Abstract
Isoprene epoxydiols (IEPOX), formed from the photooxidation of isoprene under low-NOx conditions, have recently been proposed as precursors of secondary organic aerosol (SOA) on the basis of mass spectrometric evidence. In the present study, IEPOX isomers were synthesized in high purity (> 99%) to investigate their potential to form SOA via reactive uptake in a series of controlled dark chamber studies followed by reaction product analyses. IEPOX-derived SOA was substantially observed only in the presence of acidic aerosols, with conservative lower-bound yields of 4.7–6.4% for β-IEPOX and 3.4–5.5% for δ-IEPOX, providing direct evidence for IEPOX isomers as precursors to isoprene SOA. These chamber studies demonstrate that IEPOX uptake explains the formation of known isoprene SOA tracers found in ambient aerosols, including 2-methyltetrols, C5-alkene triols, dimers, and IEPOX-derived organosulfates. Additionally, we show reactive uptake on the acidified sulfate aerosols supports a previously unreported acid-catalyzed intramolecular rearrangement of IEPOX to cis- and trans-3-methyltetrahydrofuran-3,4-diols (3-MeTHF-3,4-diols) in the particle phase. Analysis of these novel tracer compounds by aerosol mass spectrometry (AMS) suggests that they contribute to a unique factor resolved from positive matrix factorization (PMF) of AMS organic aerosol spectra collected from low-NOx, isoprene-dominated regions influenced by the presence of acidic aerosols.
1. Introduction
Isoprene (2-methyl-1,3-butadiene, C5H8) is the single largest biogenic non-methane hydrocarbon emitted into the Earth’s atmosphere,1 and has been estimated to contribute substantially to the global SOA budget.2, 3 In remote forested regions where the environments are characterized by high isoprene emissions and low-NOx concentrations, reactions with hydroxyl radicals (OH) are responsible for the vast majority of isoprene oxidation, thereby forming considerable amounts of low-volatility products that readily form SOA.3, 4 Isoprene-derived SOA species are likely to be formed by heterogeneous reactions;5, 6 however, there is little agreement regarding the mechanism(s) responsible for SOA formation following isoprene oxidation under low-NOx conditions.
Laboratory investigations based on mass spectrometric analysis and modeling studies have led to the hypothesis that gas-phase IEPOX isomers arising from the oxidation of isoprene hydroxyhydroperoxides (ISOPOOH) are likely to be key intermediates in the formation of isoprene SOA under low-NOx conditions.5, 6 Paulot et al.5, for example, reported the formation of gaseous IEPOX in high yield (~ 50%) from the OH-initiated oxidation of isoprene. Additionally, the identification of isoprene-derived SOA constituents including 2-methyltetrols,4 C5-alkene triols,7 dimers,8 as well as their organosulfate derivatives9 support an intermediary role for IEPOX, as these species can be formed by acid-catalyzed oxirane ring-opening of IEPOX. Such ring-opening reactions are reported to be kinetically favorable under typical tropospheric conditions, with reaction rates enhanced by acidity.10–12 The importance of IEPOX reactions has been also supported by Surratt et al.6 who performed reactive uptake chamber experiments with 1,4-dihydroxy-2,3-epoxybutane (BEPOX), a structurally related surrogate of IEPOX, and observed its rapid and enhanced uptake onto acidified sulfate seed aerosols.
In the present work, we investigate the reactive uptake of authentic synthesized IEPOX isomers onto pre-existing sulfate seed aerosols in a series of controlled dark chamber studies under acidic and neutral conditions. The objectives of this work were to demonstrate SOA formation through the IEPOX route in the low-NOx regime, and to examine the influence of aerosol acidity on IEPOX-derived SOA formation. SOA yields from reactive uptake of cis-β-IEPOX and a mixture of racemic diastereomers of δ-IEPOX were measured, as well as the detailed chemical composition of the resultant SOA. The SOA constituents chemically characterized from the chamber studies were directly compared to fine aerosol samples collected from the rural southeastern U.S. (Yorkville, GA) during the summer of 2010, to confirm the atmospheric relevance of the chamber findings. The results of this comparison provide substantial support for the role of IEPOX in forming organic aerosol in the Earth’s troposphere. In addition, reactive uptake of the IEPOX isomers on acidified sulfate seed aerosol was observed to yield cis- and trans-3-methyltetrahydrofuran-3,4-diols (3-MeTHF-3,4-diols), heretofore unreported products of an intramolecular rearrangement in the particle phase. Analysis of these tracer compounds by aerosol mass spectrometry (AMS) suggests the possibility that they contribute to a unique factor resolved from the positive matrix factorization (PMF) analysis of AMS data collected from low-NOx, isoprene-dominated regions.
2. Experimental Section
Synthesis of Reactive Intermediates
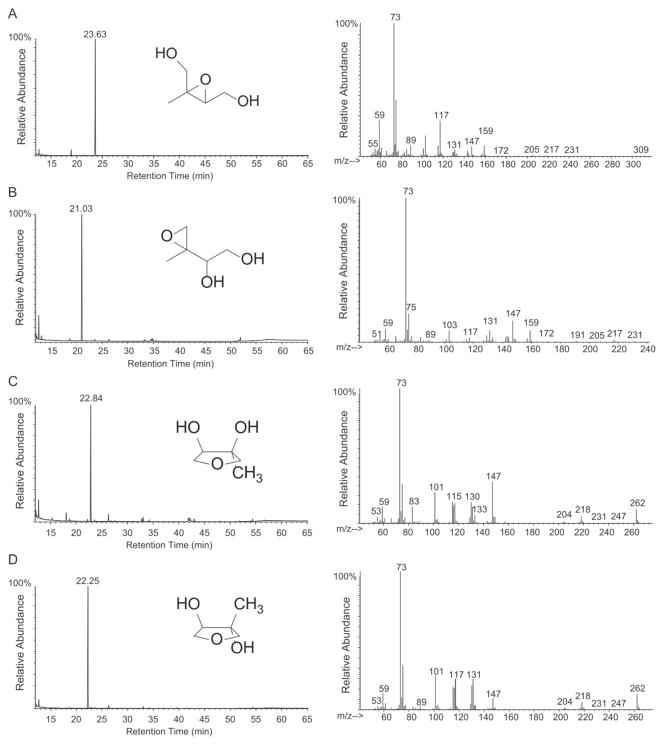
cis-β-IEPOX, the racemic mixture of δ-IEPOX diastereomers ([(2′R)-1S]/[(2′S)-1R]- and [(2′S)-1S]/[(2′R)-1R]-1-(2-methyloxiranyl)-1,2-ethanediols), and cis- and trans-3-methyltetrahydrofuran-3,4-diols were synthesized according to schemes developed in-house. Complete synthetic procedures and characterization will be described in a forthcoming publication. The identity and purity of each synthesized compound was confirmed by 1H- and 13C-NMR (Figures S1-S2), and gas chromatography/mass spectrometry (GC/MS) with electron ionization (EI) (Figure 1).
Figure 1.
GC/MS TIC traces and mass spectra of TMS-derivatized authentic standards. (A) cis-β-IEPOX, (B) δ-IEPOX, (C) cis-3-MeTHF-3,4-diol, (D) trans-3-MeTHF-3,4-diol. δ-IEPOX isomers could not be separated on this GC column.
Chamber Experiments
Chamber experiments were conducted in an indoor 10-m3 flexible Teflon chamber at UNC. Prior to the start of each experiment, the chamber was flushed continuously with clean house air for over 24 h corresponding to a minimum of 5 chamber volumes. A scanning mobility particle sizer (SMPS) system equipped with a cylindrical differential mobility analyzer (DMA, Model 3081, TSI, Inc.) and a condensation particle counter (CPC, Model 3022, TSI, Inc.) was used to measure aerosol size distributions and particle volume concentrations inside the chamber. Chamber background aerosol concentrations were monitored before all experiments to ensure that there was no pre-existing aerosol in the chamber. Either acidic or neutral seed aerosols were introduced into the chamber by atomizing 0.06 M MgSO4+ 0.06 M H2SO4 (aq) and 0.06 M (NH4)2SO4 (aq) solutions, respectively. Glass microliter syringes were used to inject known amounts of reactive intermediates (β- and δ-IEPOX) into a 10 mL glass manifold. ~15 mg of IEPOX was injected for each experiment (the mixing ratios of β- and δ-IEPOX were ~300 ppbv). The manifold was wrapped with calibrated heating tapes heated to 60°C, and flushed with N2 (pre-heated to 60°C) at 5 L min−1 for at least 2 h until no additional increase in aerosol volume was observed by the SMPS. After stabilization of particle volume concentrations, aerosol samples were collected on 47 mm diameter, 1.0-μm pore size Teflon membrane filters (Pall Life Science) for product analyses, at a sampling flow rate of ~20 L min−1 for 2 hours. For each experiment, two Teflon filters were stacked in the filter holder. The front filter was collected to examine particle-phase reaction products, whereas the back filter was collected to correct for any gas-phase IEPOX absorption on the filters. All experiments were carried out in the dark at a constant temperature (20–25°C) under dry (RH<5%) conditions. Control experiments were also performed to rule out potential artifacts. These included chamber blank experiments along with addition of reactive intermediates (β- and δ-IEPOX) or seed aerosol (i.e., acidic and neutral seed) to the chamber in isolation. Details regarding experimental conditions are fully listed in Table 1.
Table 1.
Experimental Conditions and Results for IEPOX Reactive Uptake Experiments
| exp. # | reactive intermediates | seed aerosol | IEPOX reacted (μg m−3) | initial seed (μm3 cm−3) | SOA formeda (μg m−3) | SOA yield |
|---|---|---|---|---|---|---|
| 1 | δ-IEPOX | Acidic | 1320 | 45.8 | 55 | 4.2% |
| 2 | δ-IEPOX | Acidic | 1240 | 45.0 | 68 | 5.5% |
| 3 | δ-IEPOX | Acidic | 1340 | 45.9 | 45 | 3.4% |
| 4 | δ-IEPOX | Neutral | 1430 | 44.9 | 7.6 | 0.5% |
| 5 | δ-IEPOX | Neutral | 1400 | 45.3 | 3.8 | 0.3% |
| 6 | β-IEPOX | Acidic | 1310 | 45.6 | 84 | 6.4% |
| 7 | β-IEPOX | Acidic | 1430 | 50.5 | 90 | 6.3% |
| 8 | β-IEPOX | Acidic | 1360 | 46.6 | 64 | 4.7% |
| 9 | β-IEPOX | Neutral | 1410 | 45.8 | 17 | 1.2% |
| 10 | β-IEPOX | Neutral | 1400 | 40.7 | 12 | 0.9% |
SOA density of 1.25 g cm−3 is assumed from Kroll et al.21
Ambient Aerosol Collection
Ambient PM2.5 filter samples collected from the Southeastern Aerosol Research and Characterization Study (SEARCH) network during the summer of 2010 were analyzed for IEPOX-derived SOA tracer compounds to confirm atmospheric relevancy of the chamber findings. The detailed site descriptions are provided elsewhere.13 Briefly, high-volume quartz filters were collected at 1 m3 min−1 in Yorkville, GA, a rural site located approximately 60 km WNW of Atlanta, GA with high isoprene emissions, to examine acidity-dependent heterogeneous reactions on ambient organic aerosol formation. Filter samples were collected by conditional sampling strategies, defined by pre-selected environmental SO2 thresholds, rather than time integration. Specifically, samples were only collected when the ambient SO2 mixing ratio was ≥ 0.50 ppbv during the day time, in order to examine how IEPOX-derived SOA formed under atmospherically relevant aerosol acidities.
Aerosol-Phase Chemical Analyses
Teflon filters collected from the chamber experiments were extracted with 20 mL high-purity methanol (LC-MS CHROMASOLV-grade, Sigma-Aldrich) under 45 min of sonication. The filter extracts were blown dry under a gentle N2 stream at room temperature. Residues were then trimethylsilylated by reacting with 100 μL of BSTFA + TMCS (99:1 v/v, Supelco) and 50 μL of pyridine (anhydrous, 99.8%, Sigma-Aldrich). The reaction mixture was heated at 70°C for 1 h, and analyzed by GC/MS within 24 h after extraction. GC/MS analysis was performed using a Hewlett-Packard (HP) 5890 Series II Gas Chromatograph coupled to a HP 5971A Mass Selective Detector. An Econo-Cap™-EC™-5 Capillary Column (30 m × 0.25 mm i.d.; 0.25 μm film thickness) was used to separate the trimethylsilyl (TMS) derivatives before MS detection. 1 μL of each derivatized sample was injected onto the GC column. Operating conditions and temperature program of the GC/MS were as described previously by Surratt et al.6 Characterization of IEPOX-derived organosulfates was performed using ultra performance liquid chromatography interfaced to a high-resolution quadrupole time-of-flight mass spectrometer (Agilent 6500 Series) equipped with an electrospray ionization source (UPLC/ESI-HR-Q-ToFMS) operated in the negative (−) ion mode. A Waters ACQUITY UPLC HSS T3 column (2.1 × 100 mm, 1.8 μm particle size) was used for chromatographic separations. Detailed UPLC/ESI-HR-Q-ToFMS operating conditions can be found in Zhang et al.14 Teflon filters for LC/MS analyses were extracted in the same manner as those for GC/MS analyses. After the filter extracts were blown dry, the extract residues were reconstituted with 150 μL of a 50:50 (v/v) solvent mixture of methanol containing 0.1% acetic acid (LC-MS CHROMASOLV-grade, Sigma-Aldrich) and water containing 0.1% acetic acid (LC-MS CHROMASOLV-grade, Sigma-Aldrich), and 5 μL of each sample was injected onto the LC column eluted with solvent of the same composition. Ambient quartz filter samples were extracted and analyzed in the same manner as Teflon filters from the chamber studies, except the sample extracts were filtered through 0.2 μm PTFE syringe filters (Pall Life Science, Acrodisc®) to remove suspended quartz filter fibers and insoluble particles (likely soot and soil). The efficiency of the extraction protocols was evaluated by spiking 5 replicates of pre-baked blank quartz filters with standards, including meso-erythritol (≥99%, Sigma), cis- and trans-3-MeTHF-3,4-diols, and sodium propyl sulfate (electronic grade, City Chemical LLC). Extraction efficiencies (62–82%) are taken into account for each IEPOX-derived SOA constituent that is quantified in the field samples.
High-Resolution Aerosol Mass Spectrometry of Aerosolized Standards
High-resolution mass spectra of synthesized standards (β- IEPOX, δ-IEPOX, and cis- and trans-3-MeTHF-3,4-diols) were acquired using a high-resolution time-of-flight aerosol mass spectrometer (HR-AMS).15 Standards were diluted in distilled water and atomized directly into the HR-AMS from a TSI (St. Paul, MN, USA) model 9302A aerosol atomizer after being passed through a silica-filled TSI model 3062 diffusion drier. Prior to adding each standard in the atomizer, a mass spectrum of the distilled water solvent was collected to both ensure low contributions from organics and to correct mass spectra of synthesized standards for any such contributions. Relative to the contribution of signal from the standards themselves, contributions from water were minor. After collecting the background water mass spectrum, a single synthesized sample was added directly to the atomizer and the mass spectrum of the sample was then collected. The atomizer and sampling lines were rinsed extensively with several aliquots of distilled water between each analysis. All spectra were collected with the HR-AMS operating in W-mode (m/Δm=4000–5000) and all spectra were averaged over a period of 5–10 min depending on the resulting particle concentration (with the exception of β-IEPOX, all samples were averaged for 5 min). Unit resolution and HR-AMS data was analyzed with the Squirrel and Pika software packages, respectively, developed by the Jimenez group at the University of Colorado-Boulder.16
3. Results and Discussion
Authentic Standards
Although synthesis of cis-β-IEPOX,17 the racemic δ-IEPOX diastereomers,18 and cis-3-MeTHF-3,4-diol19 has been reported, original routes were devised to provide standards in high purity via streamlined procedures. The 1H-NMR spectra are either identical to those reported for the published compounds (Figures S1A, S1B and S2A) or in agreement with the structure assigned (Figure S2B). Total ion current (TIC) chromatograms and GC/MS spectra of the TMS-derivatized standards shown in Figure 1 demonstrate purity. Noteworthy are the differences in retention times of the IEPOX and 3-MeTHF-3,4-diol isomers, and the presence of a strong molecular ion (m/z 262) in the EI mass spectrum of the 3-MeTHF-3,4-diol isomers, the significance of which will be discussed below.
Aerosol Growth from Reactive Uptake Experiments
Blank experiments (e.g., clean chamber only, seed aerosol only, and reactive intermediates only) were performed for quality control to ensure all observed aerosol growth is truly from the reactive uptake of IEPOX, during which no aerosol growth was observed. In addition, chemical analyses of filters collected from these blank experiments revealed no detectable SOA constituents.
Comparisons of aerosol growth under acidic and neutral conditions are shown in Figure S3, in which ~ 15 mg IEPOX was injected in the presence of acidified or neutral sulfate seed aerosols. From the wall-loss uncorrected SMPS data, reactive uptake of both β- and δ-IEPOX is enhanced under acidic conditions relative to that under neutral conditions. Measured SOA yields are summarized in Table 1. SOA yields (Y) are defined as the ratio of mass concentration of SOA formed (ΔM0) to mass concentration of IEPOX reacted (ΔROG).20 SOA density of 1.25 g cm−3 was assumed when converting the measured volume concentrations to mass concentrations in order to determine the SOA yields.21 To calculate SOA yields, all the measured aerosol volumes were corrected for wall losses. The chamber-dependent wall loss coefficient was characterized by conducting a number of the seed aerosol only experiments in the absence of IEPOX. It is worth noting that the wall loss correction accounts only for the decay of seed aerosols as a function of time without size dependence, and does not take into account the wall losses of IEPOX vapors. Reacted IEPOX (ΔROG) was estimated by gravimetric methods. Specifically, the weight of the injection manifold was measured before and after experiments to determine how much IEPOX had been introduced into the chamber, with the assumption that injected IEPOX is equivalent to ΔROG. It should be noted that this approach only attempts to provide a conservative lower-bound estimate of SOA yields, since IEPOX may not completely react in the chamber. As a result, ΔROG could have been overestimated if some IEPOX remained in the gas phase, or was lost to the chamber walls. A more accurate SOA yield calculation requires use of an on-line soft ionization MS technique, such as a chemical ionization, as previously employed by Paulot et al.,5 to accurately quantify the mixing ratios of IEPOX during the course of the experiments. Another limitation of this study that should be noted is that all chamber experiments were conducted under low RH (< 5%) conditions, and as a result, the sulfuric acid seed aerosol atomized into the chamber could be much more acidic than a sulfuric acid seed aerosol in the atmosphere. Further studies are required to estimate aerosol acidity and measure SOA yields under atmospherically relevant conditions of aerosol acidity.
Chemical Characterization of Aerosol-Phase Reaction Products
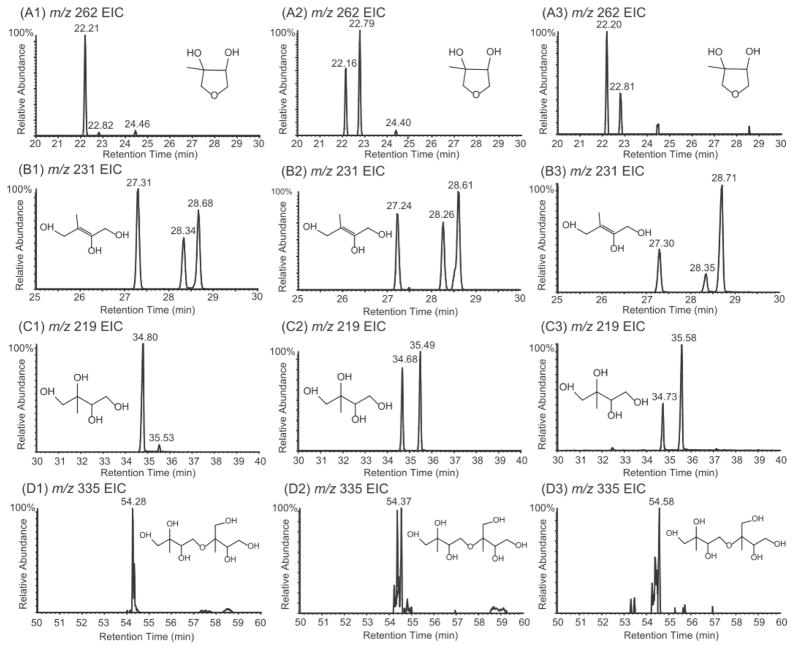
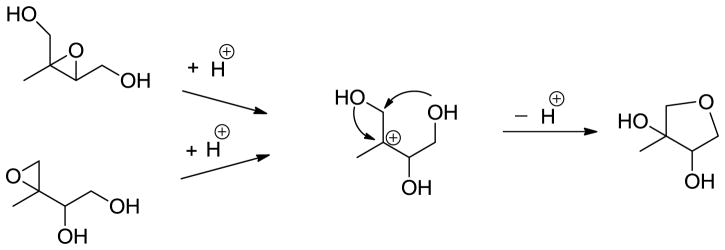
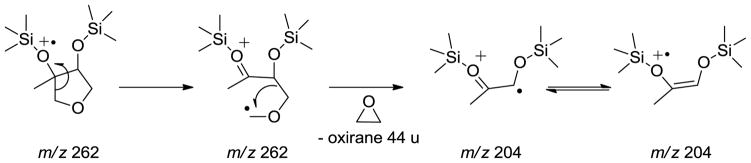
Figure 2 shows the GC/MS extracted ion chromatograms (EICs) of TMS-derivatized particle-phase reaction products from reactive uptake of cis-β-IEPOX (A1-D1) and δ-IEPOX (A2–D2) chamber experiments in the presence of acidified sulfate seed aerosol, as well as a selected field sample (A3–D3) collected from the Yorkville, GA SEARCH site. Qualitatively, retention times of IEPOX-derived SOA tracer ions detected from chamber samples agree with those from field samples. However, retention times of the peaks in the EIC of m/z 262, which have been attributed to unreacted particle-phase IEPOX isomers,6, 22 do not correspond to the retention times observed in the TIC chromatograms of the authentic IEPOX isomers (ref. Figure 1). Furthermore, a molecular ion at m/z 262 is not observed in the EI mass spectra of either IEPOX isomer. As a consequence, a different set of isomers must be responsible for the EIC of m/z 262 in the field sample. Based on acid-catalyzed cyclization of tetraols,23–25 3-MeTHF-3,4-diol isomers were synthesized as likely candidates and confirmed as the source of the EIC at m/z 262 in the field sample. A mechanism for the acid-catalyzed rearrangement of IEPOX and an EI-MS fragmentation pathway are suggested in Schemes 1 and 2, respectively. Scheme 2 leads to the diagnostic ion at m/z 204. Consistent with formation through acid-catalyzed cyclization, the concentrations of the 3-MeTHF-3,4-diols were measured to increase from 0.27 to 2.29 μg m−3 on changing from neutral to acidic conditions in δ-IEPOX reactive uptake chamber experiments, and from non-detectable (n.d.) to 1.25 μg m−3 in β-IEPOX experiments. In a prior study, Wang et al.7 have proposed the 3-MeTHF-3,4-diols as intermediates in the formation of C5-alkene triols through acid-catalyzed rearrangement of epoxydiol derivatives of isoprene via δ-IEPOX, and indicated C5-alkene triols are possibly formed in acidic ambient conditions with low RH that do not allow the complete hydrolysis of IEPOX. Our findings support this plausible mechanism, and further demonstrate the formation of 3-MeTHF-3,4-diols in the particle phase. An important implication stemming from the identification of the 3-MeTHF-3,4-diols in the particle phase is the suggestion of a mechanism for trapping gaseous IEPOX in the generation of SOA from isoprene. The EICs associated with the remaining TMS-derivatives in Figure 2 correspond to the products observed in the chamber study. Thus, our chamber studies demonstrate that in addition to the 3-MeTHF-3,4-diols, the previously identified low-NOx isoprene SOA tracer compounds are formed from reactive uptake of gaseous IEPOX.
Figure 2.
GC/MS extracted ion chromatograms (EICs) of TMS-derivatized particle-phase reaction products from reactive uptake of cis-β-IEPOX (A1–D1) and δ-IEPOX (A2–D2) experiments in the presence of acidic seed aerosol, and from field samples (A3–D3) collected in Yorkville, GA during the summer of 2010. Specifically, (A) shows EIC of m/z 262 from 3-MeTHF-3,4-diols, (B) shows the EIC of m/z 231 from C5-alkene triols, (C) shows EIC of m/z 219 from 2-methyltetrols, and (D) shows EIC of m/z 335 from dimers.
Scheme 1.
Proposed Acid-Catalyzed Intramolecular Rearrangement of IEPOX
Scheme 2.
Fragmentation Pathways of Trimethylsilylated 3-MeTHF-3,4-diols in the EI-Mass Spectra
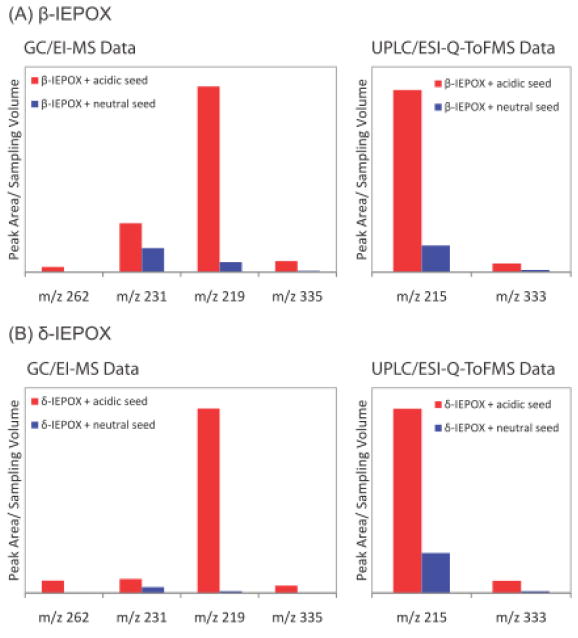
Figure 3 shows the relative abundance of IEPOX-derived SOA tracers, expressed as peak areas normalized by sampling volume detected under acidic and neutral conditions. Quantitatively, all of the measured reaction products are significantly enhanced in the presence of acidified sulfate seed aerosols. As a specific example, the 2-methyltetrols, which are quantified by using meso-erythritol as a surrogate standard, increase from 0.12 under neutral conditions to 0.61 μg m−3 under acidic conditions in the δ-IEPOX reactive uptake chamber experiments.
Figure 3.
Normalized peak area of IEPOX-derived SOA tracers detected under acidic and neutral conditions. Aerosol-phase reaction products analyzed by GC/MS: 3-MeTHF-3,4-diols (m/z 262); C5-alkene triols (m/z 231); 2-methyltetrols (m/z 219); dimers (m/z 335). Organosulfate derivatives analyzed by UPLC/(−)ESI-Q-ToFMS: organosulfate derivative of the 2-methyltetrols (m/z 215); organosulfate derivative of the dimers (m/z 333).
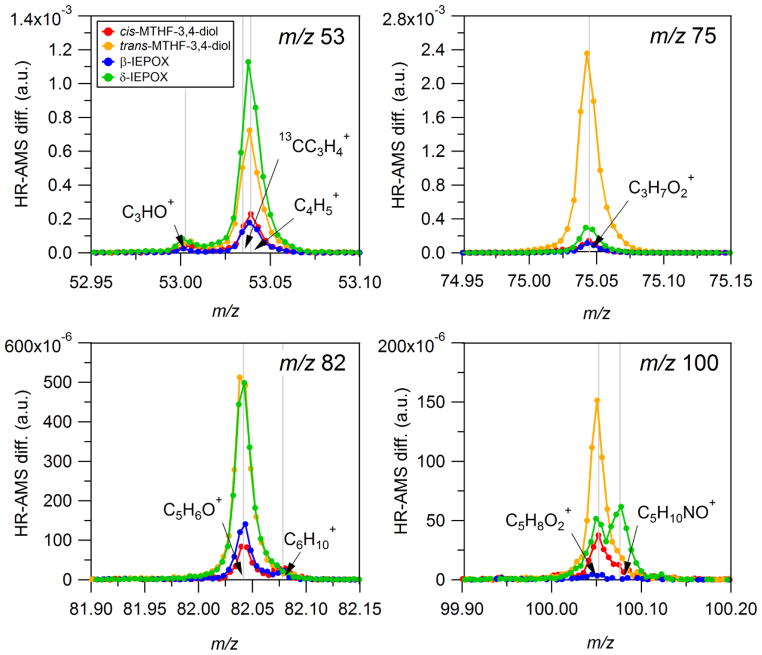
A recent study by Robinson et al.26 shows that methylfuran (MeF) contributes a significant fraction (up to 15% by mass) of submicron aerosol (PM1) above a Malaysian maritime tropical rainforest. PMF analysis of AMS organic mass spectral data resolved a robust factor that accounted for upwards of 53% of the PM1 organic mass.26 The time series of this factor was highly correlated with the time series of the first-generation isoprene oxidation products (methylvinyl ketone and methacrolein) identified by proton transfer reaction mass spectrometry, and its spectrum was characterized by prominent MeF fragments at m/z 53 and m/z 82 (mostly C5H6O+). These observations led Robinson et al.26 to suggest that isoprene oxidation plays a significant role in the formation of SOA in this remote forested region. A similar factor has also been observed by Slowik et al.27 from PMF analysis of AMS organic mass spectra from Ontario. While the origin of MeF has not been established in the Robinson et al.26 and Slowik et al.27 studies, we considered that 3-MeTHF-3,4-diols might be a source of MeF under conditions of analysis by AMS and 2D-GC/MS techniques when thermally desorbed and subjected to EI, especially since the atmospheric conditions in both the Robinson et al.26 and Slowik et al.27 studies are favorable for IEPOX formation (i.e., high isoprene emissions and low NOx levels). This possibility was investigated by atomizing solutions of our pure synthetic standards into a HR-AMS. Full AMS unit mass resolution (UMR) mass spectra of synthesized standards are available in Supporting Information (Figures S7 and S8). High resolution spectra at m/z’s 53, 75, 82, and 100 are shown in Figure 4. The m/z 82 fragment, corresponding to 3-methylfuran (3-MeF), is present in the HR-AMS mass spectra of the 3-MeTHF-3,4-diols and is mostly due to C5H6O+. The elemental composition of m/z 53 (mostly C4H5+), m/z 75 (mostly C3H7O+), and m/z 100 (mostly C5H8O2+), might aid in interpreting PMF analyses of AMS field datasets. HR-AMS mass spectra of β- and δ-IEPOX authentic standards also show similar mass spectral patterns, indicating further that this series of compounds may be useful for interpreting PMF results from prior studies. Ratios of m/z 82 to both the average signal at m/z 81 and m/z 83 (82:avg(81,83)) and total organic signal (82:org) as well as the ratio of C5H6O+ to m/z 82 (C5H6O+:m/z 82) and total organic signal (C5H6O+:org) from HR-AMS mass spectra of the synthesized standards are reported in Table 2. These ratios correspond well to those reported by Robinson et al.26 for the 82Fac resolved from Borneo where maritime air in this remote tropical rainforest could have provided acidic aerosol surfaces due to dimethyl sulfide (DMS) oxidation, thus providing conditions favorable to the formation of increased amounts of isomeric 3-MeTHF-3,4-diols and other IEPOX-derived SOA constituents.
Figure 4.
High-resolution difference spectra of synthesized standards at nominal m/z’s 53, 75, 82, and 100 showing the contributions of various ions. Note that each spectrum has been normalized by the corresponding total mass spectral signal. As a result, values on the y-axis represent fractional contributions to total signal. Overall, the largest contributions at these masses arise from oxidized organic fragments including C3H7O+ (m/z 75), C5H6O+ (m/z 82), and C5H8O2+ (m/z 100). The exception is at m/z 53 where the majority of signal from the reduced fragment C4H5+.
Table 2.
Ratios of m/z 82 Signal to Both the Average Signal at m/z 81 and m/z 83 (82:avg(81,83)) and Total Organic Signal (82:org) and C5H6O+ to m/z 82 (C5H6O+:m/z 82) and Total Organic Signal (C5H6O+:org) Obtained from HR-AMS Mass Spectra of Synthesized Standards.
| cis-3-MeTHF-3,4-diol | trans-3-MeTHF-3,4-diol | β-IEPOX | δ-IEPOX | |
|---|---|---|---|---|
| 82:avg(81,83) | 0.72 ± 0.05 | 2.04 ± 0.04 | 1.57 ± 0.05 | 1.02 ± 0.02 |
| 82:org | 0.77 ± 0.03% | 1.70 ± 0.01% | 2.32 ± 0.02% | 1.83 ± 0.01% |
| C5H6O+:m/z 82 | 67.9 ± 0.04% | 95.3 ± 0.02% | 90.9 ± 0.03% | 93.7 ± 0.02% |
| C5H6O+:org | 0.52 ± 0.02% | 1.62 ± 0.01% | 2.11 ± 0.02% | 1.71 ± 0.01% |
Atmospheric Abundance
Mass concentrations of IEPOX-derived SOA tracers quantified in the field sample from Yorkville are reported in Table 3. Cis- and trans-3-MeTHF-3,4-diols were quantified using authentic standards, while meso-erythritol was used as a surrogate to quantify 2-methyltetrols, C5-alkene triols, and dimers. Sodium propyl sulfate was used as a surrogate standard to quantify IEPOX-derived organosulfates. For each tracer compound, mass concentrations are expressed as the sum of all corresponding isomers. The identified low-NOx IEPOX-derived SOA tracers are estimated to account for 7.9 % of the total organic carbon (OC) in this field sample. This value is higher than that reported in a prior study by Chan et al.22 who analyzed ambient aerosol samples collected from the same site during the summer of 2008. Estimated mass concentrations of isomeric 3-MeTHF-3,4-diols and the organosulfate derivatives are higher than previously reported, possibly due to the sampling of high SO2 plumes during the conditional-sampling field campaign, as well as the use of authentic quantifying standards in our study. Taken together, these observations suggest that IEPOX routes are atmospherically relevant and play an important role in the formation of isoprene SOA in the low-NOx regime.
Table 3.
Mass Concentrations of IEPOX-derived SOA Tracer Compounds in a Representative Ambient PM2.5 Sample Collected in Yorkville, GA (YRK) during the Summer of 2010
| IEPOX SOA tracers | mass conc. (ng m−3) |
|---|---|
| 2-methyltetrols | 330 |
| C-5 alkene triols | 290 |
| 3-MeTHF-3,4-diols | 27 |
| dimers | 0.50 |
| Organosulfate derivatives of 2-methyltetrols | 72 |
| Organosulfate derivatives of dimers | 5.0 |
| Σ IEPOX SOA tracers (ngC m−3) | 340 |
| OC (μgC m−3) | 4.3 |
| Σ IEPOX SOA tracers/OC | 7.9 % |
Supplementary Material
Acknowledgments
Richard Kamens and Elias Rosen are gratefully acknowledged for their assistance in the design of the UNC indoor smog chamber. We thank Glenn Walters and Dennis Fedor of the UNC ESE Design Center for their assistance in building the indoor chamber, as well as Leonard Collins and Wanda Bodnar of the UNC Biomarker Mass Spectrometry Facility (NIEHS Grant 5P20-ES10126) for their assistance on the Agilent LC/ESI-Q-ToFMS. We thank the Electric Power Research Institute (EPRI) for their support in analyzing the field samples collected from the Yorkville, GA site located within the SEARCH network. The U.S. Environmental Protection Agency through its Office of Research and Development collaborated in the research described here under Contract EP-D-05-065 to Alion Science and Technology. The manuscript is subjected to external peer review and has not been cleared for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation. S. H. Budisulistiorini is supported in part by a Fulbright Presidential Fellowship (2010-2013). C. L. Rubitschun is supported in part by a Weiss Urban Livability Fellowship (2010-2012) and a Johanssen Scholarship (2011). Lastly, we thank Manjula Canagaratna, John Offenberg, Mohammed Jaoui, and Michael Lewandowski for helpful discussions.
Footnotes
Supporting Information Available. Additional information regarding NMR spectra (Figures S1–S2), SMPS data for the acidic and neutral aerosol experiments (Figure S3), GC/MS EI mass spectra for all IEPOX-derived SOA constituents found in the lab and field samples (Figures S4–S6), and full AMS unit mass resolution (UMR) mass spectra (Figures S7–S8) are provided. Additionally, Table S1 provides a summary of all IEPOX-derived SOA tracers characterized in this study. This material is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Guenther A, Karl T, Harley P, Wiedinmyer C, Palmer P, Geron C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature) Atmos Chem Phys. 2006;6(11):3181–3210. [Google Scholar]
- 2.Hallquist M, Wenger J, Baltensperger U, Rudich Y, Simpson D, Claeys M, Dommen J, Donahue N, George C, Goldstein A. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos Chem Phys. 2009;9(14):5155–5236. [Google Scholar]
- 3.Carlton A, Wiedinmyer C, Kroll J. A review of Secondary Organic Aerosol (SOA) formation from isoprene. Atmos Chem Phys. 2009;9(14):4987–5005. [Google Scholar]
- 4.Claeys M, Graham B, Vas G, Wang W, Vermeylen R, Pashynska V, Cafmeyer J, Guyon P, Andreae MO, Artaxo P. Formation of secondary organic aerosols through photooxidation of isoprene. Science. 2004;303(5661):1173–1176. doi: 10.1126/science.1092805. [DOI] [PubMed] [Google Scholar]
- 5.Paulot F, Crounse JD, Kjaergaard HG, Kürten A, St Clair JM, Seinfeld JH, Wennberg PO. Unexpected epoxide formation in the gas-phase photooxidation of isoprene. Science. 2009;325(5941):730–733. doi: 10.1126/science.1172910. [DOI] [PubMed] [Google Scholar]
- 6.Surratt JD, Chan AWH, Eddingsaas NC, Chan MN, Loza CL, Kwan AJ, Hersey SP, Flagan RC, Wennberg PO, Seinfeld JH. Reactive intermediates revealed in secondary organic aerosol formation from isoprene. Proc Natl Acad Sci USA. 2010;107(15):6640–6645. doi: 10.1073/pnas.0911114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Kourtchev I, Graham B, Cafmeyer J, Maenhaut W, Claeys M. Characterization of oxygenated derivatives of isoprene related to 2-methyltetrols in Amazonian aerosols using trimethylsilylation and gas chromatography/ion trap mass spectrometry. Rapid Commun Mass Spectrom. 2005;19(10):1343–1351. doi: 10.1002/rcm.1940. [DOI] [PubMed] [Google Scholar]
- 8.Surratt JD, Murphy SM, Kroll JH, Ng NL, Hildebrandt L, Sorooshian A, Szmigielski R, Vermeylen R, Maenhaut W, Claeys M. Chemical composition of secondary organic aerosol formed from the photooxidation of isoprene. J Phys Chem A. 2006;110(31):9665–9690. doi: 10.1021/jp061734m. [DOI] [PubMed] [Google Scholar]
- 9.Surratt JD, Kroll JH, Kleindienst TE, Edney EO, Claeys M, Sorooshian A, Ng NL, Offenberg JH, Lewandowski M, Jaoui M. Evidence for organosulfates in secondary organic aerosol. Environ Sci Technol. 2007;41(2):517–527. doi: 10.1021/es062081q. [DOI] [PubMed] [Google Scholar]
- 10.Eddingsaas NC, VanderVelde DG, Wennberg PO. Kinetics and products of the acid-catalyzed ring-opening of atmospherically relevant butyl epoxy alcohols. J Phys Chem A. 2010;114(31):8106–8113. doi: 10.1021/jp103907c. [DOI] [PubMed] [Google Scholar]
- 11.Minerath EC, Elrod MJ. Assessing the potential for diol and hydroxy sulfate ester formation from the reaction of epoxides in tropospheric aerosols. Environ Sci Technol. 2009;43(5):1386–1392. doi: 10.1021/es8029076. [DOI] [PubMed] [Google Scholar]
- 12.Minerath EC, Schultz MP, Elrod MJ. Kinetics of the reactions of isoprene-derived epoxides in model tropospheric aerosol solutions. Environ Sci Technol. 2009;43(21):8133–8139. doi: 10.1021/es902304p. [DOI] [PubMed] [Google Scholar]
- 13.Hansen DA, Edgerton ES, Hartsell BE, Jansen JJ, Kandasamy N, Hidy GM, Blanchard CL. The southeastern aerosol research and characterization study: Part 1-overview. J Air Waste Manag Assoc. 2003;53(12):1460–1471. doi: 10.1080/10473289.2003.10466318. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Surratt JD, Lin YH, Bapat J, Kamens RM. Effect of relative humidity on SOA formation from isoprene/NO photooxidation: enhancement of 2-methylglyceric acid and its corresponding oligoesters under dry conditions. Atmos Chem Phys. 2011;11(13):6411–6424. [Google Scholar]
- 15.DeCarlo PF, Kimmel JR, Trimborn A, Northway MJ, Jayne JT, Aiken AC, Gonin M, Fuhrer K, Horvath T, Docherty KS. Field-deployable, high-resolution, time-of-flight aerosol mass spectrometer. Anal Chem. 2006;78(24):8281–8289. doi: 10.1021/ac061249n. [DOI] [PubMed] [Google Scholar]
- 16.Sueper D. ToF-AMS Analysis Software. 2009 available online at http://cires.colorado.edu/jimenez-group/wii/index.php/ToF-AMS_Analysis_Software.
- 17.Cole-Filipiak NC, O’Connor AE, Elrod MJ. Kinetics of the hydrolysis of atmospherically relevant isoprene-derived hydroxy epoxides. Environ Sci Technol. 2010;44(17):6718–6723. doi: 10.1021/es1019228. [DOI] [PubMed] [Google Scholar]
- 18.Adam W, Peters K, Renz M. Titanium-catalyzed diastereoselective epoxidations of ene diols and allylic alcohols with β-hydroperoxy alcohols as novel oxygen donors. J Org Chem. 1997;62(10):3183–3189. doi: 10.1021/jo970110x. [DOI] [PubMed] [Google Scholar]
- 19.Robinson TV, Pedersen DS, Taylor DK, Tiekink ERT. Dihydroxylation of 4-substituted 1,2-dioxines: A concise route to branched erythro sugars. J Org Chem. 2009;74(14):5093–5096. doi: 10.1021/jo900669u. [DOI] [PubMed] [Google Scholar]
- 20.Odum JR, Hoffmann T, Bowman F, Collins D, Flagan RC, Seinfeld JH. Gas/particle partitioning and secondary organic aerosol yields. Environ Sci Technol. 1996;30(8):2580–2585. [Google Scholar]
- 21.Kroll JH, Ng NL, Murphy SM, Flagan RC, Seinfeld JH. Secondary organic aerosol formation from isoprene photooxidation. Environ Sci Technol. 2006;40(6):1869–1877. doi: 10.1021/es0524301. [DOI] [PubMed] [Google Scholar]
- 22.Chan MN, Surratt JD, Claeys M, Edgerton ES, Tanner RL, Shaw SL, Zheng M, Knipping EM, Eddingsaas NC, Wennberg PO. Characterization and quantification of isoprene-derived epoxydiols in ambient aerosol in the southeastern United States. Environ Sci Technol. 2010;44(12):4590–4596. doi: 10.1021/es100596b. [DOI] [PubMed] [Google Scholar]
- 23.Narayan RS, Sivakumar M, Bouhlel E, Borhan B. Regiochemical control in intramolecular cyclization of methylene-interrupted epoxydiols. Org Lett. 2001;3(16):2489–2492. doi: 10.1021/ol016118h. [DOI] [PubMed] [Google Scholar]
- 24.Fukuyama T, Vranesic B, Negri D, Kishi Y. Synthetic studies on polyether antibiotics. II. Stereocontrolled syntheses of epoxides of bishomoallylic alcohols. Tetrahedron Lett. 1978;19(31):2741–2744. [Google Scholar]
- 25.Yamaguchi A, Hiyoshi N, Sato O, Bando KK, Shirai M. Enhancement of cyclic ether formation from polyalcohol compounds in high temperature liquid water by high pressure carbon dioxide. Green Chem. 2009;11(1):48–52. [Google Scholar]
- 26.Robinson NH, Hamilton JF, Allan JD, Langford B, Oram DE, Chen Q, Docherty K, Farmer DK, Jimenez JL, Ward MW, et al. Evidence for a significant proportion of Secondary Organic Aerosol from isoprene above a maritime tropical forest. Atmos Chem Phys. 2011;11(3):1039–1050. [Google Scholar]
- 27.Slowik J, Brook J, Chang R, Evans G, Hayden K, Jeong C, Li S, Liggio J, Liu P, McGuire M. Photochemical processing of organic aerosol at nearby continental sites: contrast between urban plumes and regional aerosol. Atmos Chem Phys. 2011;11(6):2991–3006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.