp90α are still required for function of eHsp90α in outside of the cells. Focusing on the pro-motility activity of eHsp90α on primary human skin cells, Cheng and colleagues undertook mutagenesis approach to address the same issue. First, they compared recombinant proteins of the wild type and E47A, E47D, and D93N mutants of Hsp90α for pro-motility activity on human keratinocytes. As previously reported, Hsp90α-wt has a full ATPase activity, Hsp90α-E47D mutant loses half of the ATPase activity, whereas Hsp90α-E47A and Hsp90α-D93N mutants lose the entire ATPase activity [52]. Cheng et al found that all the ATPase mutant proteins retained a similar pro-motility activity as the Hsp90α-wt. Second, they used sequential deletion mutagenesis to have narrowed down the pro-motility domain to a region between the linker region (LR) and the middle (M) domain of human Hsp90α [11]. Finally, their latest study has identified a 115-amino acid fragment, called F-5 (aa-236 to aa-350), that promotes skin cell migration in vitro and wound healing in vivo as effectively as the full-length Hsp90α-wt [12]. Collectively, these findings demonstrate that the N-terminal ATPase domain and the C-terminal dimer-forming and co-factor-binding domain are dispensable for eHsp90α to promote cell migration. A schematic representation of the structure and function requirements for intracellular Hsp90α and eHsp90α is shown in Figure 2A. It should be pointed out that stimulation of cell migration might not be the only function reported for eHsp90. The 115 amino acid sequence of F-5 is highly conserved during evolution, as shown in Figure 2B. However, no more than 20% identity of F-5 was found in other Hsp family genes.
Figure 2. A schematic distinction of the functional elements for intracellular vs. extracellular Hsp90α.
(A) The intracellular chaperone function of Hsp90 requires almost the entire molecule, especially the amino terminal (green) and the carboxyl terminal (blue) domains. The extracellular pro-motility function of Hsp90α depends on less than a 115 amino acid fragment (F-5) located at the boundary between the LR and the M domains. This epitope appears at the surface of Hsp90 protein [ref. 13].
(B) F-5 is highly conserved during evolution of Hsp90 genes. Green, identify; Yellow: similarity; Red, distinction.
On the other hand, the observations made by Eustace, Tsutsumi and their colleagues were independently confirmed by studies of others. Cheng and colleagues in collaboration with Isaacs's group verified that the DMAG-N-oxide inhibitor could indeed block the full-length Hsp90α-stimulated human skin cell migration. However, as expected, DMAG-N-oxide showed little inhibition of the F-5 peptide-induced cell migration [Cheng, C-F, J. Isaacs and W. Li, unpublished] or migration induced by the middle domain of eHsp90α [11, 21]. While the reason for the apparent discrepancy remains unknown, we suggest that binding of DMAG-N-oxide to the N'-terminal ATPase domain of the full-length e Hsp90α may cause a conformational change in eHsp90α, so that the real functional epitope within eHsp90α, i.e. the F-5 region, becomes cryptic. While this hypothesis remains to be tested, it can be concluded that eHsp90 is no chaperone.
Downstream targets of eHsp90
How eHsp90α promotes cell migration has just begun to be appreciated. Eustace and colleagues reported that Hsp90α, but not Hsp90β, promotes cancer cell migration and invasion by binding and activating the matrix metalloproteinase 2 (MMP2) [42]. Two independent groups have recently provided additional support for this observation [53, 54] and, furthermore, showed that the M domain (aa-272 toaa-617) of Hsp90α is responsible for the activation (54). Following their identification of eHsp90α in conditioned media of human skin cells in 2007 [10], Li and colleagues have also been attempting to identify target that is essential for hypoxia- and eHsp90α-stimulated human skin cell migration. First, they first wanted to verify the involvement of MMP2 or any other MMPs by utilizing two broad MMP inhibitors, GM6001 (N-[(2R)-2-(hydroxamidocarbonylmethyl)-4-methylpentanoyl]-l-tryptophan methylamide, or Galardin) and MMP Inhibitor III (hydroxamido-carbonylmethyl)-4-methylpentanoyl]-l-tryptophan). However, they found that presence of either GM6001 or MMP inhibitor III showed little inhibitory effect on human recombinant Hsp90α-stimulated human keratinocyte migration [11]. Whether this discrepancy is due to differences in cellular contexts between the normal and the tumor cells remains unclear. Besides MMP2, Sidera and colleagues showed that a pool of cell membrane-bound Hsp90α interacts with the HER-2 tyrosine kinase receptor in breast cancer cells, leading to increased cell motility and invasion [53, 54]. Suzuki and Kulkarni reported that eHsp90β blocks the conversion from latent TGFβ to its active form, leading to decreased tumor suppressing effect of TGFβ [43]. McCready et al reported that eHsp90α exogenously delivered by added exosomes participated in plasminogen activation and tumor cell migration [57]. Moreover, Chung et al. showed that eHsp90 secreted by stressed vascular SMCs regulates transcription of IL-8 gene [58]. While it is still distant from understanding the mechanisms of action, these results at the least suggest that eHsp90 has multiple downstream targets in distinct extracellular environment.
Among all the studies on eHsp90 targets since 2004, the investigation of LRP-1 as a cell surface receptor for eHsp90α has gone into relatively more details and, therefore, is selected out here for mentioning with a few extra sentences. Cheng et al estimated that eHsp90α could readily reach the optimal working concentrations of 0.05–0.1μM that maximally stimulates cell migration in vitro [11]. In their cell migration assays, human recombinant Hsp90α exhibited a saturating and subsequently declined effect on human skin cells, when increasing amounts of eHsp90α were added. This was an important observation that suggests that eHsp90α acts by binding to a receptor-like molecule on the cell surface with certain Km (50% of equilibrium) and Kd (dissociation constant) values. Then, Cheng et al used four independent approaches (neutralizing antibodies, RAP (LRP-1-associated protein) inhibitor, RNAi, and somatic LRP-1 mutant cell line), to prove that the widely expressed cell surface receptor, LRP-1, mediates the eHsp90α signaling to promote cell migration. In vitro, GST-eHsp90 directly pulled down LRP-1 via its pro-motility fragment between the LR and the M domain of Hsp90α [11]. More convincingly, when these authors re-introduced an RNAi-resistant mini-LRP-1 cDNA into the LRP1-downregulated cells, they were able to rescue the migration response of the cells to either eHsp90α or hypoxia. These findings led the investigators to propose the following working model: “Hypoxia > HIF-1α > Hsp90α secretion > LRP-1 > cell motility” autocrine pathway for tissue repair and tumor invasion and metastasis [37], as previously shown in Figure 1.
However, it is equally important to point out the complexity of LRP-1 receptor signaling. First, LRP-1 (also called CD91) is a large heterotrimeric protein consisting of a 515-kDa extracellular domain and an 85-kDa trans-membrane subunit (59). Its expression was initially found in monocytes, hepatocytes, fibroblasts, and keratinocytes [60, 61, 62]. Second, LRP-1 is a bona fide cell surface receptor/co-receptor (with other receptors) for signal transduction across the membrane (59). However, the complexity of LRP-1 signal transduction mainly comes from its large ligand binding repertoire. Besides gp96 and eHsp90, other extracellular heat shock proteins that also bind LRP-1 include calreticulin, eHsp60 and eHsp70 [63, 64, 65, 66]. In addition, opposite roles for LRP-1 signaling have been reported. For instance, LRP-1 has been shown to play a critical role in PDGF-BB-stimulated ERK1/2 activation and cell proliferation and also in TGFβ-stimulated anti-proliferation [67, 68]. Cheng et al reported that, while eHsp90α dramatically increased cell migration, human recombinant Hsp70, gp96 and calreticulin exhibited either a modest or no stimulation of cell migration [11]. There is a clear need to identify the specific binding site in LRP-1 for eHsp90α, in order to understand its mechanism of action.
Is eHsp90 a design of Mother Nature?
Whenever the extracellular conditions less than normal, become such as heat shock, the cells don-regulate overall protein synthesis and yet selectively up-regulate Hsp expressions. This selective increase in chaperones has long been interpreted as helping deal with protein folding and stability. In fact, there has been little evidence to support this long-standing claim. Csermely and colleagues argued that evolution would not have tolerated such an abundant storage of Hsp90 in the cells, if the function of Hsp90 had had only been an intracellular chaperone [13]. Therefore, many suspected that those “extra” Hsp90 serve the similar role as cytoskeletal proteins. Based on recent studies on eHsp90, the $64,000 question is whether Mother Nature purposely designed the increase in an already high level of intracellular Hsp90 to supply the “eHsp90 pool” for the cells to deal with environmental stress. Currently, there has been limited knowledge for or against this previously unthinkable possibility, either. The answer to the above question for sure will not come in the near future.
If we jump one step forward to assume the “eHsp90 hypothesis” is correct, why had Mother Nature chosen Hsp90 (instead of the numerous known cell migration-promoting factors) for this job? What unique qualifications was eHsp90 given by Mother Nature and those qualifications are absent from those conventional pro-motility factors, such as growth factors. A possible answer to these questions merged from a surprising finding of our group a few years ago. It is known for long that TGFβ family cytokines block conventional growth factor-stimulated proliferation in many cell types and migration in certain cell types. For instance, Bandyopadhay et al found that TGFβ3 blocks growth factor-stimulated human dermal fibroblast and endothelial cell, but not human keratinocyte, migration [69].
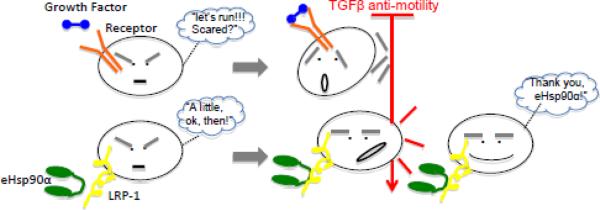
This inhibition by TGFβ3 was believed to be a major reason for why conventional growth factor therapy in wound healing has largely failed [12]. However, our laboratory found that TGFβ3 was unable to inhibit eHsp90α-induced migration of any type of human skin cells [11]. This unique property of eHsp90α is schematically represented in Figure 3. Such a superior effect of eHsp90α over growth factors was also reflected in in vivo wound healing stduies. Topical application of recombinant Hsp90α proteins to diabetic mouse wounds dramatically shortened the time of wound closure from 35 days to ~18 days, significantly stronger than becaplermin gel (PDGF-BB) treatment [12]. These authors proposed that it is eHsp90α, but not the conventional wisdom of growth factors (such as PDGF-BB), that drives inward migration of the dermal cells into the wound against the TGFβ inhibition. As previously emphasized, moving into the wound by these dermal cells is essential for the wound remodeling and new blood vessel formation.
Figure 3. eHsp90α-driven, not growth factor-driven, cell migration overrides TGF β inhibition.
(Upper part) Conventional growth factor-induced cell migration is sensitive to the anti-motility signal of TGFβ, which is abundantly present in wounded tissues or at tumor site. Unless mutations take place along the TGFβ signaling pathway, growth factors will not be able to be effective on the cells in vivo. (Lower part) eHsp90 is the first ligand-like peptide that is able to continue promoting cell migration in the presence of TGF-beta. The mechanism of action remains to be studied. This finding explains 1) why eHsp90 is more effective than growth factors in skin wound healing and 2) cancers without mutation in TGFβ signaling pathway progress.
Similarly, cancer has also a love-and-hate relationship with TGFβ, which is regarded as both tumor suppressor and tumor promoter [70]. Hanahan and Weinberg in their most cited review of 2000 organized the traits of cancer into six distinct yet overlapping events; 1) self-sufficiency in growth signals; 2) insensitivity to anti-growth signals; 3) tissue evasion and metastasis; 4) unlimited proliferative potential; 5) sustained angiogenesis; and 6) preventing apoptosis [71]. They cited that one of the most recognized anti-growth signals is TGFβ. TGFβ prevents an inactivating phosphorylation of the tumor suppressor gene product Rb in cells, thereby blocking the cell's advance through the G1 phase of the cell cycle. In this case, TGFβ induces increased gene expression of p15INK4B and p21, which in turn inhibit cyclin:CDK complex's kinase activity responsible for the inactivating phosphorylation of Rb. In certain cell types, TGFβ also suppresses expression of the c-myc proto-oncogene, a positive regulator of the cell cycle progression. These inhibitory capabilities of TGFβ are obviously bad news for cancer cells [70]. To sabotage the tumor suppressing effect of TGFβ, a number of tumors choose to mutate either the type II (TβRII) or type I (TβRI) TGFβ receptor. Whereas other tumors choose to eliminate downstream signaling molecule, Smad4, which forms complex with activated Smad2/3 to regulate gene expression in the nucleus. These TGFβ pathway-mutated tumors include gastrointestinal and colorectal cancer [72], gastric ovarian cancer [73, 74], breast cancer [72] and pancreative cancer [75, 76]. Those alterations in TGFβ signaling pathway have presumably made the cancer cells no longer sensitive to the anti-proliferation and anti-migration signals of TGFβ. However, in many other human cancers, no mutations in TGFβ signaling components are found. How could these cancers bypass the TGFβ's inhibitory signals? There have been little studies that address this question. If we put the importance of HIF-1 in cancer and the recent finding of the “HIF-1 > Hsp90α secretion > LRP-1 > tumor cell invasion” axis into perspective, one may extrapolate a possible answer to the puzzle from the following facts: 1) Approximately 40% of all human tumors has constitutively elevated expression of HIF-1α, the critical subunit of the master transcription factor for tissue oxygen homeostasis [41]; 2) HIF-1α is a central controller of Hsp90α secretion [10, 39]; and 3) eHsp90α is required for cancer cell invasion in vitro [30] and tumor formation in vivo [32, 45, 54]. Therefore, it is conceivable that secretion of Hsp90α is an alternative strategy for cancer cells to bypass the anti-motility of TGFβ without mutating TGFβ signaling components.
How was eHsp90 connected to wound healing?
What is the natural driving force of skin wound healing? For decades, the conventional wisdom has been that cell type-specific growth factors represent Mother Nature's design for repairing wounds [2, 3]. These growth factors often appear only when skin is wounded or their concentrations rise significantly from a basal level in response to injury, such as TGFα and KGF (FGF7) for keratinocytes, PDGF-BB for dermal fibroblasts and VEGF-A for dermal microvascular endothelial cells. Based on this belief, more than 30 growth factors have been subjected to extensive pre-clinical studies and/or clinical trials alone or in combinations [4]. Despite these enormous efforts, the in vivo functions for many of these growth factors remained unconfirmed and their efficacy in human trials mostly fell short of providing significant clinical benefits. Among them all, only recombinant PDGF-BB received the FDA approval (becaplermin gel) for treatment of diabetic ulcers, as previously mentioned. Subsequent studies found becaplermin gel low efficacy and higher risks of causing cancer in patients, resulting in its declined use in clinical practice (http://www.medicalnewstoday.com/releases/110442.php). This significant side-effect may not be surprising to cancer researchers, since it was already known years before the FDA approval of becaplermin gel that overexpression of PDGF-BB (c-sis) or autocrine of its viral form, v-sis, will cause cell transformation and that yet the recommended dosage of PDGF-BB in becaplermin gel is more than 1000 fold higher than the range of the physiological PDGF-BB levels in human circulation. The reason for this stern reality has never been investigated, even though it challenges whether continued emphasis on growth factors is the right thing to do for wound healing.
In 2006, Badyopadhay and colleagues made a surprising observation that had ultimately led to the discovery of an unconventional wound-healing factor, the eHsp90α. Having noticed that fetal bovine serum (FBS) has been used to culture human cells for years, the researchers argued that human cells are never in contact with FBS in reality and, instead, it is the human serum that represents the main soluble environment in wounded skin. They compared the effect of FBS versus human serum on migration of three major human skin cell types, epidermal keratnocytes and dermal fibroblasts and microvascular endothelial cells. They found that FBS non-discriminatively stimulated migration of all three types of human skin cells, as expected. However, they were surprised to find that human serum only promoted keratinocyte migration, whereas halted migration of the two dermal cell types. They further revealed that the blocking signal in human serum comes from TGFβ3 (not TGFβ1 or TGFβ2) and the selective sensitivity of the human dermal cells to TGFβ3 is due to their 7–15 fold higher levels of TβIIR than that in epidermal keratinocytes [69]. An important message of this finding was that conventional growth factors may not be able to induce proliferation and migration of dermal fibroblasts and microvascular endothelial cells in vivo, as they do in vitro, due to abundant presence of TGFβ3 in human serum. These authors speculated that this defect in growth factors represents a reason for why the majority of the growth factor trials in the past failed to show any promising efficacy.
If it were not the action of growth factors, what would be the factor that drives dermal cell migration against TGFβ3 inhibition in the wound and where would it come from? The researchers reasoned that this critical factor does not come from human serum, because the latter inhibits dermal cell migration. Instead, they suggested that it comes from secretion of the migrating keratinocytes in response to injury-generated stress signals, such as hypoxia, and factors that promote keratinocyte migration, such as TGFα (77, 78). More importantly, they proposed that this factor must satisfy the following two criteria: 1) being a common pro-motility factor for all three types of human skin cells and 2) being able to override TGFβ3 inhibition. First, they detected a strong pro-motility activity in serum-free conditioned medium of TGFα-stimulated keratinocytes and hypoxia-treated dermal fibroblasts, which drives migration of all three types of human skin cells. In subsequent 18 months, from ten liters of serum-free conditioned medium of primary human keratinocytes, protein purification allowed them to discover eHsp90α responsible for the entire pro-motility activity in the conditioned medium [10, 11]. That was a total surprise to these researchers.
eHsp90 carries three unique properties, absent from conventional growth factors, to effectively heal acute and diabetic wounds
In vivo, topical application of recombinant Hsp90α proteins shortened the time of 1cm × 1cm full thickness acute wound closure from more than two weeks to ~10 days in nude mice and of similar wound in db/db (diabetic) mice closure from 35 days to ~15 days [10, 12]. In comparison, bercaplermin gel treatment showed little improvement on acute (normal) wound healing and only shortened the time for diabetic wound closure from 35 days to ~28 days. What has made eHsp90α superior to the conventional growth factor therapy? Cheng et al provided three unique properties of eHsp90α. First, eHsp90 is a common pro-motility factor for all three types of human skin cells involved in wound healing. Following skin injury, the lateral migration of keratinocytes closes wound and subsequent inward migration of dermal fibroblasts and dermal microvascular endothelial cells into the wound remodels the damaged tissue and builds new blood vessels. Therefore, ideally, a single factor-based wound-healing agent should be a molecule that is capable of recruiting all the three types of human skin cells into the wound bed. eHsp90 is a common pro-motility factor for all the three major types of human skin cells, because all of the cells express a compatible level of LRP-1 [11, 12]. In contrast, if an agent, such as a growth factor, that selectively acts on some, but not all, the cell types, it would be less effective in the multi-cell-type participated process of wound healing. For instance, PDGF-BB only acts on dermal fibroblasts, but not keratinocytes and dermal microvascular endothelial cells, due to a complete lack of both PDGFRα and PDGFRβ on the latter two types of cells. Only human dermal fibroblasts express the PDGFRs. If one extrapolates these in vitro findings to equivalent wound healing events, it suggests that PDGF-BB cannot have a direct role in recruitment of keratinocyte for wound re-epithelialization and dermal microvascular endothelial cells for wound neovascularization.
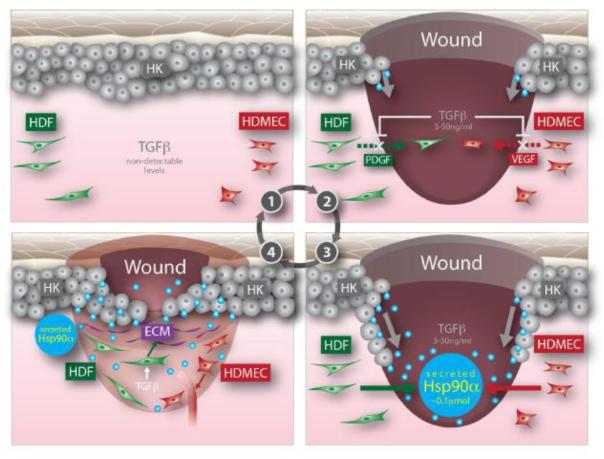
Second, in addition to its selected cell type target, PDGF-BB is unable to override inhibition of TGFβ3, which is co-present in the wound, to promote migration of dermal fibroblasts. This defect creates an additional hurdle for PDGF-BB to overcome when it is added to the wound as a therapeutic. In contrast, even in the presence of TGFβ3, eHsp90α remains equally effective to promote migration of all three types of human skin cells. The mechanism by which eHsp90α is able to do so remains to be studied. Third, it is known that all forms of diabetes are characterized by chronic hyperglycemia in circulation, which is blamed as one of the possibly many factors that delay the healing of diabetic wounds [79]. A reported damage by hyperglycemia was to destabilize HIF-1α (hypoxia-inducible factor-1alpha) protein, the key regulator of Hsp90α secretion, in the wound [80, 81]. Cheng et al recently found that hyperglycemia blocked hypoxia and serum-stimulated human dermal fibroblast migration. However, eHsp90 not only enhanced hypoxia-driven migration under normal glycemia, but also partially “rescued” migration of the cells cultured under hyperglycemia [12]. We believe that the eHsp90 promotes diabetic wound healing by bypassing the hyperglycemia-caused HIF-1α down-regulation and jumpstarting migration of the cells that otherwise cannot respond to the environmental hypoxia. Based on these findings, we propose a new wound-healing paradigm, as schematically shown in Figure 4, to explain what (factor) drives epidermal and dermal cells to migrate into the wound, leading to wound closure. Prior to injury, eHsp90α, TGFβ3 or cell motility remain minimal in intact skin (Step 0). Within hours following injury, keratinocytes start to migrate laterally across the wound (possibly induced by acute hypoxia or TGFα from serum). At the same time, however, dermal fibroblasts and dermal macrovascular endothelial cells at the wound edge cannot immediately move into the wound bed due to the presence of TGFβ3 in serum (Step 1). Migrating keratinocytes start secreting Hsp90α and once the eHsp90α reaches the threshold concentration of 100 nM [35], the dermal cells migrate inwardly into the wound bed from the surrounding wound edge even in the presence of TGFβ3 (Step 2). Finally, the migrating keratinocytes completely close the wound and the newly moved-in dermal fibroblasts cells start to remodel the wounded tissue and dermal microvascular endothelial cells to re-build new blood vessels (Step 3). The dermal neovascularization and remodeling processes would take many months to complete. Thus, migrating keratinocyte-secreted eHsp90α, instead of conventional growth factors from serum, may be the bona fide recruiting factor of the dermal cells into the wound.
Figure 4. A model of how eHsp90 promotes re-epithelialization and recruits dermal cells into the wound during wound healing.
(Step 0) Uninjured intact skin with little detectable TGFβ, cell migration or stress; (Step 1) Injury triggers release of TGFβ from several sources, the immotile to motile transition of keratinocytes and release conventional growth factors. However, the growth factors will not be able to recruit the dermal cells at the wound edge to the wound bed due to the presence of TGFβ; (Step 2) while keratinocytes are migrating, they release/secrete Hsp90α. Whence the secreted Hsp90α reaches the threshold concentration of >0.1 μM, it will drive inward migration of HDFs and HDMECs; (Step 3) The HKs are about to close the wound and the moved-in HDFs will start remodel the wound and HDMECS to build new blood vessels. HK, human keratinocyte, HDF, human dermal fibroblast and HDMECs, human dermal microvascular endothelial cells (Taken with permission from ref. 12).
Finally, the unique property of eHsp90α being a motogen, but not a mitogen (i.e. it does not stimulate cell proliferation) [10] also makes a physiological sense. First, keratinocyte migration occurs almost immediately following skin injury, whereas the inward migration of dermal cells is not detected until four days later [2]. Second, it is known that when a cell is migrating, it cannot proliferate at the same time. Beside, any attempt by on-site growth factors to stimulate proliferation of both epidermal and dermal cells would be inhibited by TGFβ that appears whence skin is wounded (12, 69). Third, cell migration proceeds cell proliferation during wound healing. Then, when and where does cell proliferation take place in the wounded skin? Based on the above three facts, we believe that, while the cells at the wound edge are moving toward the wound bed, they left “empty space” between themselves and the cells behind them. The cells behind the migrating cells start to proliferate after losing contact inhibition with the front moving cells. The stimuli of the cell proliferation likely come from plasma growth factors diffused from surrounding unwounded blood vessels, where TGFβ levels are low or undetectable. Thus, the role of cell proliferation in wound healing is to re-fill the space generated by the front-migrating cells. The specific role of eHsp90α is to help to achieve the initial wound closure as quickly as possible to prevent infection, water loss, and severe environmental stress. Many other factors (including growth factors and TGFβ) must participate in the remaining long and tedious wound remodeling processes (up to 12 months).
How was eHsp90 connected to cancer and to what types of cancer?
Although eHsp90 was reported as a cell surface-bound tumor antigen as early as the late 70's, the first direct evidence for eHsp90 in tumor cell invasion in vitro and tumor formation in nude mice came from two relatively recent studies. Both of these studies used membrane impermeable 17-AAG inhibitors to block the action of eHsp90 and reported for the first time requirement of eHsp90 for tumor progression [42, 45]. Stellas et al. used a mAb, 4C5, against Hsp90α to confirm the importance of eHsp90 in breast cancer cell “deposits” in nude mice [54]. Sahu and colleagues found that permanent down-regulation of the LRP-1 receptor in MDA-MB-231 cells dramatically reduced lung colonization of the cells in nude mice [42]. However, all these studies suffered from technical limitations that should have made the investigators refrain from making any definitive conclusions, like those they had had made. First, DMAG-N-oxide treated melanoma cells were reported to have decreased lung colonization in vivo [45]. However, it is hard to understand how a single pre-treatment of the cells with the drug in vitro could have had the reported long lasing effect after the cells were injected into mice. Second, Patsavoudi and colleagues reported that mixing breast cancer cells with 4C5 in vitro prior to injection into nude mice resulted in reduced lung deposits of the cells within hours following injection [54]. Under these conditions, there would have been no way to know whether the co-injected 4C5 could have worked by continuously binding and neutralizing constantly secreted Hsp90α and Hsp90α by the tumor cells for the entire period of the experiments. It would make more sense to inject and maintain a steady-state amount of DMAG-N-oxide or 4C5 in circulation, prior to injection with the tumor cells. For DMAG-N-oxide, toxicity in the animals was cited as the reason for excluding injection into blood. Third, while the data was convincing that breast cancer cells lacking the LRP-1 receptor were unable to effectively form tumors in nude mice, the effect of down-regulation of LRP-1 may not necessarily be due to specific blockade of eHsp90α signaling. LRP-1 is known to bind a number of other ligands (82). Thus, to prove the essential role of eHsp90, there is a need to develop more specific and stable inhibitors against the pro-motility function of eHsp90.
Nonetheless, it has come to the clinically important question of what tumors do or do not secrete Hsp90. So far, at least one critical regulator of Hsp90 secretion has been established. Li et al showed that HIF-1α mediates hypoxia-induced Hsp90α secretion in human dermal fibroblasts and keratnocytes. Forced expression of a constitutively activated HIF-1α, CA-HIF-1α, was sufficient to replace hypoxia to cause HIF-1α secretion [10, 39]. This finding is relevant to cancer, since HIF-1α overexpression is associated with increased patient mortality in approximately 40% of human solid tumors, independent of other specific mechanisms [41]. Taking breast caner as an example, Dales et al carried out anti-HIF-1α immunohistochemical assays on frozen sections of 745 breast cancer samples and found that the levels of HIF-1α expression correlated to poor prognosis, lower overall survival and high metastasis risk among both node-negative and node-positive patients [83]. By using HIF-1α expression as a marker, it was estimated that approximately 25–40% of all invasive breast cancer samples are hypoxic, suggesting that HIF-1α may be used as a broader marker for breast cancers [82]. Sahu et al have recently shown that down-regulation of the endogenous and constitutively expressed HIF-1α in breast cancer cell lines, MDA-MB-231 and MDA-MB-468, completely blocked Hsp90α secretion, and the secretion could be rescued by re-introducing RNAi-insensitive WT-HIF-1α and CA-HIF-1α, but not DN-HIF-1α, genes (32). These data establish that HIF-1α is a crucial and direct upstream regulator of Hsp90α secretion. If we extrapolate these findings and numbers on HIF-1α and cancer progression, it suggests that eHsp90α plays an important role at least in those HIF-1α-overexpressing (≥40%) tumors in humans.
Is eHsp90 a more effective and less toxic target than intracellular Hsp90 for treatment of the tumors?
In many, but not all, tumor cells, Hsp90 has been found either quantitatively overexpressed or qualitatively overactivated (with similar expressing levels of normal cells) [85]. In either case, these seemingly “cancer-related” Hsp90 proteins are believed to bind and protect the stability and, therefore, oncogenecity of the oncogene products [84]. Such higher degrees of protection of the oncoproteins by these Hsp90 proteins in tumor cells than that of cellular proteins in normal cells are viewed as an opportunity for anti-tumor drugs. Geldanamycin (GM, or benzoquinone ansamycin) and its derivatives inhibit the ATP-binding and ATP hydrolysis functions of Hsp90 and have been the focus of anti-tumor drug development for two decades [87]. Many of the earlier trials did not advance. Several newer generations of chemically modified and less toxic GM-related drugs are being developed and tested in a dozen of new clinical trials [88, 89]. An obvious key hurdle of these trials is how to minimize their potential interference with the physiological chaperone function of Hsp90 in surrounding normal tissue and cells and selectively harm the “cancer-related” Hsp90 proteins in tumor cells embedded next to the normal cells. It has proven difficult for Hsp90 inhibitors of this nature [89].
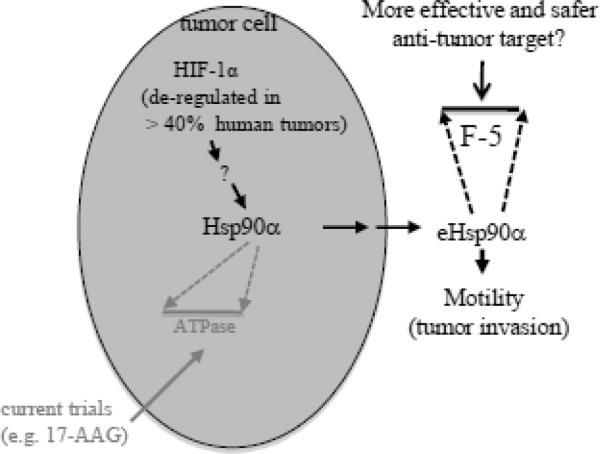
In contrast, no physiological function has been reported for the action of eHsp90, which requires the F-5 epitope within the highly charged linker region and part of the middle domain in Hsp90α. Using gene rescue experiments, Picard and colleagues showed that a highly charged linker region in yeast Hsp90 (Hsp82), which overlaps with the F-5 epitope of human Hsp90α, was dispensable for viability in yeast [90]. This genetic datum, together with all the studies for the past seven years, suggests that secretion of Hsp90 by normal cells is an emergency response of the cells to environmental insults, such as normal cells in a wounded tissue or cancer cells in a hypoxic environment. Furthermore, eHsp90 does not require the ATPase region, which is the target for the GM-related inhibitors and the reason for the drug-caused cytotoxicity. Therefore, the F-5 epitope in eHsp90α may represent an excellent target for design of safer, effective and more specific inhibitors for treatment of HIF-1α-positive tumors. Therefore, we propose that new anti-cancer drugs should i) selectively inhibit eHsp90 (not its intracellular counterpart) and ii) specifically target at the pro-motility activity located at the F-5 region. Drugs that bear both properties should achieve a higher efficacy and pose minimum toxicity to cancer patients. A schematic representation of this simplified thought is depicted in Figure 5.
Figure 5. A model of secreted Hsp90α as a potential target for HIF-1α-positive cancers.
The severe hypoxia often found at the center of a stroma-surrounded tumor. Gene mutations in the tumor cells have caused constitutive accumulation of HIF-1α, even under normoxia. The deregulated HIF-1α triggers secretion of Hsp90α via exosomal protein trafficking pathway. The secreted Hsp90α binds, via F-5 epitope, to cell surface LRP-1 receptor and promotes motility and invasion of tumor cells in an autocrine fashion. While current clinical trials focus on intracellular Hsp90α, we propose that drugs that target the F-5 region of secreted Hsp90α are more effective and safer in treatment of cancer patients (Modified with permission from ref.32).
Wound healing and tumor progression: similar strategy used by peacemaker and terror
What is the relationship between wound healing and cancer? Wound healing is a physiological repair process by inflammatory, epithelial, fibroblastic and endothelial cells that is only activated in response to injury or a surgeon's knife. Cancer is in many ways a similar process by a similar group of cells (called tumor stroma cells as a distinction) in response to the “injury” that is caused by the invading tumor cells. Wound healing usually has a beginning and an ending, whereas cancer has a beginning but often an open ending (patient death) if left untreated. In his seminar-converted analytical article published in The New England Journal of Medicine, Dvotak listed lines of similarities (and distinctions) between wound healing and tumor stroma generation and suggested that tumor stroma formation is subversion of the normal wound healing process. Therefore, he called tumors “wounds that do not heal” (91).
Such a reverse relationship of “healing” and “no healing” between wound healing and cancer should be taken into account, whence eHsp90 becomes a clinical target. For instance, topical application of F-5 peptide to wounds has to consider if the peptide goes into the blood circulation and travels to the site where an early-stage tumor is in progress. Under this circumstance, F-5 might aid the tumor cell invasion and speed up its growth. On the other hand, administration of inhibitors (a mAb, for instance) of F-5 for blockade of the tumor growth might interfere with the normal wound healing process in the same patient. This is a legitimate and realistic concern. For example, numerous studies showed that the people with type II diabetes are more likely to die from cancer than non-diabetic people. Therefore, for a diabetic patient who has cancer and a foot ulcer, if the patient is treated with inhibitors of F-5 by an oncologist to slow the cancer progression, the administered inhibitor could interfere with the healing process of the chronic wound managed by a wound specialist. The reverse is true. If the diabetic ulcer is treated with F-5 peptide, the peptide may travel through the blood circulation to the tumor site and to aid invasion and metastasis of the tumor. It is important to know what patients can receive the treatments and what cannot.
Conclusion and Perspective
It is becoming clear that Hsp90 has two Mother Nature-signed roles to play. One is as an intracellular chaperone and the other is as an extracellular tissue-repairing factor, both of which seem to be designed for the cells to cope with environmental changes, such as tissue injury. Both functions have been taken advantage of by tumor cells during invasion and metastasis. A central controller of Hsp90 secretion inside the cells is HIF-1α, which is overexpressed in more than 40% solid tumors in humans. LRP-1 is essential for eHsp90 signaling to promote cell migration and tumor invasion. More importantly, the recognition of eHsp90's existence and its role in tissue repair and cancer invasion has revealed a new line of therapeutic intervention. Evidence-based advantages of targeting eHsp90 over conventional growth factors for wound healing have been shown in pre-clinical studies. Predicted advantages of targeting eHsp90 over intracellular Hsp90 in prevention of tumor progression remain to be tested, upon availability of specific inhibitors. A crucial tool for the future studies is to develop inhibitors that specific target the F-5 region of eHsp90 for both in vitro and in vivo experiments. Meanwhile, for the next five years, important questions such as mechanisms of Hsp90 secretion and eHsp90 action will continue keeping researchers busy and challenged.
Highlights
Normal cells secrete Hsp90 only under a stress, whereas tumor cells secrete Hsp90 always.
Secreted Hsp90α is an unconventional pro-motility factor, independent of ATPase.
Secreted Hsp90α, but not conventional growth factors, works against TGFβ inhibition.
Topical application of Hsp90α protein promotes acute and chronic wound healing.
Drugs that selectively target F-5 region of Hsp90 may be more effective and less toxic than those targeting the ATPase.
Acknowledgment
We thank many of our previous lab colleagues who made contributions to some of the work described in this review. We apologize if we failed to acknowledge every publication on eHsp90, while this review was being written. This study was supported by NIH grants GM066193 and GM067100 (to W. L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- [2].Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- [3].Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- [4].Grose R, Werner S. Wound-healing studies in transgenic and knockout mice. Mol Biotechnol. 2004;28:147–166. doi: 10.1385/MB:28:2:147. [DOI] [PubMed] [Google Scholar]
- [5].LeGrand EK. Preclinical promise of becaplermin (rhPDGF-BB) in wound healing. Am J Surg. 1998;176:48S–54S. doi: 10.1016/s0002-9610(98)00177-9. [DOI] [PubMed] [Google Scholar]
- [6].Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J Vasc Surg. 1995;21:71–78. doi: 10.1016/s0741-5214(95)70245-8. discussion 79–81. [DOI] [PubMed] [Google Scholar]
- [7].Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21:822–827. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- [8].Nagai MK, Embil JM. Becaplermin: recombinant platelet derived growth factor, a new treatment for healing diabetic foot ulcers. Expert Opin Biol Ther. 2002;2:211–218. doi: 10.1517/14712598.2.2.211. [DOI] [PubMed] [Google Scholar]
- [9].Mandracchia VJ, Sanders SM, Frerichs JA. The use of becaplermin (rhPDGF-BB) gel for chronic nonhealing ulcers. A retrospective analysis. Clin Podiatr Med Surg. 2001;18:189–209. viii. [PubMed] [Google Scholar]
- [10].Li W, Li Y, Guan S, Fan J, Cheng CF, Bright AM, Chinn C, Chen M, Woodley DT. Extracellular heat shock protein-90alpha: linking hypoxia to skin cell motility and wound healing. EMBO J. 2007;26:1221–1233. doi: 10.1038/sj.emboj.7601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cheng CF, Fan J, Fedesco M, Guan S, Li Y, Bandyopadhyay B, Bright AM, Yerushalmi D, Liang M, Chen M, Han YP, Woodley DT, Li W. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol Cell Biol. 2008;28:3344–3358. doi: 10.1128/MCB.01287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cheng CF, Sahu D, Tsen F, Zhao Z, Fan J, Wang X, Kim R, Kuang Y, Chen M, Woodley DT, Li W. J. Clin. Inv. 2011. A fragment of secreted Hsp90alpha carries unique properties to accelerate acute and chronic wound healing. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- [14].Cheng CF, Fan J, Zhao Z, Woodley DT, Li W. Secreted Heat Shock Protein-90alpha: A More Effective and Safer Target for Anti-Cancer Drugs? Current Signal Transduction Therapy. 2010;5:121–127. [Google Scholar]
- [15].Tsutsumi S, Neckers L. Extracellular heat shock protein 90: a role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 2007;98:1536–1539. doi: 10.1111/j.1349-7006.2007.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liao DF, Jin ZG, Baas AS, Daum G, Gygi SP, Aebersold R, Berk BC. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem. 2000;275:189–196. doi: 10.1074/jbc.275.1.189. [DOI] [PubMed] [Google Scholar]
- [17].Kuroita T, Tachibana H, Ohashi H, Shirahata S, Murakami H. Growth stimulating activity of heat shock protein 90 alpha to lymphoid cell lines in serum-free medium. Cytotechnology. 1992;8:109–117. doi: 10.1007/BF02525493. [DOI] [PubMed] [Google Scholar]
- [18].Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81:15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]
- [19].Basu S, Srivastava PK. Heat shock proteins: the fountainhead of innate and adaptive immune responses. Cell Stress Chaperones. 2000;5:443–451. doi: 10.1379/1466-1268(2000)005<0443:hsptfo>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Binder RJ, Vatner R, Srivastava P. The heat-shock protein receptors: some answers and more questions. Tissue Antigens. 2004;64:442–451. doi: 10.1111/j.1399-0039.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- [21].Gopal U, Bohonowych JE, Lema-Tome C, Liu A, Garrett-Mayer E, Wang B, Isaacs JS. A novel extracellular Hsp90 mediated co-receptor function for LRP1 regulates EphA2 dependent glioblastoma cell invasion. PLoS One. 2011;6:e17649. doi: 10.1371/journal.pone.0017649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gasc JM, Renoir JM, Radanyi C, Joab I, Tuohimaa P, Baulieu EE. Progesterone receptor in the chick oviduct: an immunohistochemical study with antibodies to distinct receptor components. J Cell Biol. 1984;99:1193–1201. doi: 10.1083/jcb.99.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Welch WJ, Feramisco JR. Purification of the major mammalian heat shock proteins. J Biol Chem. 1982;257:14949–14959. [PubMed] [Google Scholar]
- [24].Catelli MG, Joab I, Renoir JM, Mester J, Binart N, Radanyi C, Buchou T, Zoorob R, Baulieu EE. 402 Immunological studies of chick oviduct progesterone receptor. J. Steroid Biochem. 1983;19:134. [Google Scholar]
- [25].Lai BT, Chin NW, Stanek AE, Keh W, Lanks KW. Quantitation and intracellular localization of the 85K heat shock protein by using monoclonal and polyclonal antibodies. Mol Cell Biol. 1984;4:2802–2810. doi: 10.1128/mcb.4.12.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ferrarini M, Heltai S, Zocchi MR, Rugarli C. Unusual expression and localization of heatshock proteins in human tumor cells. Int J Cancer. 1992;51:613–619. doi: 10.1002/ijc.2910510418. [DOI] [PubMed] [Google Scholar]
- [27].Jameel A, Skilton RA, Campbell TA, Chander SK, Coombes RC, Luqmani YA. Clinical and biological significance of HSP89 alpha in human breast cancer. Int J Cancer. 1992;50:409–415. doi: 10.1002/ijc.2910500315. [DOI] [PubMed] [Google Scholar]
- [28].Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O'Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- [29].Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, Kluger HM. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007;67:2932–2937. doi: 10.1158/0008-5472.CAN-06-4511. [DOI] [PubMed] [Google Scholar]
- [30].Wang X, Heuvelman DM, Carroll JA, Dufield DR, Masferrer JL, Geldanamycin-induced JL. PCNA degradation in isolated Hsp90 complex from cancer cells. Cancer Invest. 2010;28:635–641. doi: 10.3109/07357901003630983. [DOI] [PubMed] [Google Scholar]
- [31].Kubota H, Yamamoto S, Itoh E, Abe Y, Nakamura A, Izumi Y, Okada H, Iida M, Nanjo H, Itoh H, Yamamoto Y. Increased expression of co-chaperone HOP with HSP90 and HSC70 and complex formation in human colonic carcinoma. Cell Stress Chaperones. 2010;15:1003–1011. doi: 10.1007/s12192-010-0211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sahu D, Zhao Z, Tsen F, Cheng C-F, Ryan P, Fan J, Dai J, Eginli A, Shams S, Chen M, Conti P, Woodley DT, Li W. Mol. Biol. Cell. 2011. Identification of a novel tumor epitope in secreted Hsp90α for HIF-1α-overexpressing breast cancer. in press. [Google Scholar]
- [33].Multhoff G, Hightower LE. Cell surface expression of heat shock proteins and the immune response. Cell Stress Chaperones. 1996;1:167–176. doi: 10.1379/1466-1268(1996)001<0167:cseohs>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hightower LE, Guidon PT., Jr. Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- [35].Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- [36].Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- [37].Tsutsumi S, Mollapour M, Graf C, Lee CT, Scroggins BT, Xu W, Haslerova L, Hessling M, Konstantinova AA, Trepel JB, Panaretou B, Buchner J, Mayer MP, Prodromou C, Neckers L. Hsp90 charged-linker truncation reverses the functional consequences of weakened hydrophobic contacts in the N domain. Nat Struct. Mol. Biol. 2009;16:1141–1147. doi: 10.1038/nsmb.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang X, Song X, Zhuo W, Fu Y, Shi H, Liang Y, Tong M, Chang G, Luo Y. The regulatory mechanism of Hsp90alpha secretion and its function in tumor malignancy. Proc Natl Acad Sci U S A. 2009;106:21288–21293. doi: 10.1073/pnas.0908151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Woodley DT, Fan J, Cheng CF, Li Y, Chen M, Bu G, Li W. Participation of the lipoprotein receptor LRP1 in hypoxia-HSP90alpha autocrine signaling to promote keratinocyte migration. J Cell Sci. 2009;122:1495–1498. doi: 10.1242/jcs.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today. 2007;12:853–859. doi: 10.1016/j.drudis.2007.08.006. [DOI] [PubMed] [Google Scholar]
- [42].Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, Zehetmeier C, Lain B, Torella C, Henning SW, Beste G, Scroggins BT, Neckers L, Ilag LL, Jay DG. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- [43].Suzuki S, Kulkarni AB. Extracellular heat shock protein HSP90beta secreted by MG63 osteosarcoma cells inhibits activation of latent TGF-beta1. Biochem Biophys Res Commun. 2010;398:525–5331. doi: 10.1016/j.bbrc.2010.06.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen JS, Hsu YM, Chen CC, Chen LL, Lee CC, Huang TS. Secreted heat shock protein 90alpha induces colorectal cancer cell invasion through CD91/LRP-1 and NF-kappaB-mediated integrin alphaV expression. J Biol Chem. 2010;285:25458–25466. doi: 10.1074/jbc.M110.139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tsutsumi S, Scroggins B, Koga F, Lee MJ, Trepel J, Felts S, Carreras C, Neckers L. A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene. 2008;27:2478–2487. doi: 10.1038/sj.onc.1210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(Pt 19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- [47].Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- [48].Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- [49].Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- [50].Mignot G, Roux S, Thery C, Segura E, Zitvogel L. Prospects for exosomes in immunotherapy of cancer. J Cell Mol Med. 2006;10:376–388. doi: 10.1111/j.1582-4934.2006.tb00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol. 2004;164:1807–1815. doi: 10.1016/S0002-9440(10)63739-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Young JC, Moarefi I, Hartl FU. Hsp90: a specialized but essential protein-folding tool. J Cell Biol. 2001;154:267–273. doi: 10.1083/jcb.200104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Song X, Wang X, Zhuo W, Shi H, Feng D, Sun Y, Liang Y, Fu Y, Zhou D, Luo Y. The regulatory mechanism of extracellular Hsp90{alpha} on matrix metalloproteinase-2 processing and tumor angiogenesis. J Biol Chem. 2010;285:40039–40049. doi: 10.1074/jbc.M110.181941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Stellas D, El Hamidieh A, Patsavoudi E. Monoclonal antibody 4C5 prevents activation of MMP2 and MMP9 by disrupting their interaction with extracellular HSP90 and inhibits formation of metastatic breast cancer cell deposits. BMC Cell Biol. 2010;11:51. doi: 10.1186/1471-2121-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sidera K, Samiotaki M, Yfanti E, Panayotou G, Patsavoudi E. Involvement of cell surface HSP90 in cell migration reveals a novel role in the developing nervous system. J Biol Chem. 2004;279:45379–45388. doi: 10.1074/jbc.M405486200. [DOI] [PubMed] [Google Scholar]
- [56].Sidera K, Gaitanou M, Stellas D, Matsas R, Patsavoudi E E. A critical role for HSP90 in cancer cell invasion involves interaction with the extracellular domain of HER-2. J Biol Chem. 2008;283:2031–2041. doi: 10.1074/jbc.M701803200. [DOI] [PubMed] [Google Scholar]
- [57].McCready J, Sims JD, Chan D D, Jay DG. Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010;10:294–299. doi: 10.1186/1471-2407-10-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chung SW, Lee JH JH, Choi KH, Park YC, Eo SK, Rhim BY, Kim K. Extracellular heat shock protein 90 induces interleukin-8 in vascular smooth muscle cells. Biochem Biophys Res Commun. 2009;378:444–449. doi: 10.1016/j.bbrc.2008.11.063. [DOI] [PubMed] [Google Scholar]
- [59].Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, Argraves WS. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265:17401–17404. [PubMed] [Google Scholar]
- [61].Kristensen T, Moestrup SK, Gliemann J, Bendtsen L, Sand O, Sottrup-Jensen L. Evidence that the newly cloned low-density-lipoprotein receptor related protein (LRP) is the alpha 2-macroglobulin receptor. FEBS Lett. 1990;276:151–155. doi: 10.1016/0014-5793(90)80530-v. [DOI] [PubMed] [Google Scholar]
- [62].Van Leuven F, Stas L, Raymakers L, Overbergh L, De Strooper B, Hilliker C, Lorent K, Fias E, Umans L, Torrekens S, et al. Molecular cloning and sequencing of the murine alpha-2-macroglobulin receptor cDNA. Biochim Biophys Acta. 1993;1173:71–74. doi: 10.1016/0167-4781(93)90244-8. [DOI] [PubMed] [Google Scholar]
- [63].Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- [64].Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169:3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- [66].Habich C, Baumgart K, Kolb H, Burkart V. The receptor for heat shock protein 60 on macrophages is saturable, specific, and distinct from receptors for other heat shock proteins. J Immunol. 2002;168:569–576. doi: 10.4049/jimmunol.168.2.569. [DOI] [PubMed] [Google Scholar]
- [67].Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lillis AP, Mikhailenko I, Strickland DK. Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J Thromb Haemost. 2005;3:1884–1893. doi: 10.1111/j.1538-7836.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- [69].Bandyopadhyay B, Fan J, Guan S, Li Y, Chen M, Woodley DT, Li W. A “traffic control” role for TGFbeta3: orchestrating dermal and epidermal cell motility during wound healing. J Cell Biol. 2006;172:1093–1105. doi: 10.1083/jcb.200507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bachman KE, Park BH. Duel nature of TGF-beta signaling: tumor suppressor vs. tumor promoter. Curr Opin Oncol. 2005;17:49–54. doi: 10.1097/01.cco.0000143682.45316.ae. [DOI] [PubMed] [Google Scholar]
- [71].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [72].Papadopoulos N, Nicolaides NC, Liu B, Parsons R, Lengauer C, Palombo F, D'Arrigo A, Markowitz S, Willson JK, Kinzler KW, et al. Mutations of GTBP in genetically unstable cells. Science. 1995;268:1915–1917. doi: 10.1126/science.7604266. [DOI] [PubMed] [Google Scholar]
- [73].Chen T, Triplett J, Dehner B, Hurst B, Colligan B, Pemberton J, Graff JR, Carter JH. Transforming growth factor-beta receptor type I gene is frequently mutated in ovarian carcinomas. Cancer Res. 2001;61:4679–4682. [PubMed] [Google Scholar]
- [74].Pinto M, Oliveira C, Cirnes L, Carlos Machado J, Ramires M, Nogueira A, Carneiro F, Seruca R. Promoter methylation of TGFbeta receptor I and mutation of TGFbeta receptor II are frequent events in MSI sporadic gastric carcinomas. J Pathol. 2003;200:32–38. doi: 10.1002/path.1327. [DOI] [PubMed] [Google Scholar]
- [75].Hahm KB, Im YH, Lee C, Parks WT, Bang YJ, Green JE, Kim SJ. Loss of TGF-beta signaling contributes to autoimmune pancreatitis. J Clin Invest. 2000;105:1057–1065. doi: 10.1172/JCI8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Howe JR, Roth S, Ringold JC, Summers RW, Jarvinen HJ, Sistonen P, Tomlinson IP, Houlston RS, Bevan S, Mitros FA, Stone EM, Aaltonen LA. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science. 1998;280:1086–1088. doi: 10.1126/science.280.5366.1086. [DOI] [PubMed] [Google Scholar]
- [77].O'Toole EA, Marinkovich MP, Peavey CL, Amieva MR, Furthmayr H, Mustoe TA, Woodley DT. Hypoxia increases human keratinocyte motility on connective tissue. J. Clin. Invest. 1997;100:2881–2891. doi: 10.1172/JCI119837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Li Y, Fan J, Chen M, Li W, Woodley DT. Transforming growth factor-alpha: a major human serum factor that promotes human keratinocyte migration. J. Invest. Dermatol. 2006;126:2096–2105. doi: 10.1038/sj.jid.5700350. [DOI] [PubMed] [Google Scholar]
- [79].Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- [80].Catrina SB, Okamoto K, Pereira T, Brismar K, Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes. 2004;53:3226–3232. doi: 10.2337/diabetes.53.12.3226. [DOI] [PubMed] [Google Scholar]
- [81].Botusan IR, Sunkari VG, Savu O, Catrina AI, Grunler J, Lindberg S, Pereira T, Yla-Herttuala S, Poellinger L, Brismar K, Catrina SB. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A. 2008;105:19426–19431. doi: 10.1073/pnas.0805230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- [83].Dales JP, Garcia S, Meunier-Carpentier S, Andrac-Meyer L, Haddad O, Lavaut MN, Allasia C, Bonnier P, Charpin C. Overexpression of hypoxia-inducible factor HIF-1alpha predicts early in breast cancer: retrospective study in a series of 745 patients. Int J Cancer. 2005;116:734–739. doi: 10.1002/ijc.20984. [DOI] [PubMed] [Google Scholar]
- [84].Lundgren K, Holm C, Landberg G. Hypoxia and breast cancer: prognostic and therapeutic implications. Cell Mol Life Sci. 2007;64:3233–3247. doi: 10.1007/s00018-007-7390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- [86].Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Neckers L, Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutic agents. Expert Opin Emerg Drugs. 2002;7:277–288. doi: 10.1517/14728214.7.2.277. [DOI] [PubMed] [Google Scholar]
- [88].Solit DB, Chiosis G. Development and application of Hsp90 inhibitors. Drug Discov Today. 2008;13:38–43. doi: 10.1016/j.drudis.2007.10.007. [DOI] [PubMed] [Google Scholar]
- [89].Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev. Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Louvion JF, Warth R, Picard D. Two eukaryote-specific regions of Hsp82 are dispensable for its viability and signal transduction functions in yeast. Proc Natl Acad Sci U S A. 1996;93:13937–13942. doi: 10.1073/pnas.93.24.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dvorack HF. Tumors: wounds that do not heal. The New Eng. J. Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]