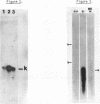
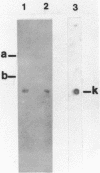
Abstract
The myeloma variant NS-1n has lost the functional immunoglobulin kappa gene which is present in its parent, myeloma MOPC-21. The variant retains a nonfunctional rearranged gene, M.21N, which undergoes RNA transcription and processing to yield a mature size kmRNA. This kRNA, however, is not translated into kappa polypeptide chains. The nonfunctional gene was cloned into Charon 4A to determine the basis for its inactivity. Nucleotide sequence analysis of a DNA fragment overlapping the V-J recombination site in the M.21N gene indicated that a misalignment had taken place during somatic recombination. This misalignment results in a deletion of four nucleotides at the 3' end of the V gene and, thus, a translational reading frame shift. In other respects the M.21n V gene, which corresponds to a different VK subgroup than the functional gene of MOPC-21, appears normal.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho R., Weissman I. L., Early P., Cole J., Hood L. Organization of kappa light chain genes in germ-line and somatic tissue. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1106–1110. doi: 10.1073/pnas.77.2.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhard-Schuller R., Hohn B., Brack C., Hirama M., Tonegawa S. DNA clones containing mouse immunoglobulin kappa chain genes isolated by in vitro packaging into phage lambda coats. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4709–4713. doi: 10.1073/pnas.75.10.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Miller H., Leder P. Variation in the crossover point of kappa immunoglobulin gene V-J recombination: evidence from a cryptic gene. Cell. 1980 Oct;21(3):793–799. doi: 10.1016/0092-8674(80)90442-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near R. I., Storb U. RNA sequences homologous to the 3' portion of immunoglobulin alpha-chain mRNA in thymus-derived lymphocytes. Biochemistry. 1979 Mar 20;18(6):964–972. doi: 10.1021/bi00573a005. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. Organization and complete sequence of identical embryonic and plasmacytoma kappa V-region genes. J Biol Chem. 1980 Apr 25;255(8):3691–3694. [PubMed] [Google Scholar]
- Potter M. Antigen-binding myeloma proteins of mice. Adv Immunol. 1977;25:141–211. [PubMed] [Google Scholar]
- Putnam D., Clagett J., Storb U. Immunoglobulin synthesis by T cells: quantitative and qualitative aspects. J Immunol. 1980 Feb;124(2):902–912. [PubMed] [Google Scholar]
- Rose S. M., Kuehl W. M., Smith G. P. Cloned MPC 11 myeloma cells express two kappa genes: a gene for a complete light chain and a gene for a constant region polypeptide. Cell. 1977 Oct;12(2):453–462. doi: 10.1016/0092-8674(77)90121-0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell H., Steinmetz M., Zachau H. G., Schechter I. An unusual translocation of immunoglobulin gene segments in variants of the mouse myeloma MPC11. Nature. 1980 Jul 10;286(5769):170–173. doi: 10.1038/286170a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Max E. E., Leder P. A kappa-immunoglobulin gene is formed by site-specific recombination without further somatic mutation. Nature. 1979 Aug 2;280(5721):370–375. doi: 10.1038/280370a0. [DOI] [PubMed] [Google Scholar]
- Smith A. J. The use of exonuclease III for preparing single stranded DNA for use as a template in the chain terminator sequencing method. Nucleic Acids Res. 1979 Mar;6(3):831–848. doi: 10.1093/nar/6.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Zachau H. G. Two rearranged immunoglobulin kappa light chain genes in one mouse myeloma. Nucleic Acids Res. 1980 Apr 25;8(8):1693–1707. doi: 10.1093/nar/8.8.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb U., Arp B., Wilson R. Myeloma with multiple rearranged immunoglobulin kappa genes: only one kappa gene codes for kappa chains. Nucleic Acids Res. 1980 Oct 24;8(20):4681–4687. doi: 10.1093/nar/8.20.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb U., Hager L., Wilson R., Putnam D. Expression of immunoglobulin and globin genes in B and T lymphocytes and other cells. Biochemistry. 1977 Dec 13;16(25):5432–5438. doi: 10.1021/bi00644a005. [DOI] [PubMed] [Google Scholar]
- Storb U., Near R., Putnam D., Clagett J. Immunoglobulin messenger RNAs of T lymphocytes. Mol Immunol. 1980 Jul;17(7):947–957. doi: 10.1016/0161-5890(80)90043-7. [DOI] [PubMed] [Google Scholar]
- Swan D., Aviv H., Leder P. Purification and properties of biologically active messenger RNA for a myeloma light chain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1967–1971. doi: 10.1073/pnas.69.7.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfield A. M., Storb U., Selsing E., Zentgraf H. Comparison of different rearranged immunoglobulin kappa genes of a myeloma by electronmicroscopy and restriction mapping of cloned DNA: implications for "allelic exclusion". Nucleic Acids Res. 1980 Oct 24;8(20):4689–4707. doi: 10.1093/nar/8.20.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M., Perry R., Kelley D., Hunkapiller T., Schilling J., Hood L. The joining of V and J gene segments creates antibody diversity. Nature. 1980 Jan 31;283(5746):497–499. doi: 10.1038/283497a0. [DOI] [PubMed] [Google Scholar]
- Wilson R., Miller J., Storb U. Rearrangement of immunoglobulin genes. Biochemistry. 1979 Oct 30;18(22):5013–5021. doi: 10.1021/bi00589a032. [DOI] [PubMed] [Google Scholar]