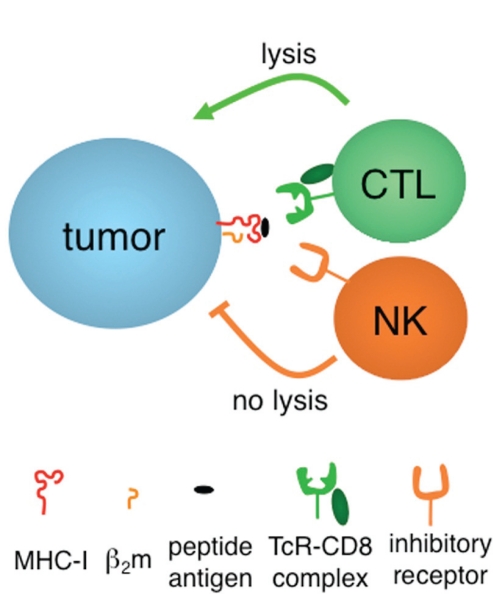
Cytotoxic T lymphocytes (ctls) and natural killer (nk) cells lyse tumours expressing and lacking, respectively, properly conformed class i molecules of the major histocompatibility complex [mhc-i (Figure 1)]. In keeping with the “missing self” hypothesis 1, a logical extrapolation would be to postulate that the primary goal of a tumour is to elude both defense lines.
FIGURE 1.
Activation–inhibition and molecules of major histocompatibility complex, class i (mhc-i). Tumours are killed either when they are able to express mhc-i molecules containing tumour peptide antigens recognized by the rearranging T-cell receptor (tcr) of cytotoxic T lymphocytes (ctls), or when self–mhc-i molecules are lacking or unable to properly engage non-rearranging inhibitory receptors, mainly (but not exclusively) expressed by natural killer (nk) cells. Peptide antigens must be processed and tailored before they can stabilize mhc-i assemblies with β2-microglobulin (β2m). Only folded mhc-i molecules serve as ligands of ctls and nk cells.
With regard to ctl evasion, tumour losses of mhci have been thoroughly studied (our group has more than 200 papers on file) and have, in most instances (although not invariably), been associated with poor outcome (reviewed in Garrido et al. 2). Interestingly, the principle of mhc-i loss also applies to the members of the so-called antigen-processing machinery, such as the transporter associated with antigen processing (tap), the endoplasmic reticulum aminopeptidase associated with antigen processing (eraap in mice and erap1 and erap2 in humans), and tapasin. These are in charge of, respectively, translocation (into the endoplasmic reticulum), final trimming, and editing of peptide antigens (Figure 1) before loading onto mhc-i. After our initial observation of linked expression patterns between mhc-i and members of the antigen-processing machinery 3, coordinated downregulation of some of these molecules was shown to correlate with poor prognosis 4.
Immunotherapeutic approaches, including the massive administration of dominant tumour antigens in peptide-based T-cell therapy (mostly pursued in melanoma and incorrectly called “vaccination”), impose an even greater selective pressure, possibly leading to an increased advantage for tumour cell variants lacking the antigen-presenting mhc-i molecule or the protein antigen that contains the immunogenic peptide epitope (or both) 2,5. Particularly when irreversible, mhc-i loss in cancer patients has been claimed to negatively affect prognosis 2.
Assuming that spontaneous and immunotherapy-induced mhc-i losses are drivers and not passengers of tumour progression, it remains to be explained why they do not incite recognition and tumour lysis by nk cells (Figure 1). Porgador et al. 6 described a very high prevalence (5 in 13 cases) of irreversible complete mhc-i losses in patients treated with various immunotherapeutic regimens. Despite the cells being very sensitive targets of autologous nk cells in vitro, clinical outcome was reported to be poor. Likewise, Pende et al. 7 observed that long-term tumour cell lines, even when established from patients not undergoing immunotherapy, do not express enough mhc-i to protect themselves from nk recognition. Why, then, can these tumours evade in the face of a brisk in vitro nk response?
A possible interpretation is that simple cytotoxicity readouts do not reflect the lytic behaviour of immune effectors in vivo. After all, if antitumour T-cell counts and activity in vitro are not entirely predictive of clinical responsiveness to vaccination 8, why should nk cell responses in vivo be faithfully recapitulated in an in vitro assay? Alternatively, it might be hypothesized that nk cells have nothing to do with tumour immune surveillance, at least in humans. Indeed, lymphoid cell infiltrates contain many more T cells than nk cells, and only T cells are positively associated with a favorable outcome 9. Whatever the interpretation, a drastic objection is that certain subsets of nk cells may be important at early stages, but may be long gone by the time the tumour becomes clinically evident and hits the pathology slide.
If nk cells are indeed important, tumours low in mhc-i may elude them either by exploiting certain “gaps” in the inhibitory nk receptor repertoire 10 or, analogous with viral immuno-evasion strategies 11, by “replacing” mhc-i self-inhibitory signals with other inhibitory ligands such as the non-classical mhc-i human leukocyte antigens G (hla-g) and E (hla-e) 12–14. However, at least hla-e behaves not only as an inhibitory, but also as a triggering ligand 15. In addition, hla-e expression may not be restricted to tumours with mhc-i loss as required by the “replacement” model 16,17. Finally, and quite surprisingly, hla-e is associated with a good prognosis, at least in certain tumour histotypes 18–20. It will be of considerable interest to find out if and how tumours use nk-decoy tactics.
Although there are simpler ways to explain mhc-i–driven tumour evasion from both ctl and nk cells, those explanations have received considerably less attention than the foregoing mechanisms. A straightforward assumption is that, besides mhc-i losses adopted by ctl-sensitive tumours, there are mechanisms of mhc-i gains, and those mechanisms are preferred by another set of tumours that are particularly sensitive to nk lysis. It might be envisaged that the opposing influences of ctl and nk cells prevent any major change in mhc-i expression, making less-aggressive tumours resemble their normal counterparts. By contrast, aggressive tumours may escape by adopting whichever immuno-evasion strategy is the most advantageous in the context of the immune response mounted by an individual host. Indeed, a Gaussian distribution of mhc-i expression around “normal” values was observed in vitro and in vivo in a variety of solid tumours 3,21, mhc-i losses and mhc-i gains both being associated with poor prognosis in colorectal carcinoma 22.
Given the opposing effects of mhc-i molecules on ctl and nk cells (Figure 1), an mhc-i phenotype efficiently triggering both effectors is a contradiction in terms. For instance, in the classical paper that pioneered the “missing self” hypothesis, a tap-defective mutant of the murine lymphoma RMA, called RMAS, was shown to be rejected essentially by nk cells 1.
Recently, rna interference of the same RMA cells for eraap (just downstream of tap in the antigen-processing machinery pathway 23) similarly resulted in tumour rejection 24, but in addition to nk cells, T cells (CD4 and CD8 alike) were also involved. It appears that poorly folded mhc-i molecules synthesized in the absence of eraap can be “seen” as abnormal by several immune effectors. Quite interestingly, only a few human tumours express low erap1 and erap2 levels 25,26, suggesting that the spontaneous occurrence of this altered, two-edge phenotype is counterselected in vivo.
In conclusion, it is fairly clear what tumours look like when they are “out of the hands” of the immune system, but we know much less of “real” tumours under immunologic scrutiny and during immunoediting in vivo. If ctl and nk cells must both be “tuned in” to reject tumours, many more immunoevasive mhc-i (and non-mhc-i 27) phenotypes remain to be discovered.
Acknowledgments
The work described herein is supported by the Italian Ministry of Health and airc (Associazione Italiana per la Ricerca sul Cancro).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to disclose.
REFERENCES
- 1.Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2–deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 2.Garrido F, Cabrera T, Aptsiauri N. “Hard” and “soft” lesions underlying the hla class i alterations in cancer cells: implications for immunotherapy. Int J Cancer. 2010;127:249–56. doi: 10.1002/ijc.25270. [DOI] [PubMed] [Google Scholar]
- 3.Giorda E, Sibilio L, Martayan A, et al. The antigen processing machinery of class i human leukocyte antigens: linked patterns of gene expression in neoplastic cells. Cancer Res. 2003;63:4119–27. [PubMed] [Google Scholar]
- 4.Ogino T, Shigyo H, Ishii H, et al. hla class i antigen downregulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006;66:9281–9. doi: 10.1158/0008-5472.CAN-06-0488. [DOI] [PubMed] [Google Scholar]
- 5.Anichini A, Mortarini R, Nonaka D, et al. Association of antigen-processing machinery and hla antigen phenotype of melanoma cells with survival in American Joint Committee on Cancer stage iii and iv melanoma patients. Cancer Res. 2006;66:6405–11. doi: 10.1158/0008-5472.CAN-06-0854. [DOI] [PubMed] [Google Scholar]
- 6.Porgador A, Mandelboim O, Restifo NP, Strominger JL. Natural killer cell lines kill autologous β2m-deficient melanoma cells: implications for cancer immunotherapy. Proc Natl Acad Sci U S A. 1997;94:13140–5. doi: 10.1073/pnas.94.24.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pende D, Accame L, Pareti L, et al. The susceptibility to natural killer cell–mediated lysis of hla class i–positive melanomas reflects the expression of insufficient amounts of different hla class i alleles. Eur J Immunol. 1998;28:2384–94. doi: 10.1002/(SICI)1521-4141(199808)28:08<2384::AID-IMMU2384>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Coulie PG, Connerotte T. Human tumor–specific T lymphocytes: does function matter more than number? Curr Opin Immunol. 2005;17:320–5. doi: 10.1016/j.coi.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez–Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 10.Demanet C, Mulder A, Deneys V, et al. Down-regulation of hla-a and hla-bw6, but not hla-bw4, allospecificities in leukemic cells: an escape mechanism from ctl and nk attack? Blood. 2004;103:3122–30. doi: 10.1182/blood-2003-07-2500. [DOI] [PubMed] [Google Scholar]
- 11.Tomasec P, Braud VM, Rickards C, et al. Surface expression of hla-e, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 2000;287:1031. doi: 10.1126/science.287.5455.1031. [DOI] [PubMed] [Google Scholar]
- 12.LeMaoult J, Le Discorde M, Rouas–Freiss N, et al. Biology and functions of human leukocyte antigen-G in health and sickness. Tissue Antigens. 2003;62:273–84. doi: 10.1034/j.1399-0039.2003.00143.x. [DOI] [PubMed] [Google Scholar]
- 13.Marín R, Ruiz–Cabello F, Pedrinaci S, et al. Analysis of hla-e expression in human tumors. Immunogenetics. 2003;54:767–75. doi: 10.1007/s00251-002-0526-9. [DOI] [PubMed] [Google Scholar]
- 14.de Kruijf EM, Sajet A, van Nes JG, et al. hla-e and hla-g expression in classical hla class i–negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol. 2010;185:7452–9. doi: 10.4049/jimmunol.1002629. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan LC, Hoare HL, McCluskey J, Rossjohn J, Brooks AG. A structural perspective on mhc class ib molecules in adaptive immunity. Trends Immunol. 2006;27:413–20. doi: 10.1016/j.it.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Lo Monaco E, Sibilio L, Melucci E, et al. hla-e: strong association with β2m and surface expression in the absence of hla class i signal sequence-derived peptides. J Immunol. 2008;181:5442–50. doi: 10.4049/jimmunol.181.8.5442. [DOI] [PubMed] [Google Scholar]
- 17.Lo Monaco E, Tremante E, Cerboni C, et al. Human leukocyte antigen E contributes to protect tumor cells from lysis by natural killer cells. Neoplasia. 2011;13:822–30. doi: 10.1593/neo.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John T, Black MA, Toro TT, et al. Predicting clinical outcome through molecular profiling in stage iii melanoma. Clin Cancer Res. 2008;14:5173–80. doi: 10.1158/1078-0432.CCR-07-4170. [DOI] [PubMed] [Google Scholar]
- 19.Kren L, Slaby O, Muckova K, et al. Expression of immunemodulatory molecules hla-g and hla-e by tumor cells in glioblastomas: an unexpected prognostic significance? Neuropathology. 2011;31:129–34. doi: 10.1111/j.1440-1789.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- 20.Benevolo M, Mottolese M, Tremante E, et al. High expression of hla-e in colorectal carcinoma is associated with a favorable prognosis. J Transl Med. 2011;9:184. doi: 10.1186/1479-5876-9-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giacomini P, Giorda E, Fraioli R, et al. Low prevalence of selective human leukocyte antigen (hla)-a and hla-b epitope losses in early-passage tumor cell lines. Cancer Res. 1999;59:2657–67. [PubMed] [Google Scholar]
- 22.Benevolo M, Mottolese M, Piperno G, et al. hla-a, -b, -c expression in colon carcinoma mimics that of the normal colonic mucosa and is prognostically relevant. Am J Surg Pathol. 2007;31:76–84. doi: 10.1097/01.pas.0000213343.55605.b9. [DOI] [PubMed] [Google Scholar]
- 23.Van Endert P. Post-proteasomal and proteasome-independent generation of mhc class i ligands. Cell Mol Life Sci. 2011;68:1553–67. doi: 10.1007/s00018-011-0662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cifaldi L, Lo Monaco E, Forloni M, et al. nk cells efficiently reject lymphoma silenced for the endoplasmic reticulum aminopeptidase associated with antigen processing. Cancer Res. 2011;71:1597–606. doi: 10.1158/0008-5472.CAN-10-3326. [DOI] [PubMed] [Google Scholar]
- 25.Fruci D, Ferracuti S, Limongi MZ, et al. Expression of endoplasmic reticulum aminopeptidases in ebv-b cell lines from healthy donors and in leukemia/lymphoma, carcinoma, and melanoma cell lines. J Immunol. 2006;176:4869–79. doi: 10.4049/jimmunol.176.8.4869. [DOI] [PubMed] [Google Scholar]
- 26.Fruci D, Giacomini P, Nicotra MR, et al. Altered expression of endoplasmic reticulum aminopeptidases erap1 and erap2 in transformed non-lymphoid human tissues. J Cell Physiol. 2008;216:742–9. doi: 10.1002/jcp.21454. [DOI] [PubMed] [Google Scholar]
- 27.Lakshmikanth T, Burke S, Ali TH, et al. ncrs and dnam-1 mediate nk cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest. 2009;119:1251–63. doi: 10.1172/JCI36022. [DOI] [PMC free article] [PubMed] [Google Scholar]