Abstract
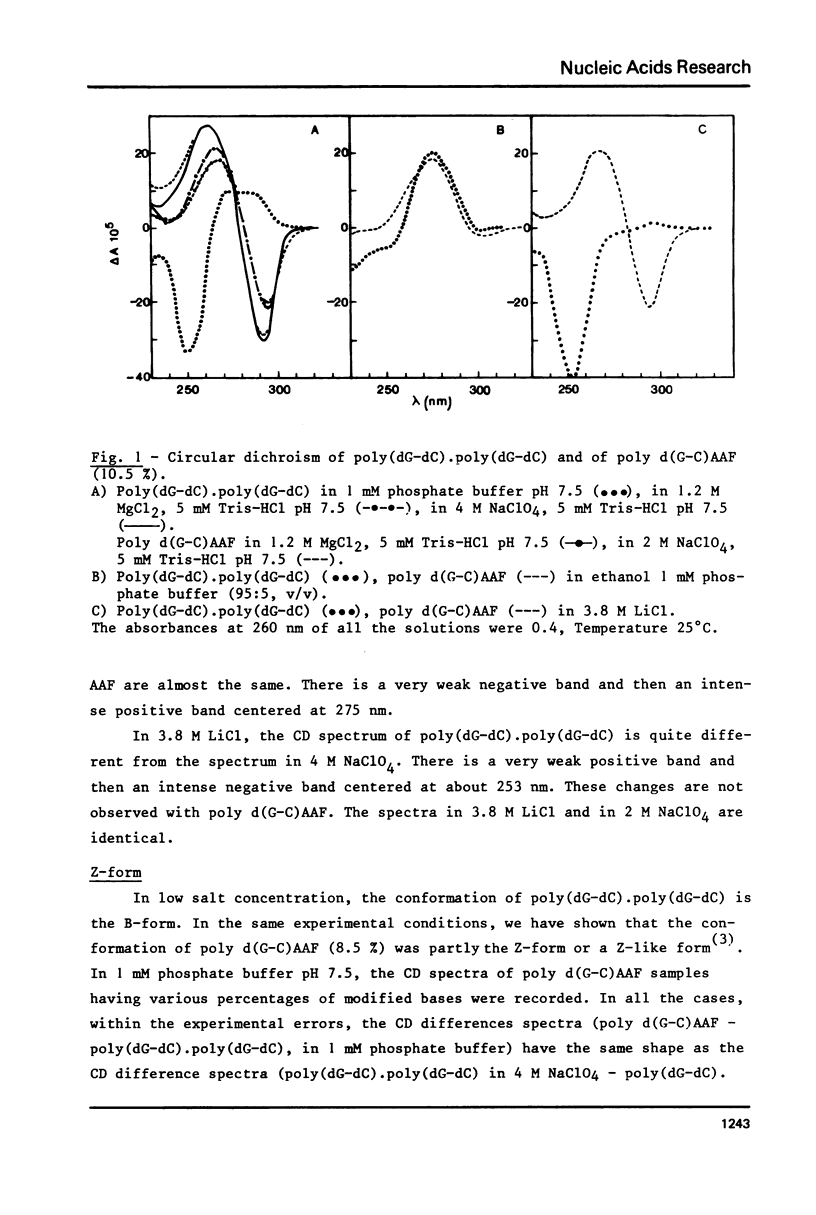
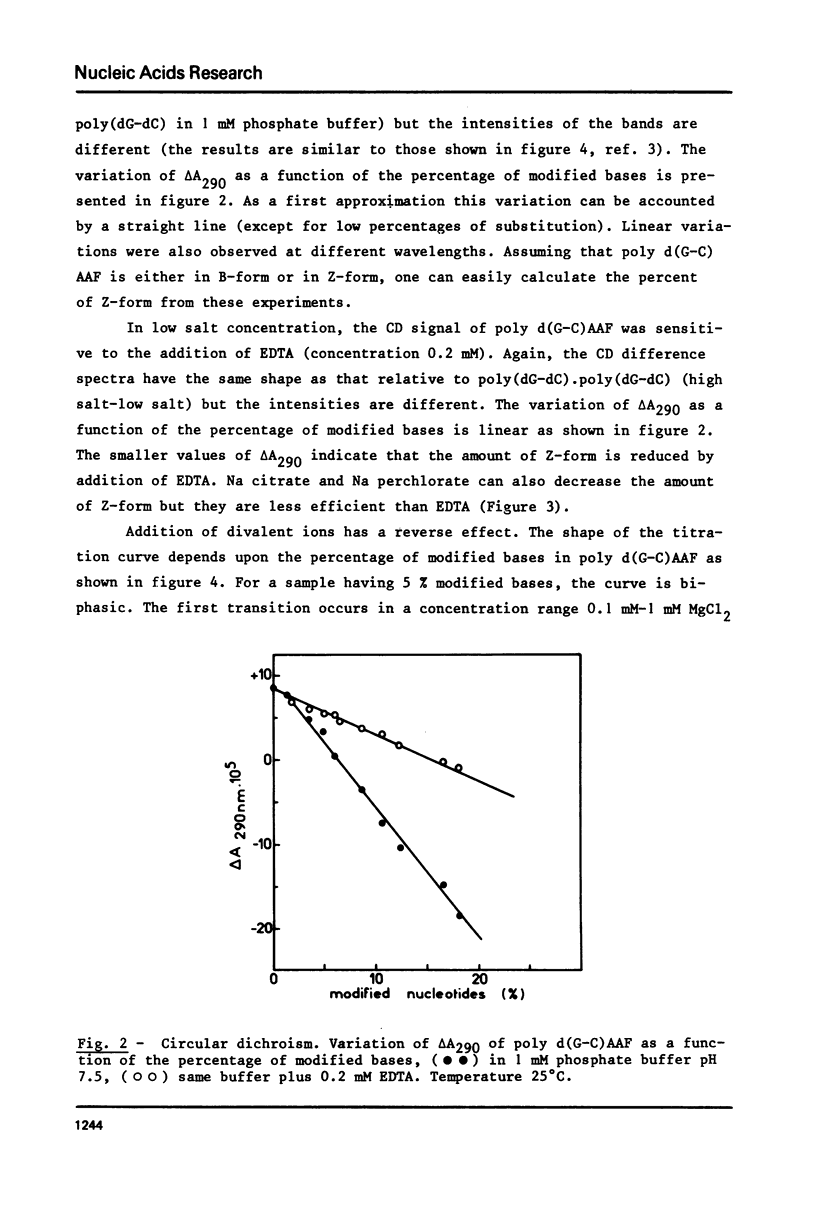
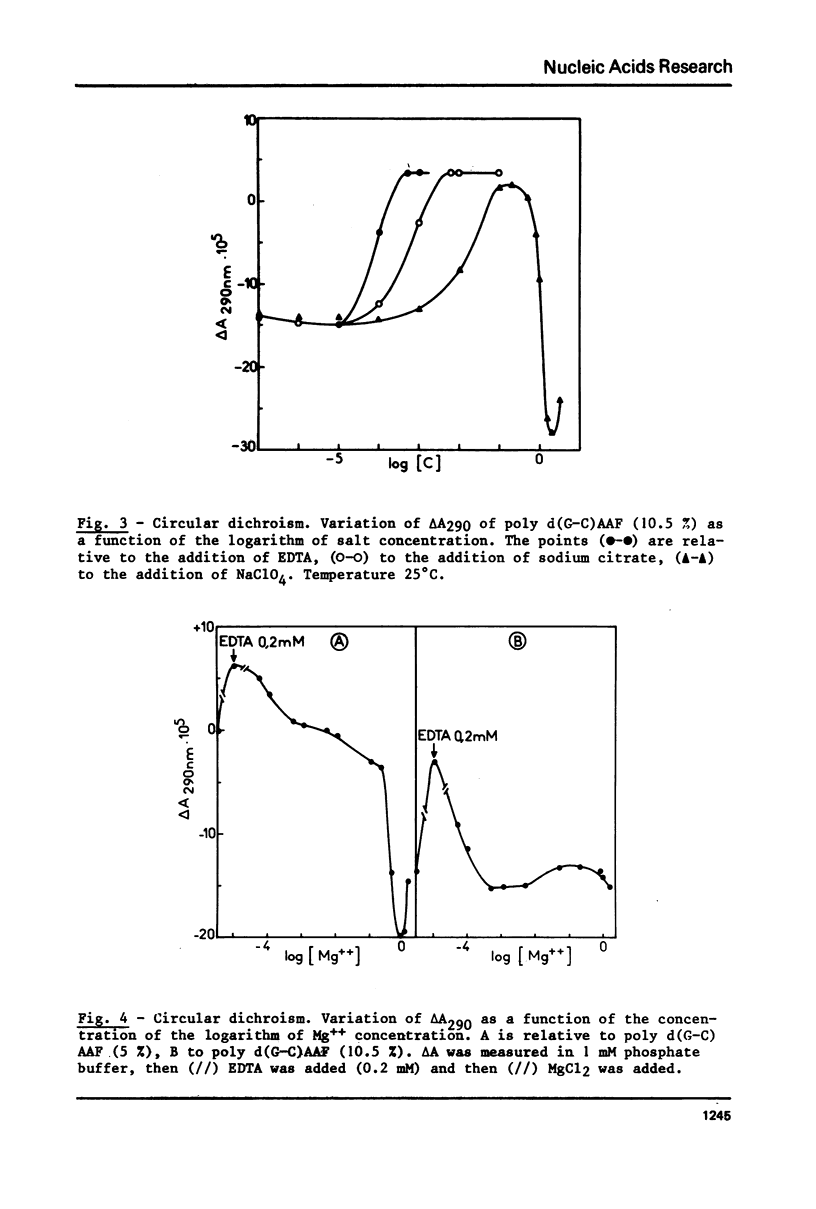
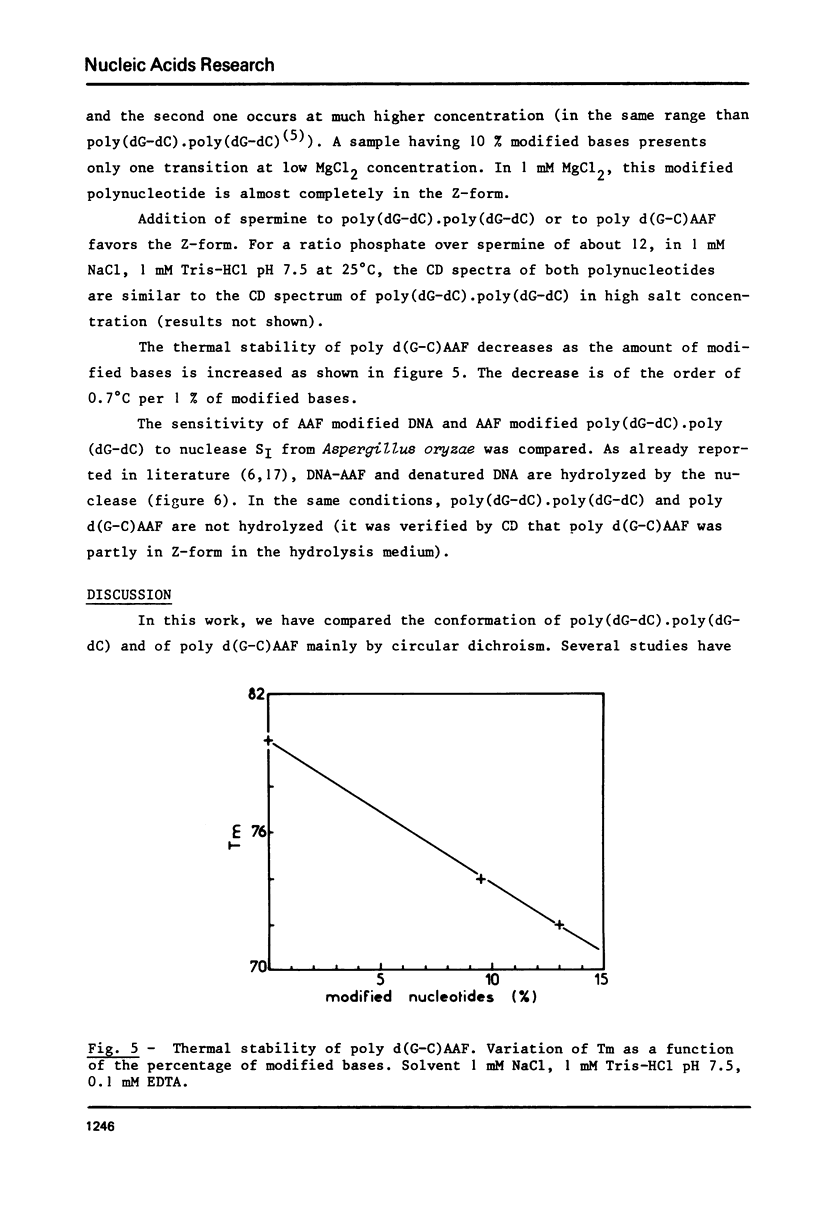
Poly (dG-dC) . poly(dG-dC) was modified by the reaction with N-acetoxy-N-acetyl-2-aminofluorene. The conformations of poly(dG-dC) . poly(dG-dC) and of poly d(G-C)AAF were studied by circular dichroism under various experimental conditions. In 95% ethanol, the two polynucleotides adopt the A-form. In 3.9 M LiCl, the transition B-form-C-form is observed with poly(dG-dC) . poly (dG-dC) but not with poly d(G-C)AAF. In 1 mM phosphate buffer, poly d(G-C)AAF behaves as a mixture of B- and Z-form, the relative percentages depending upon the amounts of modified bases. The percentage of Z-form is decreased by addition of EDTA and is increased by addition of Mg++. Spermine favors the Z-form in modified and unmodified polynucleotides. No defect in the double helix of poly d(G-C)AAF is detected by SI endonuclease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P. In vitro recognition of carcinogen-induced local denaturation sites native DNA by S1 endonuclease from Aspergillus oryzae. Nature. 1975 Sep 11;257(5522):151–152. doi: 10.1038/257151a0. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Daune M. Physical basis of chemical carcinogenesis by N-2-fluorenylacetamide derivatives and analogs. FEBS Lett. 1973 Aug 15;34(2):295–298. doi: 10.1016/0014-5793(73)80815-4. [DOI] [PubMed] [Google Scholar]
- Harvan D. J., Hass J. R., Lieberman M. W. Adduct formation between the carcinogen N-acetoxy-2-acetylaminofluorene and synthetic polydeoxyribonucleotides. Chem Biol Interact. 1977 May;17(2):203–210. doi: 10.1016/0009-2797(77)90085-0. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Lefèvre J. F., Fuchs R. P., Daune M. P. Comparative studies on the 7-iodo and 7-fluoro derivatives of N-acetoxy-N-2-acetylaminofluorene: binding sites on DNA and conformational change of modified deoxytrinucleotides. Biochemistry. 1978 Jun 27;17(13):2561–2567. doi: 10.1021/bi00606a016. [DOI] [PubMed] [Google Scholar]
- Leng M., Ptak M., Rio P. Conformation of acetylaminofluorene and aminofluorene modified guanosine and guanosine derivatives. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1095–1102. doi: 10.1016/0006-291x(80)90064-9. [DOI] [PubMed] [Google Scholar]
- Mercado C. M., Tomasz M. Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry. 1977 May 3;16(9):2040–2046. doi: 10.1021/bi00628a044. [DOI] [PubMed] [Google Scholar]
- Nelson J. H., Grunberger D., Cantor C. R., Weinstein I. B. Modification of ribonucleic acid by chemical carcinogens. IV. Circular dichroism and proton magnetic resonance studies of oligonucleotides modified with N-2-acetylaminofluorene. J Mol Biol. 1971 Dec 14;62(2):331–346. doi: 10.1016/0022-2836(71)90431-1. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Ramstein J., Leng M. Salt-dependent dynamic structure of poly(dG-dC) x poly(dG-dC). Nature. 1980 Nov 27;288(5789):413–414. doi: 10.1038/288413a0. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Shindo H. Conformation of 145 base pair length poly (dG-dC) . poly (dG-dC) in solution and in association with histones. Nucleic Acids Res. 1980 May 10;8(9):2093–2103. doi: 10.1093/nar/8.9.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunis-Schneider M. J., Maestre M. F. Circular dichroism spectra of oriented and unoriented deoxyribonucleic acid films--a preliminary study. J Mol Biol. 1970 Sep 28;52(3):521–541. doi: 10.1016/0022-2836(70)90417-1. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Leffler S., Weinstein I. B. Effect of N-2-acetylaminofluorene modification on the structure and template activity of DNA and reconstituted chromatin. Cancer Res. 1977 Mar;37(3):684–691. [PubMed] [Google Scholar]
- Zimmerman S. B., Pheiffer B. H. Does DNA adopt the C form in concentrated salt solutions or in organic solvent water mixtures? An x-ray diffraction study of DNA fibers immersed in various media. J Mol Biol. 1980 Sep 25;142(3):315–330. doi: 10.1016/0022-2836(80)90275-2. [DOI] [PubMed] [Google Scholar]
- de Murcia G., Lang M. C., Freund A. M., Fuchs R. P., Duane M. P., Sage E., Leng M. Electron microscopic visualization of N-acetoxy-N-2-acetylaminofluorene binding sites in ColE1 DNA by means of specific antibodies. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6076–6080. doi: 10.1073/pnas.76.12.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]


