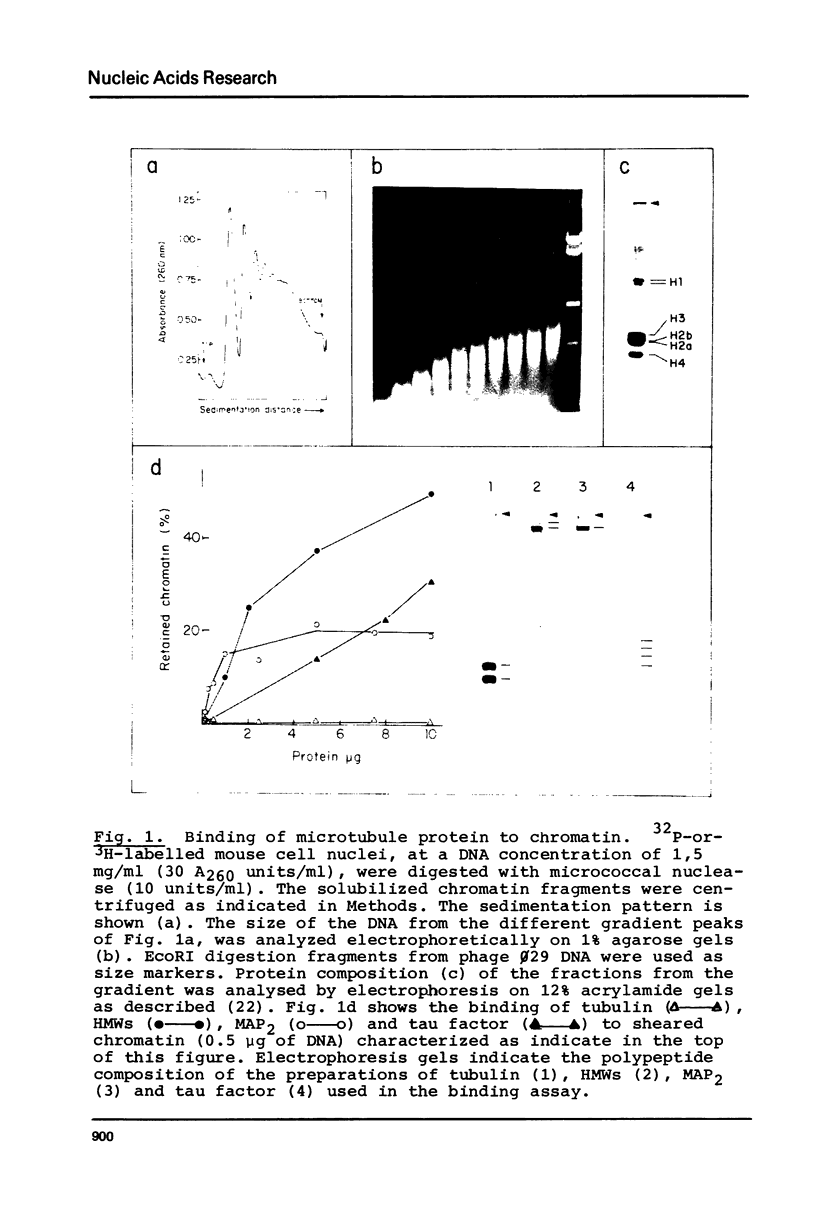
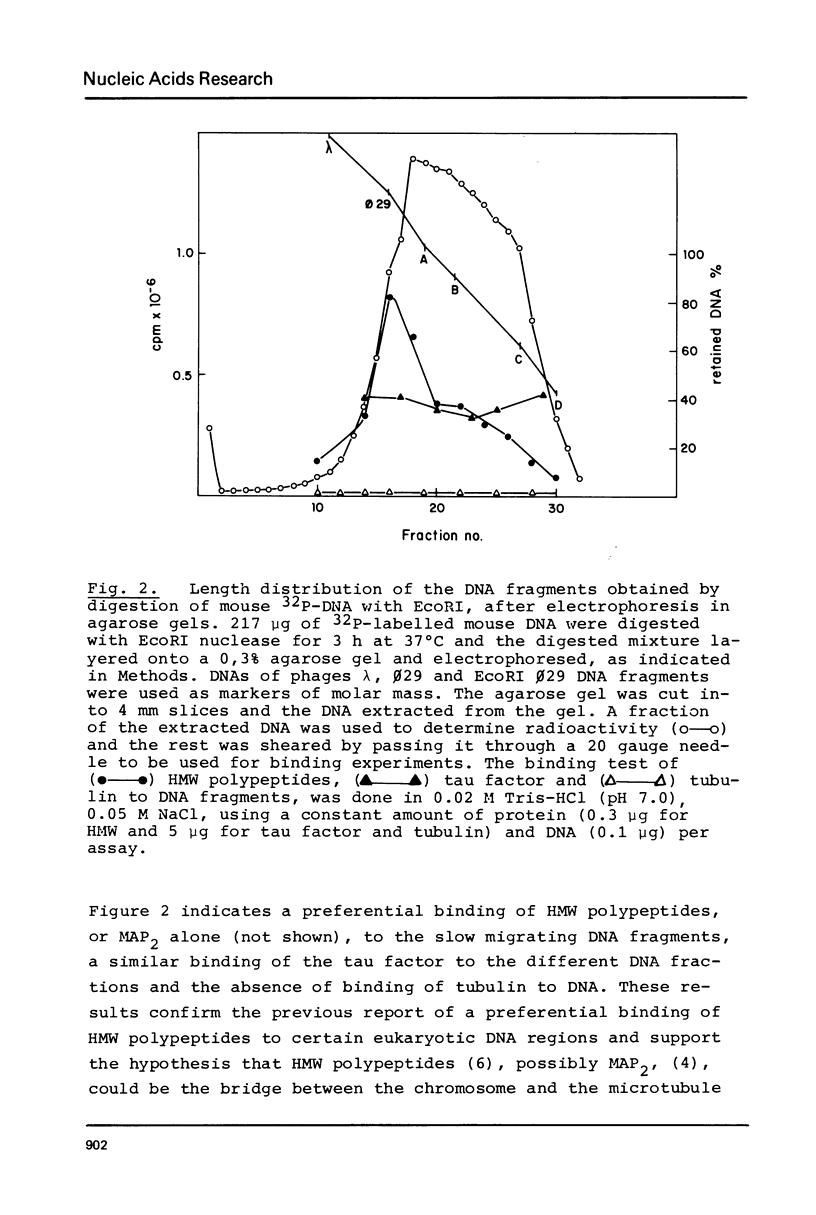
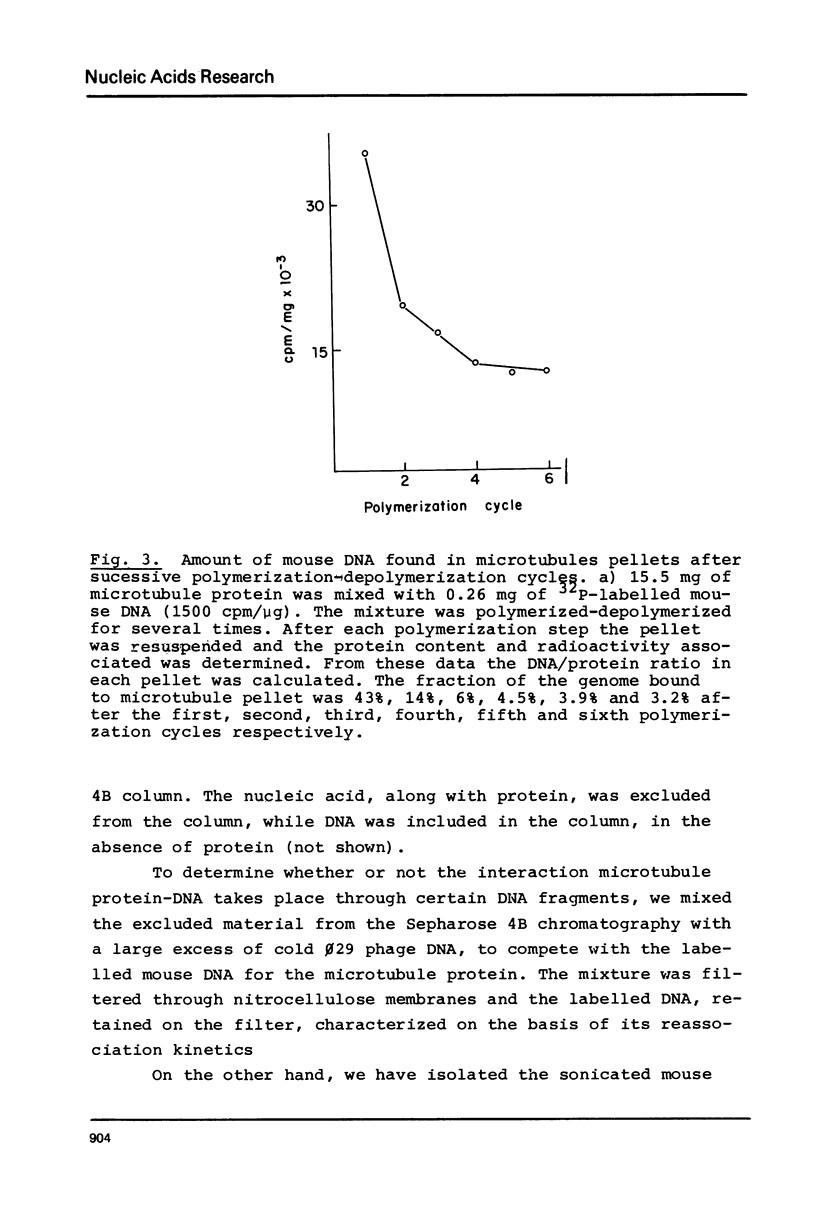
Abstract
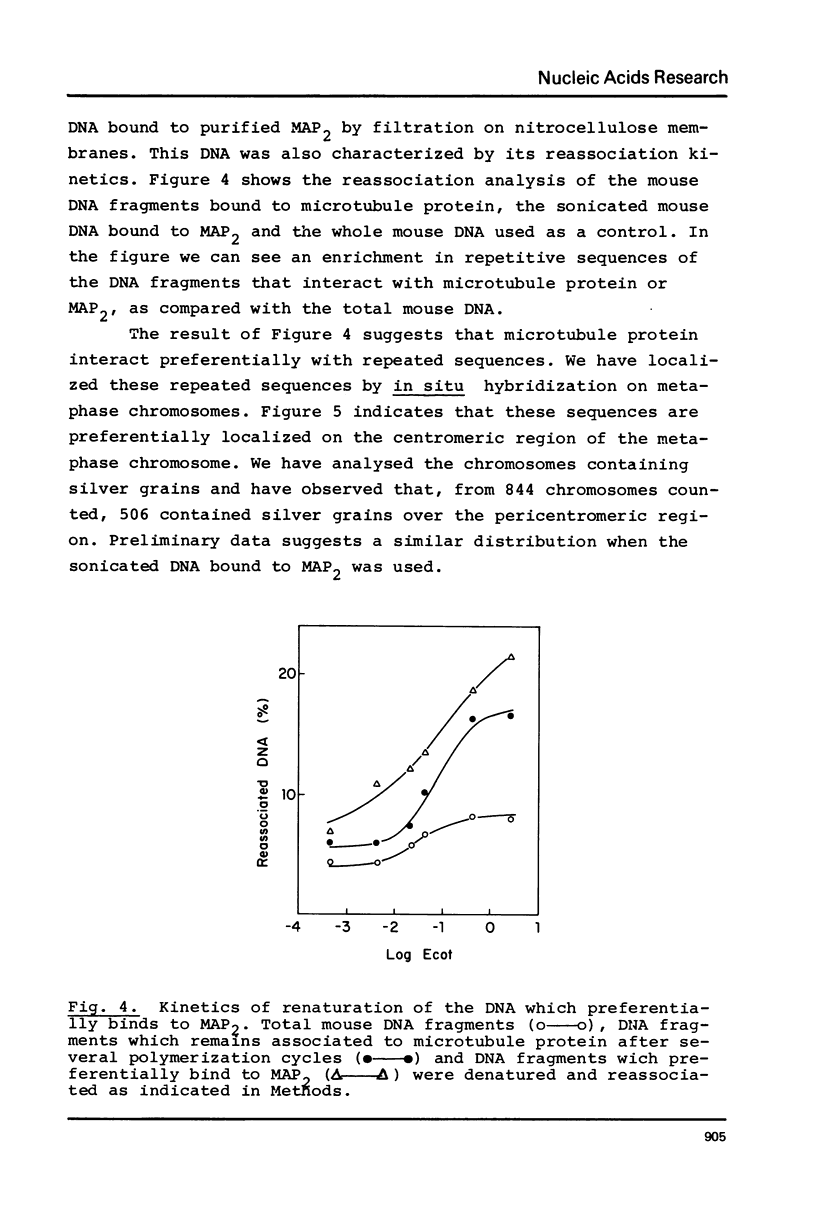
Microtubule protein binds to DNA through microtubule associated polypeptides (MAPs). Among MAPs there is one high molecular weight polypeptide (MAP2) which interacts with DNA fundamentally through certain polynucleotide sequences. This interaction is not affected by the presence of histones and other chromosomal proteins. DNA can associate to assembled microtubules and when a determinate DNA/protein ratio is reached the nucleic acid behaves as a microtubule associated molecule. The nucleic acid fragments which preferentially bind to microtubules have been isolated and characterized. These fragments contain DNA regions enriched in repetitive sequences that hybridizes preferentially to the pericentromeric zone of metaphase chromosomes. These results give further support to the model of interaction microtubule-chromosome based upon the mediator function of the microtubule associated proteins.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Botchan M., McKenna G., Sharp P. A. Cleavage of mouse DNA by a restriction enzyme as a clue to the arrangement of genes. Cold Spring Harb Symp Quant Biol. 1974;38:383–395. doi: 10.1101/sqb.1974.038.01.041. [DOI] [PubMed] [Google Scholar]
- Corces V. G., Manso R., De La Torre J., Avila J., Nasr A., Wiche G. Effects of DNA on microtubule assembly. Eur J Biochem. 1980 Mar;105(1):7–16. doi: 10.1111/j.1432-1033.1980.tb04468.x. [DOI] [PubMed] [Google Scholar]
- Corces V. G., Salas J., Salas M. L., Avila J. Binding of microtubule proteins to DNA: specificity of the interaction. Eur J Biochem. 1978 May 16;86(2):473–479. doi: 10.1111/j.1432-1033.1978.tb12330.x. [DOI] [PubMed] [Google Scholar]
- Gould R. R., Borisy G. G. Quantitative initiation of microtubule assembly by chromosomes from Chinese hamster ovary cells. Exp Cell Res. 1978 May;113(2):369–374. doi: 10.1016/0014-4827(78)90377-4. [DOI] [PubMed] [Google Scholar]
- Herzog W., Weber K. Fractionation of brain microtubule-associated proteins. Isolation of two different proteins which stimulate tubulin polymerization in vitro. Eur J Biochem. 1978 Dec 1;92(1):1–8. doi: 10.1111/j.1432-1033.1978.tb12716.x. [DOI] [PubMed] [Google Scholar]
- Jones K. W. Chromosomal and nuclear location of mouse satellite DNA in individual cells. Nature. 1970 Mar 7;225(5236):912–915. doi: 10.1038/225912a0. [DOI] [PubMed] [Google Scholar]
- Jones O. W., Berg P. Studies on the binding of RNA polymerase to polynucleotides. J Mol Biol. 1966 Dec 28;22(2):199–209. doi: 10.1016/0022-2836(66)90126-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacy E., Axel R. Analysis of DNA of isolated chromatin subunits. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3978–3982. doi: 10.1073/pnas.72.10.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manso-Martínez R., Villasante A., Avila J. Incorporation of the high-molecular-weight microtubule-associated protein 2 (MAP2) into microtubules at steady state in vitro. Eur J Biochem. 1980 Apr;105(2):307–313. doi: 10.1111/j.1432-1033.1980.tb04502.x. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Vallee R. B., Borisy G. G. Identity and polymerization-stimulatory activity of the nontubulin proteins associated with microtubules. Biochemistry. 1977 Jun 14;16(12):2598–2605. doi: 10.1021/bi00631a004. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer B. R., Moses M. J., Rosenbaum J. L. Assembly of microtubules onto kinetochores of isolated mitotic chromosomes of HeLa cells. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4023–4027. doi: 10.1073/pnas.72.10.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater W., Müller H., Unger E. Inhibition of microtubule formation by DNA. Biochem Biophys Res Commun. 1978 Oct 16;84(3):721–726. doi: 10.1016/0006-291x(78)90764-7. [DOI] [PubMed] [Google Scholar]
- Wiche G., Corces V. G., Avila J. Preferential binding of hog brain microtubule-associated proteins to mouse satellite versus bulk DNA preparations. Nature. 1978 Jun 1;273(5661):403–405. doi: 10.1038/273403a0. [DOI] [PubMed] [Google Scholar]