Abstract
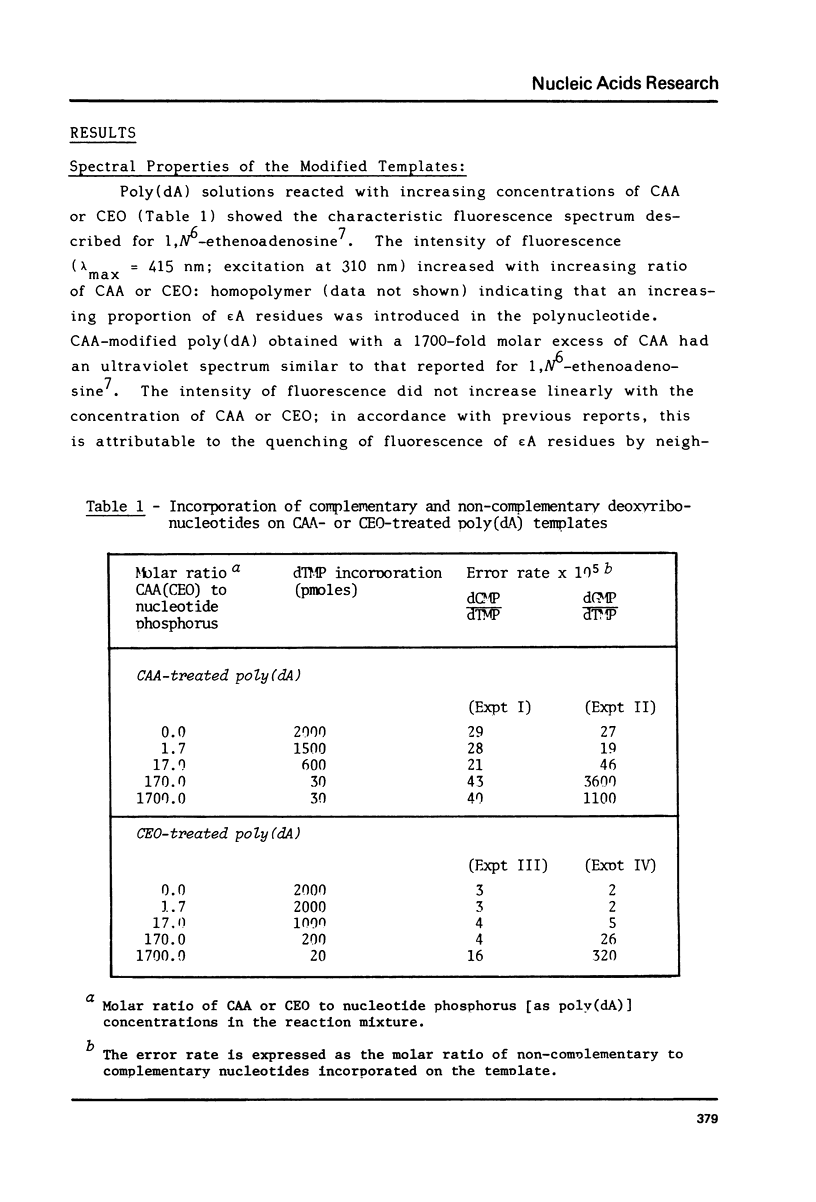
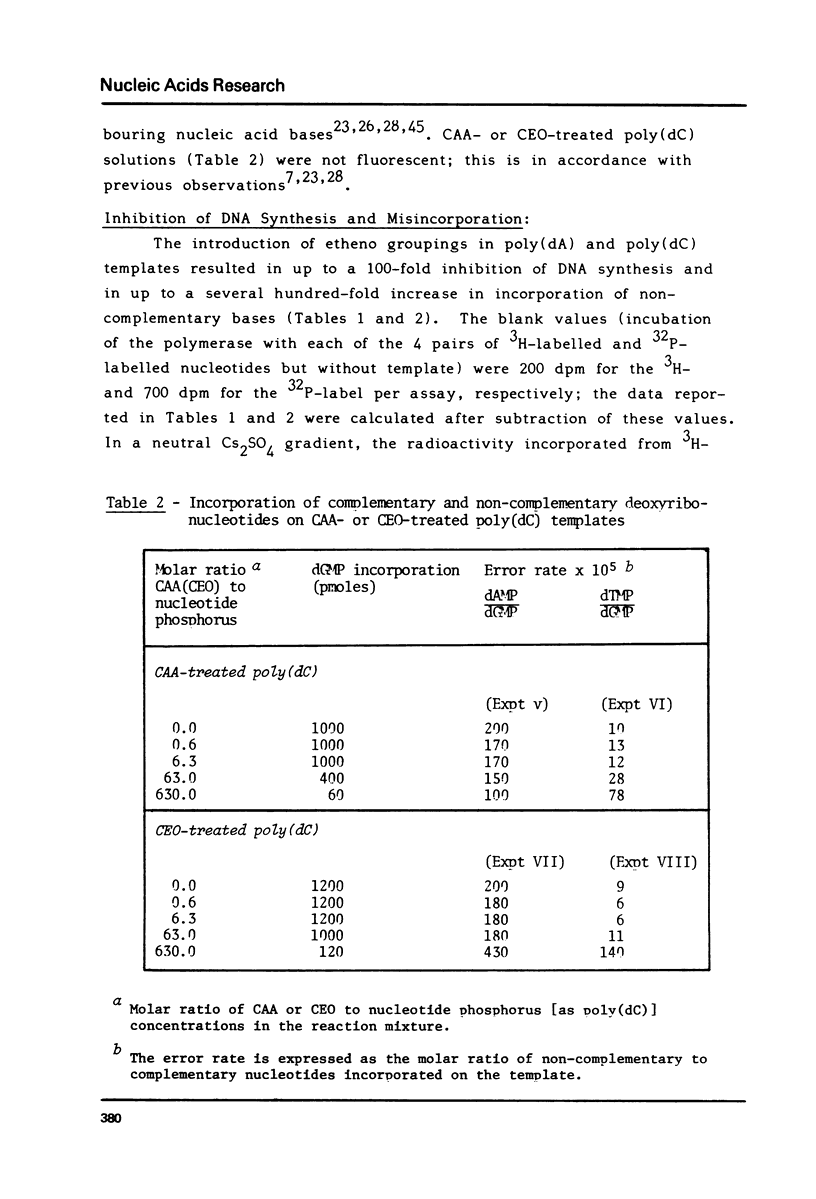
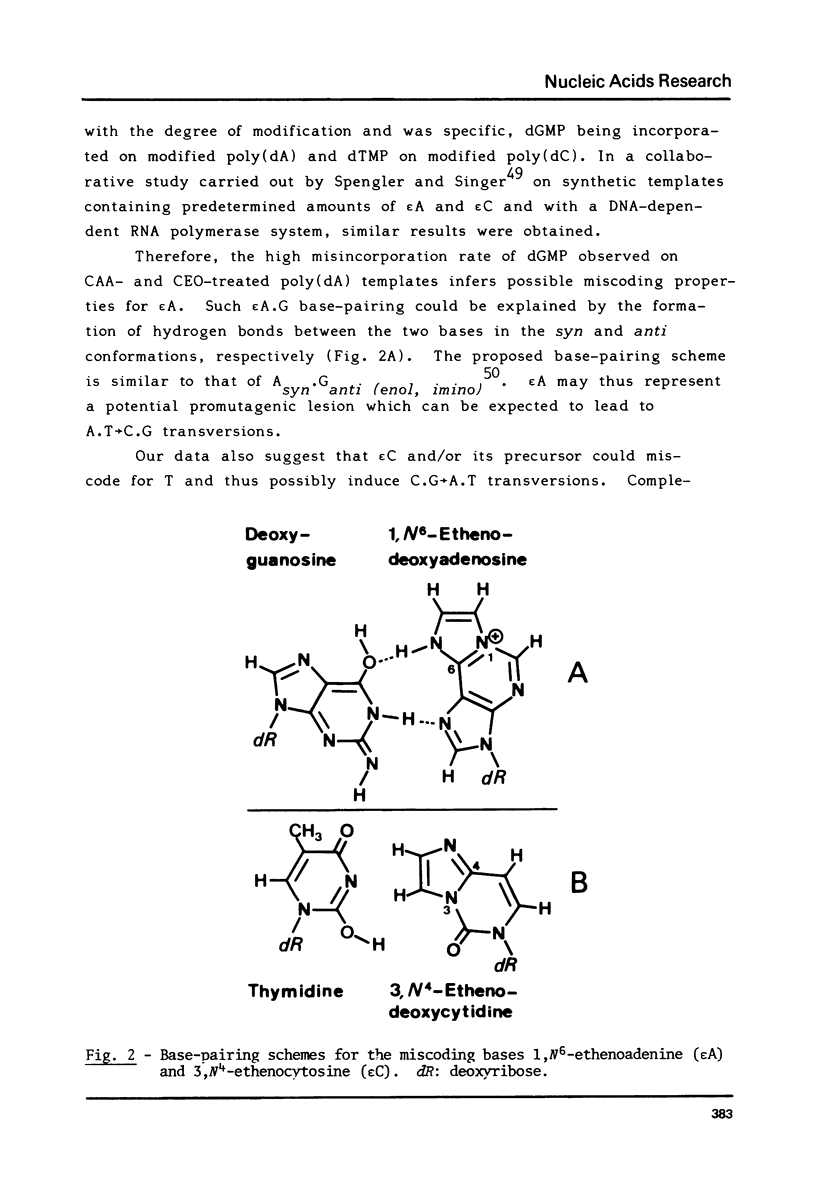
1,N6-Ethenoadenine (epsilon A) and 3,N4-ethenocytosine (epsilon C) are formed when electrophilic vinyl chloride (VC) metabolites, chloroethylene oxide (CEO) or chloroacetaldehyde (CAA) react with adenine and cytosine residues in DNA. They were assayed for their miscoding properties in an in vitro system using Escherichia coli DNA polymerase I and synthetic templates prepared by reaction of poly(dA) and poly(dC) with increasing concentrations of CEO or CAA. Following the introduction of etheno groups, an increasing inhibition of DNA synthesis was observed. dGMP was misincorporated on CAA- or CEO-treated poly(dA) templates and dTMP was misincorporated on CAA- or CEO-treated poly(dC) templates, suggesting that epsilon A and epsilon C may miscode. The error rates augmented with the extent of reaction of CEO or CAA with the templates. Base-pairing models are proposed for the epsilon A.G. and epsilon C.T pairs. The potentially miscoding properties of epsilon A and epsilon C may explain why metabolically-activated VC and its reactive metabolites specifically induce base-pair substitution mutations in Salmonella typhimurium. Promutagenic lesions may represent one of the initial steps in VC- or CEO-induced carcinogenesis.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott P. J., Saffhill R. DNA synthesis with methylated poly(dC-dG) templates. Evidence for a competitive nature to miscoding by O(6)-methylguanine. Biochim Biophys Acta. 1979 Mar 28;562(1):51–61. doi: 10.1016/0005-2787(79)90125-4. [DOI] [PubMed] [Google Scholar]
- Abbott P. J., Saffhill R. DNA-synthesis with methylated poly(dA-dT) templates: possible role of O4-methylthymine as a pro-mutagenic base. Nucleic Acids Res. 1977 Mar;4(3):761–769. doi: 10.1093/nar/4.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S. S., Dube D. K., Loeb L. A. On the fidelity of DNA replication. Accuracy of Escherichia coli DNA polymerase I. J Biol Chem. 1979 Jan 10;254(1):101–106. [PubMed] [Google Scholar]
- Barbin A., Brésil H., Croisy A., Jacquignon P., Malaveille C., Montesano R., Bartsch H. Liver-microsome-mediated formation of alkylating agents from vinyl bromide and vinyl chloride. Biochem Biophys Res Commun. 1975 Nov 17;67(2):596–603. doi: 10.1016/0006-291x(75)90854-2. [DOI] [PubMed] [Google Scholar]
- Barrio J. R., Dammann L. G., Kirkegaard L. H., Switzer R. L., Leonard N. J. Enzymatic activity and - 32 P labeling of fluorescent derivatives of cytidine triphosphate and adenosine triphosphate. J Am Chem Soc. 1973 Feb 7;95(3):961–962. doi: 10.1021/ja00784a075. [DOI] [PubMed] [Google Scholar]
- Barrio J. R., Secrist J. A., 3rd, Leonard N. J. A fluorescent analog of nicotinamide adenine dinucleotide. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2039–2042. doi: 10.1073/pnas.69.8.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio J. R., Secrist J. A., 3rd, Leonard N. J. Fluorescent adenosine and cytidine derivatives. Biochem Biophys Res Commun. 1972 Jan 31;46(2):597–604. doi: 10.1016/s0006-291x(72)80181-5. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Malaveille C., Barbin A., Planche G. Mutagenic and alkylating metabolites of halo-ethylenes, chlorobutadienes and dichlorobutenes produced by rodent or human liver tissues. Evidence for oxirane formation by P450-linked microsomal mono-oxygenases. Arch Toxicol. 1979 Feb 23;41(4):249–277. doi: 10.1007/BF00296896. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Montesano R. Mutagenic and carcinogenic effects of vinyl chloride. Mutat Res. 1975;32(2):93–114. doi: 10.1016/0165-1110(75)90001-9. [DOI] [PubMed] [Google Scholar]
- Biernat J., Ciesiołka J., Górnicki P., Adamiak R. W., Kryzosiak W. J., Wiewiórowski M. New observations concerning the chloroacetaldehyde reaction with some tRNA constituents. Stable intermediates, kinetics and selectivity of the reaction. Nucleic Acids Res. 1978 Mar;5(3):789–804. doi: 10.1093/nar/5.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami M., Conti G., Conti L., Crebelli R., Misuraca F., Puglia A. M., Randazzo R., Sciandrello G., Carere A. Mutagenicity of halogenated aliphatic hydrocarbons in Salmonella typhimurium, Streptomyces coelicolor and Aspergillus nidulans. Chem Biol Interact. 1980 Apr;30(1):9–23. doi: 10.1016/0009-2797(80)90110-6. [DOI] [PubMed] [Google Scholar]
- Bonse G., Urban T., Reichert D., Henschler D. Chemical reactivity, metabolic oxirane formation and biological reactivity of chlorinated ethylenes in the isolated perfused rat liver preparation. Biochem Pharmacol. 1975 Oct 1;24(19):1829–1834. doi: 10.1016/0006-2952(75)90468-2. [DOI] [PubMed] [Google Scholar]
- Creech J. L., Jr, Johnson M. N. Angiosarcoma of liver in the manufacture of polyvinyl chloride. J Occup Med. 1974 Mar;16(3):150–151. [PubMed] [Google Scholar]
- Gerchman L. L., Ludlum D. B. The properties of O 6 -methylguanine in templates for RNA polymerase. Biochim Biophys Acta. 1973 May 10;308(2):310–316. doi: 10.1016/0005-2787(73)90160-3. [DOI] [PubMed] [Google Scholar]
- Goldschmidt B. M., Blazej T. P., Van Duuren B. L. The reaction of guanosine and deoxyguanosine with glycidaldehyde. Tetrahedron Lett. 1968 Feb;13:1583–1586. doi: 10.1016/s0040-4039(01)99007-0. [DOI] [PubMed] [Google Scholar]
- Green T., Hathway D. E. Interactions of vinyl chloride with rat-liver DNA in vivo. Chem Biol Interact. 1978 Sep;22(2-3):211–224. doi: 10.1016/0009-2797(78)90126-6. [DOI] [PubMed] [Google Scholar]
- Huberman E., Bartsch H., Sachs L. Mutation induction in Chinese hamster V79 cells by two vinyl chloride metabolites, chloroethylene oxide and 2-chloroacetaldehyde. Int J Cancer. 1975 Oct 15;16(4):639–644. doi: 10.1002/ijc.2910160414. [DOI] [PubMed] [Google Scholar]
- Hussain S., Osterman-Golkar S. Comment on the mutagenic effectiveness of vinyl chloride metabolites. Chem Biol Interact. 1976 Mar;12(3-4):265–267. doi: 10.1016/0009-2797(76)90042-9. [DOI] [PubMed] [Google Scholar]
- Ikehara M., Hattori M. Polynucleotides,. XIV. Synthesis and properties of polynucleotides containing 2,6-bis(methylthio)purine ribonucleotides. Biochim Biophys Acta. 1972 Sep 29;281(1):11–17. doi: 10.1016/0005-2787(72)90183-9. [DOI] [PubMed] [Google Scholar]
- Ikehara M., Hattori M. Polynucleotides. XV. Synthesis and properties of polynucleotides containing N 2 -dimethylguanylic acid residues in polyinosinate and polyadenylate chains. Biochim Biophys Acta. 1972 Nov 16;287(1):9–15. doi: 10.1016/0005-2787(72)90325-5. [DOI] [PubMed] [Google Scholar]
- Janik B., Sommer R. G., Kotick M. P., Wilson D. P., Erickson J. Synthesis and properties of poly (1,N-6-ethenoadenylic acid) and poly (3,N-4-ethenocytidylic acid). Physiol Chem Phys. 1973;5(1):27–36. [PubMed] [Google Scholar]
- Kimura K., Nakanishi M., Yamamoto T., Tsuboi M. A correlation between the secondary structure of DNA and the reactivity of adenine residues with chloroacetaldehyde. J Biochem. 1977 Jun;81(6):1699–1703. doi: 10.1093/oxfordjournals.jbchem.a131629. [DOI] [PubMed] [Google Scholar]
- Laib R. J., Bolt H. M. Alkylation of RNA by vinyl chloride metabolites in vitro and in vivo: formation of 1-N(6)-etheno-adenosine. Toxicology. 1977 Oct;8(2):185–195. doi: 10.1016/0300-483x(77)90007-5. [DOI] [PubMed] [Google Scholar]
- Laib R. J., Bolt H. M. Formation of 3,N4-ethenocytidine moieties in RNA by vinyl chloride metabolities in vitro and in vivo. Arch Toxicol. 1978 Jan 25;39(3):235–240. doi: 10.1007/BF00368232. [DOI] [PubMed] [Google Scholar]
- Ledneva R. K., Razjivin A. P., Kost A. A., Bogdanov A. A. Interaction of tobacco mosaic virus protein with synthetic polynucleotides containing a fluorescent label: optical properties of poly(A,epsilonA) and poly(C,epsilonC) copolymers and energy migration from the tryptophan to 1,N6-ethenoadenine or 3,N4-ethenocytosine residues in RNP. Nucleic Acids Res. 1978 Nov;5(11):4225–4243. doi: 10.1093/nar/5.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. H., Wetmur J. G. Physical studies of chloroacetaldehyde labelled fluorescent DNA. Biochem Biophys Res Commun. 1973 Feb 5;50(3):879–885. doi: 10.1016/0006-291x(73)91327-2. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Scheit K. H. Synthesis and properties of a new fluorescent polynucleotide, poly(1,N6-ethenoadenylic acid). Biochim Biophys Acta. 1973 Apr 21;308(7):28–34. doi: 10.1016/0005-2787(73)90118-4. [DOI] [PubMed] [Google Scholar]
- Leonard N. J., Tolman G. L. Fluorescent nucleosides and nucleotides. Ann N Y Acad Sci. 1975 Aug 8;255:43–58. doi: 10.1111/j.1749-6632.1975.tb29212.x. [DOI] [PubMed] [Google Scholar]
- Loprieno N., Barale R., Baroncelli S., Bartsch H., Bronzetti G., Cammelini A., Corsi C., Frezza D., Nieri R., Leporini C. Induction of gene mutations and gene conversions by vinyl chloride metabolites in yeast. Cancer Res. 1977 Jan;37(1):253–257. [PubMed] [Google Scholar]
- Ludlum D. B., Kramer B. S., Wang J., Fenselau C. Reaction of 1,3-bis(2-chloroethyl)-1-nitrosourea with synthetic polynucleotides. Biochemistry. 1975 Dec 16;14(25):5480–5485. doi: 10.1021/bi00696a016. [DOI] [PubMed] [Google Scholar]
- Malaveille C., Bartsch H., Barbin A., Camus A. M., Montesano R., Croisy A., Jacquignon P. Mutagenicity of vinyl chloride, chloroethyleneoxide, chloroacetaldehyde and chloroethanol. Biochem Biophys Res Commun. 1975 Mar 17;63(2):363–370. doi: 10.1016/0006-291x(75)90697-x. [DOI] [PubMed] [Google Scholar]
- McCann J., Simmon V., Streitwieser D., Ames B. N. Mutagenicity of chloroacetaldehyde, a possible metabolic product of 1,2-dichloroethane (ethylene dichloride), chloroethanol (ethylene chlorohydrin), vinyl chloride, and cyclophosphamide. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3190–3193. doi: 10.1073/pnas.72.8.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenwälder H., Laib R. J., Bolt H. M. Alkylation of RNA by vinyl bromide metabolites in vitro and in vivo. Arch Toxicol. 1979 Feb 23;41(4):279–286. doi: 10.1007/BF00296897. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Formation and metabolism of alkylated nucleosides: possible role in carcinogenesis by nitroso compounds and alkylating agents. Adv Cancer Res. 1977;25:195–269. doi: 10.1016/s0065-230x(08)60635-1. [DOI] [PubMed] [Google Scholar]
- Rannug U., Göthe R., Wachtmeister C. A. The mutagenicity of chloroethylene oxide, chloroacetaldehyde, 2-chloroethanol and chloroacetic acid, conceivable metabolites of vinyl chloride. Chem Biol Interact. 1976 Mar;12(3-4):251–263. doi: 10.1016/0009-2797(76)90041-7. [DOI] [PubMed] [Google Scholar]
- Secrist J. A., 3rd, Barrio J. R., Leonard N. J., Villar-Palasi C., Gilman A. G. Fluorescent modification of adenosine 3',5'-monophosphate: spectroscopic properties and activity in enzyme systems. Science. 1972 Jul 21;177(4045):279–280. doi: 10.1126/science.177.4045.279. [DOI] [PubMed] [Google Scholar]
- Shearman C. W., Loeb L. A. Effects of depurination on the fidelity of DNA synthesis. J Mol Biol. 1979 Feb 25;128(2):197–218. doi: 10.1016/0022-2836(79)90126-8. [DOI] [PubMed] [Google Scholar]
- Singer B., Fraenkel-Conrat H., Kuśmierek J. T. Preparation and template activities of polynucleotides containing O2- and O4-alkyluridine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1722–1726. doi: 10.1073/pnas.75.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Erroneous base-pairing induced by a chemical carcinogen during DNA synthesis. Nature. 1974 Nov 29;252(5482):414–416. doi: 10.1038/252414a0. [DOI] [PubMed] [Google Scholar]
- Tolman G. L., Barrio J. R., Leonard N. J. Chloroacetaldehyde-modified dinucleoside phosphates. Dynamic fluorescence quenching and quenching due to intramolecular complexation. Biochemistry. 1974 Nov 19;13(24):4869–4878. doi: 10.1021/bi00721a001. [DOI] [PubMed] [Google Scholar]
- Tong W. P., Ludlum D. B. Mechanism of action of the nitrosoureas -- III. Reactions of bis-chloroethyl nitrosourea and bis-fluoroethyl nitrosourea with adenosine. Biochem Pharmacol. 1979 Apr 1;28(7):1175–1179. doi: 10.1016/0006-2952(79)90325-3. [DOI] [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Complementary base pairing and the origin of substitution mutations. Nature. 1976 Sep 23;263(5575):285–289. doi: 10.1038/263285a0. [DOI] [PubMed] [Google Scholar]
- Van Duuren B. L., Goldschmidt B. M., Loewengart G., Smith A. C., Melchionne S., Seldman I., Roth D. Carcinogenicity of halogenated olefinic and aliphatic hydrocarbons in mice. J Natl Cancer Inst. 1979 Dec;63(6):1433–1439. [PubMed] [Google Scholar]
- Van Duuren B. L., Katz C., Goldschmidt B. M., Frenkel K., Sivak A. Carcinogenicity of halo-ethers. II. Structure-activity relationships of analogs of bis(chloromethyl)ether. J Natl Cancer Inst. 1972 May;48(5):1431–1439. [PubMed] [Google Scholar]
- WATSON J. D., CRICK F. H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953 Apr 25;171(4356):737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- Zajdela F., Croisy A., Barbin A., Malaveille C., Tomatis L., Bartsch H. Carcinogenicity of chloroethylene oxide, an ultimate reactive metabolite of vinyl chloride, and bis(chloromethyl)ether after subcutaneous administration and in initiation-promotion experiments in mice. Cancer Res. 1980 Feb;40(2):352–356. [PubMed] [Google Scholar]


