Abstract
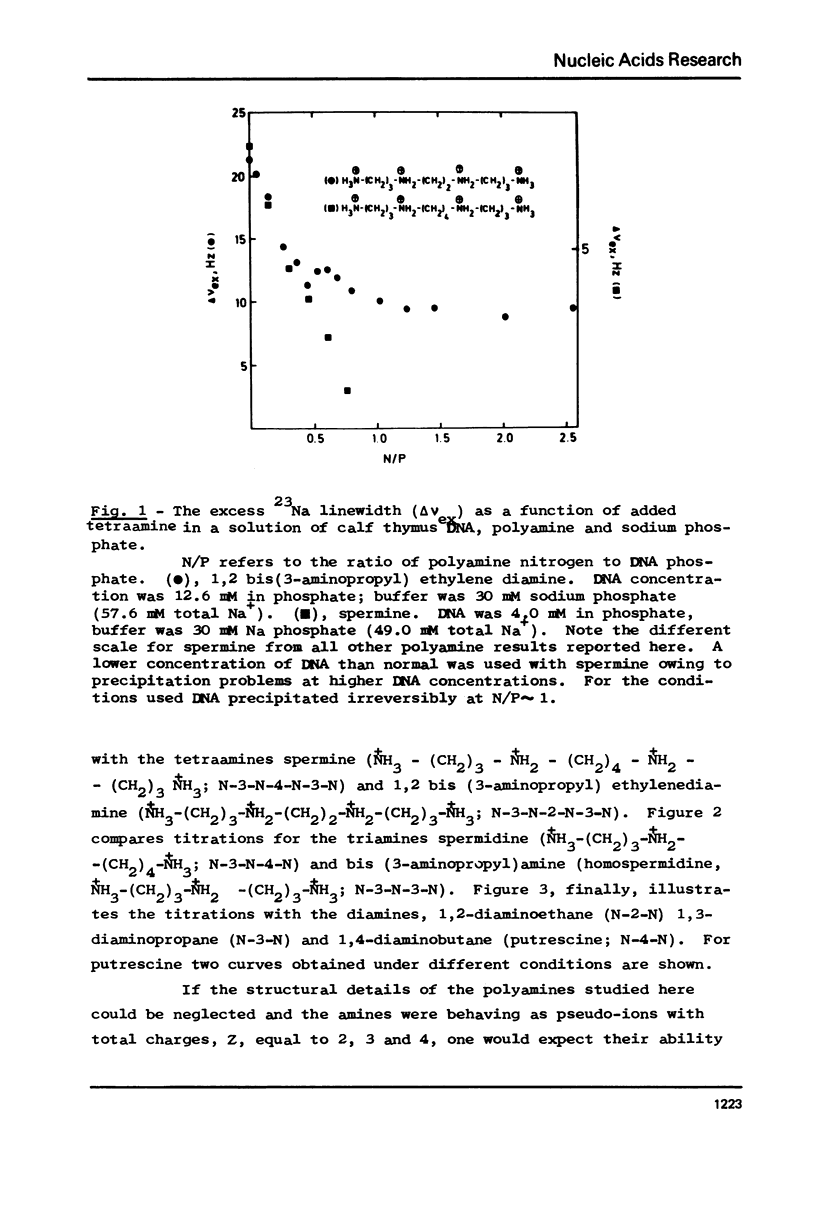
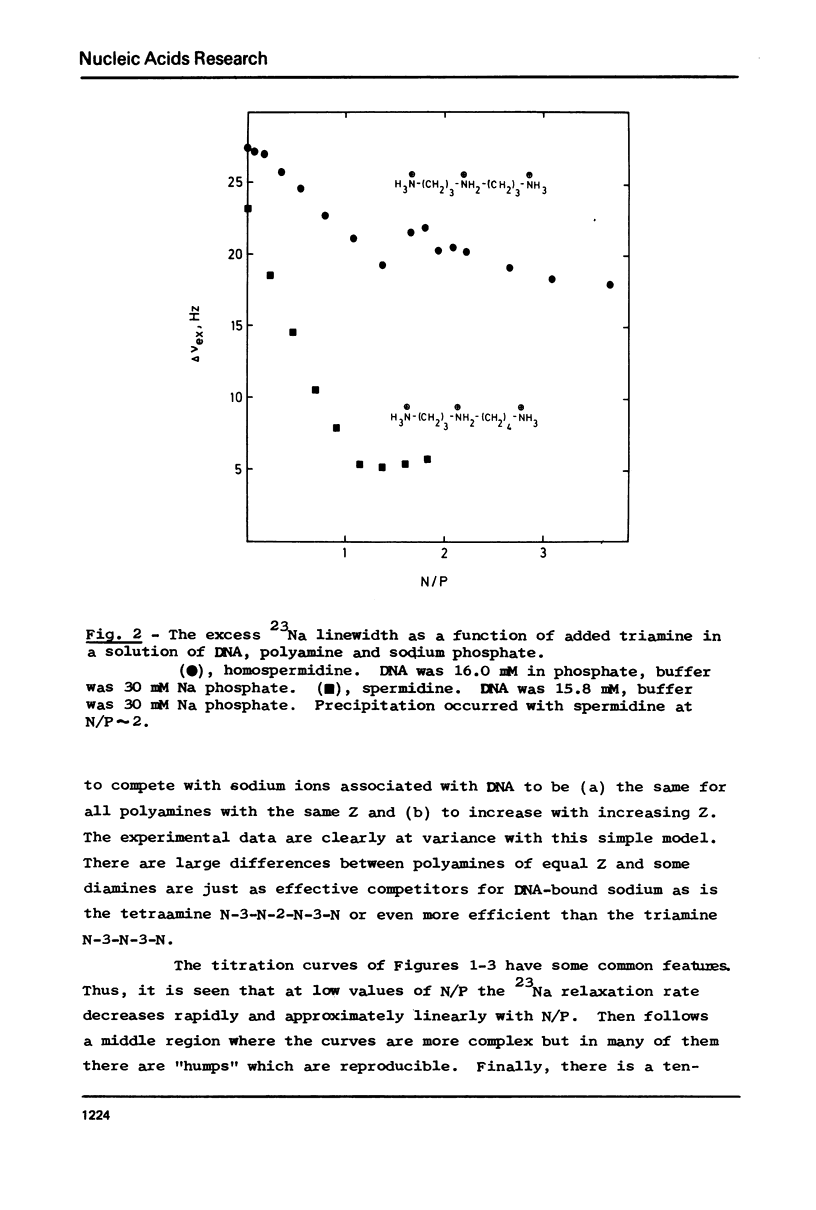
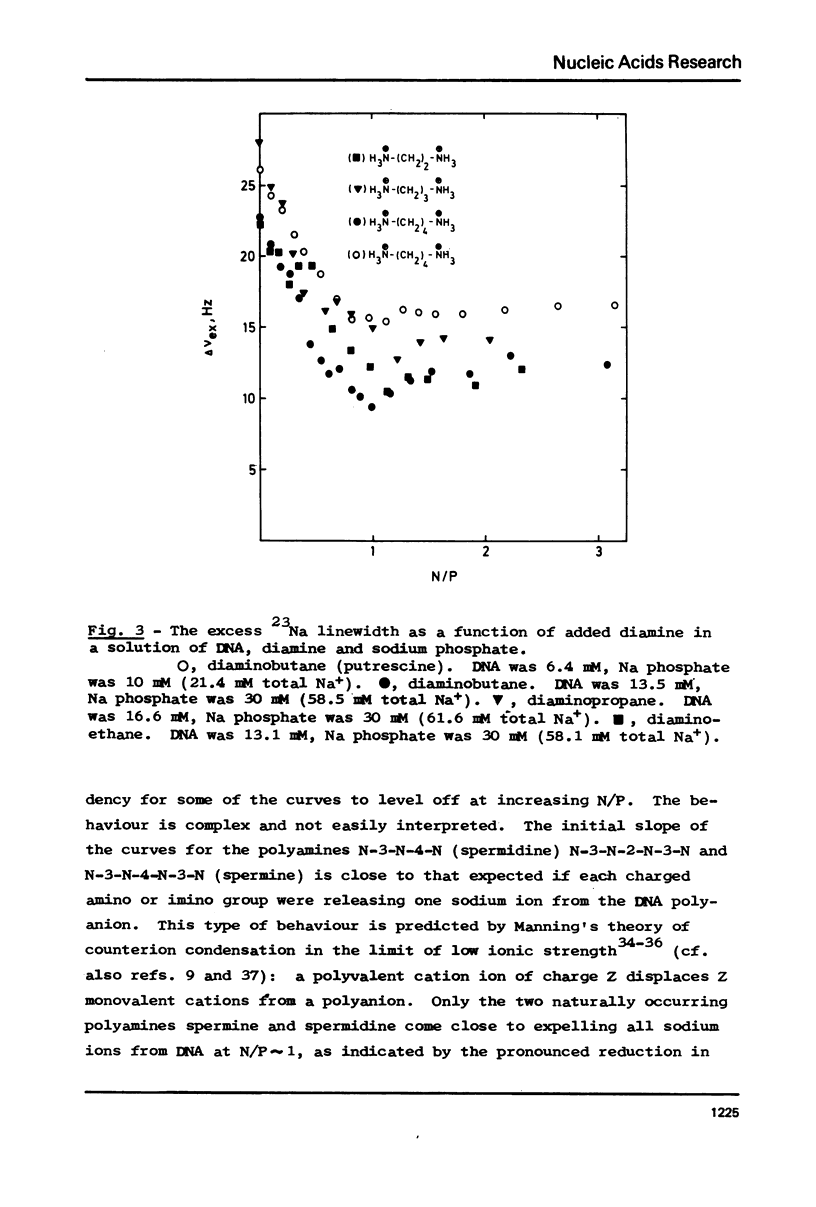
The interaction between a variety of polyamines, both naturally occurring and synthetic, and calf thymus DNA has been studied using 23Na NMR. The relaxation behaviour of 23Na reflects the extent of interaction of Na+ with DNA phosphate groups and therefore the extent of charge neutralisation of DNA phosphate groups (P) by polyamine amino and imino groups (N) in solutions of DNa, polyamine and Na+. The studies reveal that whereas spermine and spermidine are capable of expelling nearly all of the Na+ ions from DNA at N/P approximately 1, diamines such as putrescine and homologues of spermine and spermidine are capable of neutralising only roughly 50% of DNA phosphates. The results provide a challenge to current models of DNA-polyamine interactions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Anderson C. F., Record M. T., Jr, Hart P. A. Sodium-23 NMR studies of cation-DNA interactions. Biophys Chem. 1978 Jan;7(4):301–316. doi: 10.1016/0301-4622(78)85007-8. [DOI] [PubMed] [Google Scholar]
- Bleam M. L., Anderson C. F., Record M. T. Relative binding affinities of monovalent cations for double-stranded DNA. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3085–3089. doi: 10.1073/pnas.77.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield V. A., Wilson R. W., Rau D. C. Polyelectrolyte effects in DNA condensation by polyamines. Biophys Chem. 1980 Jun;11(3-4):339–343. doi: 10.1016/0301-4622(80)87006-2. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Gosule L. C., Schellman A. DNA condensation with polyamines. II. Electron microscopic studies. J Mol Biol. 1978 May 25;121(3):327–337. doi: 10.1016/0022-2836(78)90367-4. [DOI] [PubMed] [Google Scholar]
- Eickbush T. H., Moudrianakis E. N. The compaction of DNA helices into either continuous supercoils or folded-fiber rods and toroids. Cell. 1978 Feb;13(2):295–306. doi: 10.1016/0092-8674(78)90198-8. [DOI] [PubMed] [Google Scholar]
- Fresco J. R., Adams A., Ascione R., Henley D., Lindahl T. Tertiary structure in transfer ribonucleic acids. Cold Spring Harb Symp Quant Biol. 1966;31:527–537. doi: 10.1101/sqb.1966.031.01.068. [DOI] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Compartmentalization of spermine and spermidine in the herpes simplex virion. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2818–2821. doi: 10.1073/pnas.68.11.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingerty B., Brown R. S., Jack A. Further refinement of the structure of yeast tRNAPhe. J Mol Biol. 1978 Sep 25;124(3):523–534. doi: 10.1016/0022-2836(78)90185-7. [DOI] [PubMed] [Google Scholar]
- Ichikawa T., Sundaralingam M. X-ray diffraction study of a new crystal form of yeast phenylalanine tRNA. Nat New Biol. 1972 Apr 12;236(67):174–175. doi: 10.1038/newbio236174a0. [DOI] [PubMed] [Google Scholar]
- James T. L., Noggle J. H. 23Na nuclear magnetic resonance relaxation studies of sodium ion interaction with soluble RNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):644–649. doi: 10.1073/pnas.62.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenc P. C., Kurland C. G. Nucleoside triphosphate regeneration decreases the frequency of translation errors. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3174–3178. doi: 10.1073/pnas.76.7.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Quigley G., Suddath F. L., Rich A. High-resolution x-ray diffraction patterns of crystalline transfer RNA that show helical regions. Proc Natl Acad Sci U S A. 1971 Apr;68(4):841–845. doi: 10.1073/pnas.68.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Manning G. S. Theory of the delocalized binding of Mg(II) to DNA: preliminary analysis for low binding levels. Biophys Chem. 1977 Sep;7(2):141–145. doi: 10.1016/0301-4622(77)80006-9. [DOI] [PubMed] [Google Scholar]
- Manning G. S. Thermodynamic stability theory for DNA doughnut shapes induced by charge neutralization. Biopolymers. 1980 Jan;19(1):37–59. doi: 10.1002/bip.1980.360190104. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Imai N. The release of monovalent counterions by addition of divalent counterions in coulombic interaction system. Biophys Chem. 1980 Jun;11(3-4):345–352. doi: 10.1016/0301-4622(80)87007-4. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Teeter M. M., Rich A. Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):64–68. doi: 10.1073/pnas.75.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben J., Shporer M., Gabbay E. J. The Alkali Ion-DNA Interaction as Reflected in the Nuclear Relaxation Rates of Na and Rb. Proc Natl Acad Sci U S A. 1975 Jan;72(1):245–247. doi: 10.1073/pnas.72.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards K. E., Williams R. C., Calendar R. Mode of DNA packing within bacteriophage heads. J Mol Biol. 1973 Aug 5;78(2):255–259. doi: 10.1016/0022-2836(73)90114-9. [DOI] [PubMed] [Google Scholar]
- Sakai T. T., Cohen S. S. Effects of polyamines on the structure and reactivity of tRNA. Prog Nucleic Acid Res Mol Biol. 1976;17:15–42. doi: 10.1016/s0079-6603(08)60064-1. [DOI] [PubMed] [Google Scholar]
- Suwalsky M., Traub W., Shmueli U., Subirana J. A. An X-ray study of the interaction of DNA with spermine. J Mol Biol. 1969 Jun 14;42(2):363–373. doi: 10.1016/0022-2836(69)90049-7. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Zhurkin V. B., Lysov Y. P., Ivanov V. I. Interaction of spermine with different forms of DNA. A conformational study. Biopolymers. 1980 Aug;19(8):1415–1434. doi: 10.1002/bip.1980.360190802. [DOI] [PubMed] [Google Scholar]


