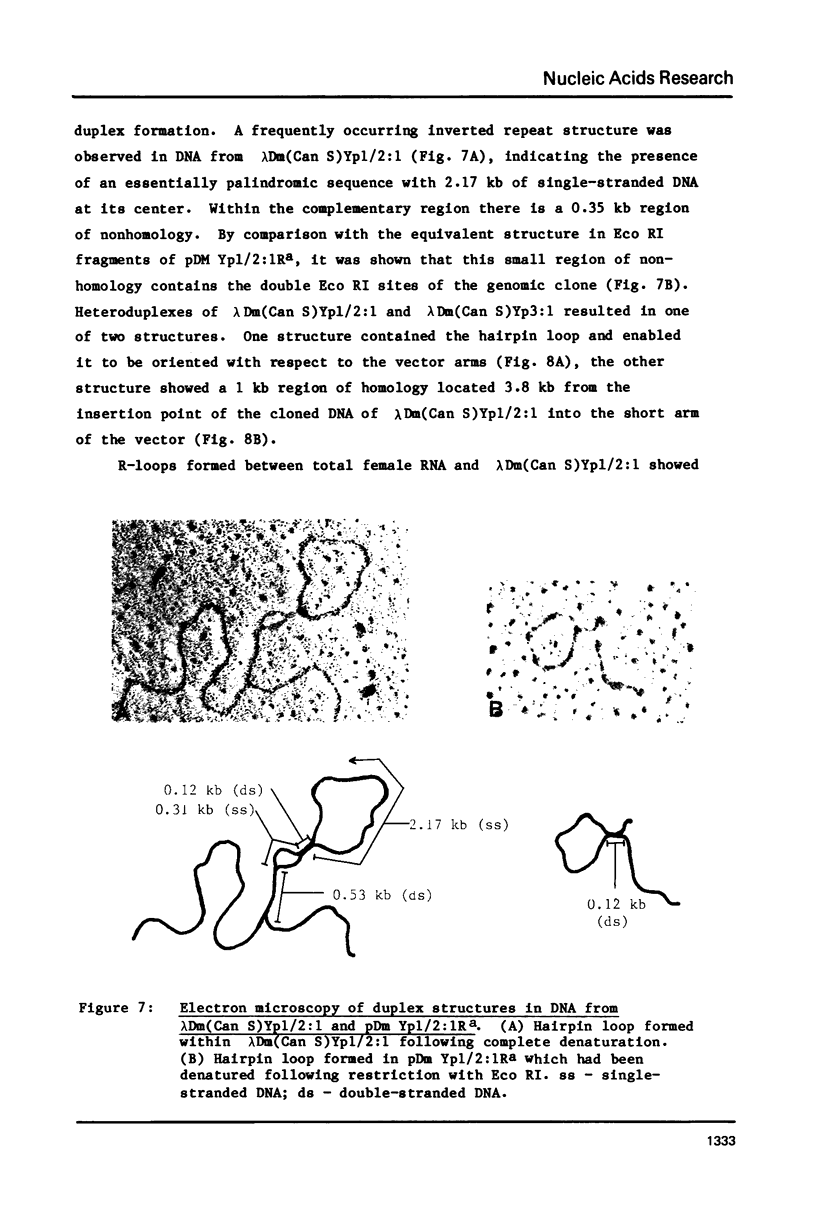
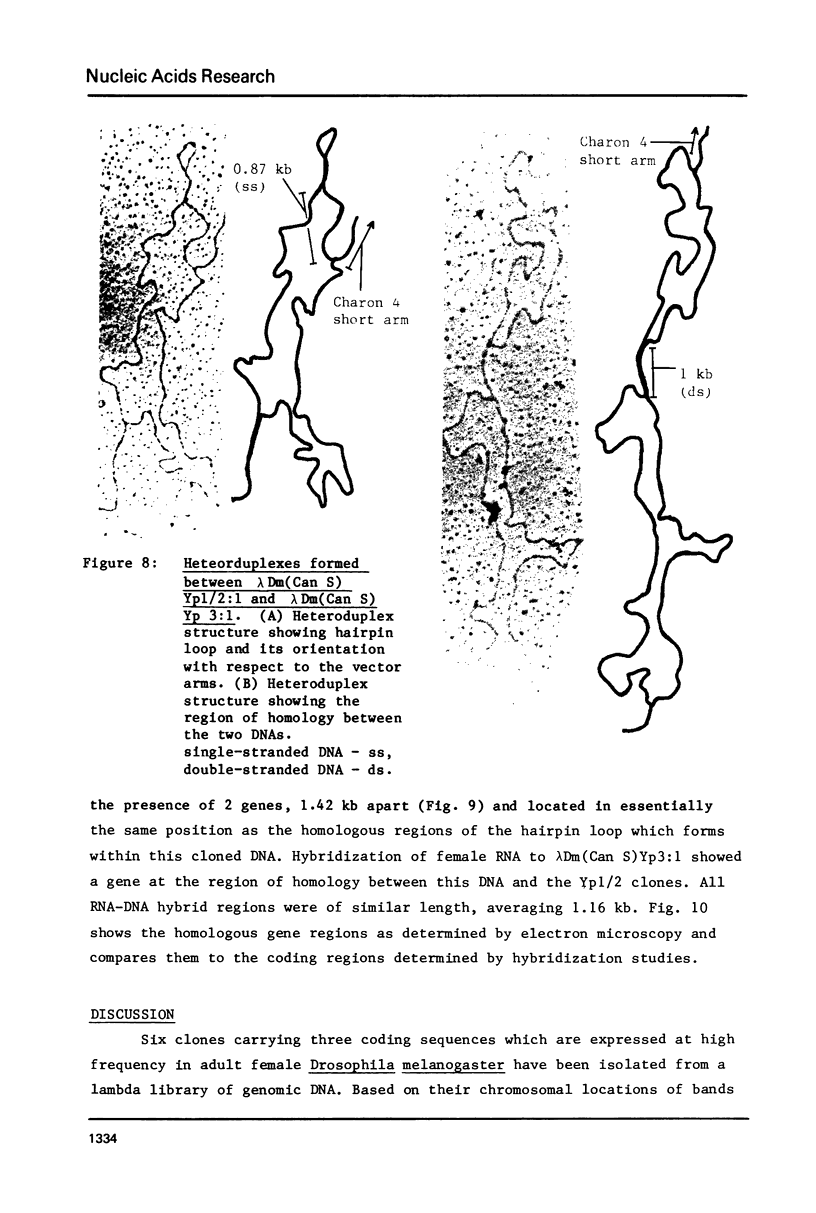
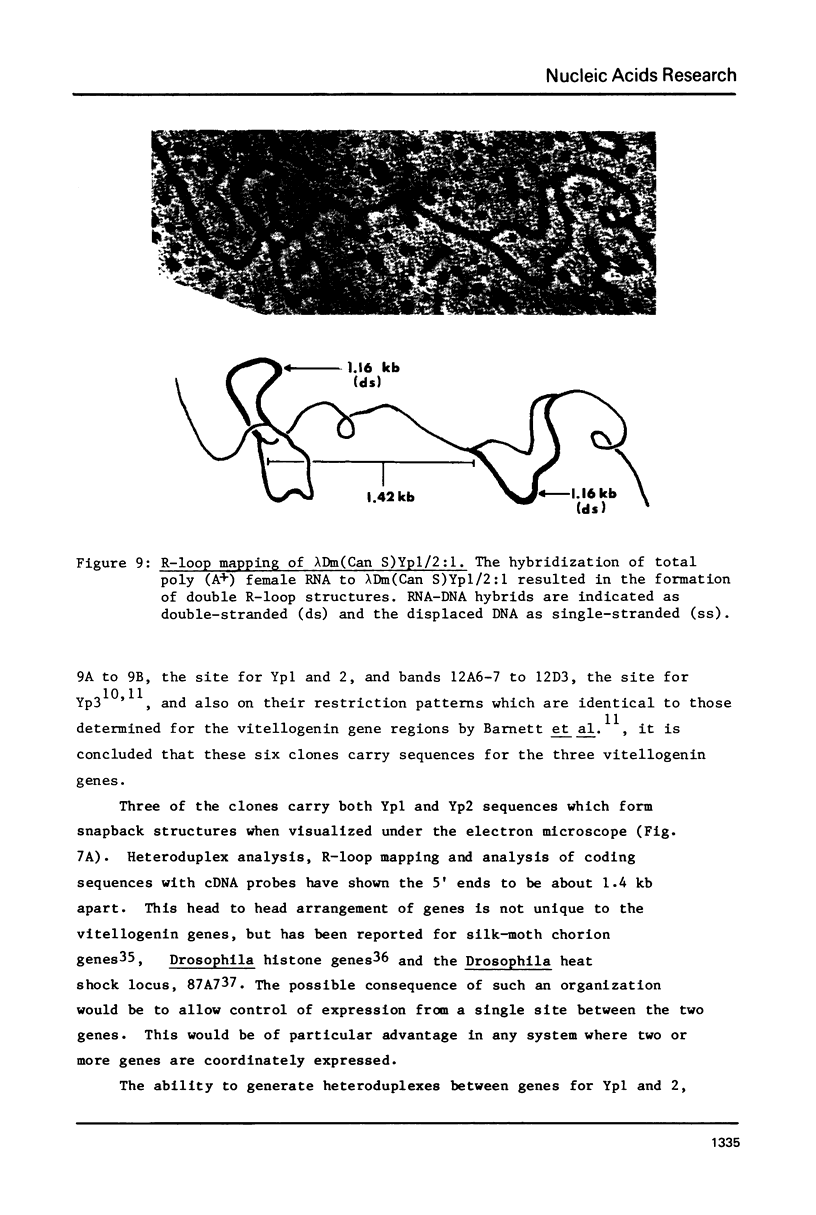
Abstract
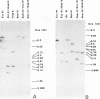
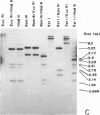
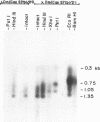
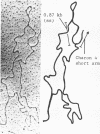
Genomic fragments coding for sequences expressed as abundant mRNA in female Drosophila melanogaster were isolated from a lambda library. Hybridization of these clones to polytene chromosomes. in situ, identified four which mapped to X chromosomal region 9A to 9B, the locus for yolk proteins 1 and 2 (Ypl,2) and two which mapped to 12A6-7 to 12D3, the locus for Yp3. These clones were mapped with restriction enzymes, and the coding regions and regions of homology determined by Southern blots probed with cDNA, 5'-end-labelled RNA and nick-translated DNA. Heteroduplex and R-loop mapping confirmed that three of the clones carried two genes separated by about 1.4 kb and oriented in opposite directions. Southern blots probed with cDNA made from alkali-hydrolyzed RNA showed that these genes had their 5' ends next to each other. All 3 genes show homology to each other and have a main coding region of about 1.3 kb, the approximate size for the mRNAs.
Full text
PDF



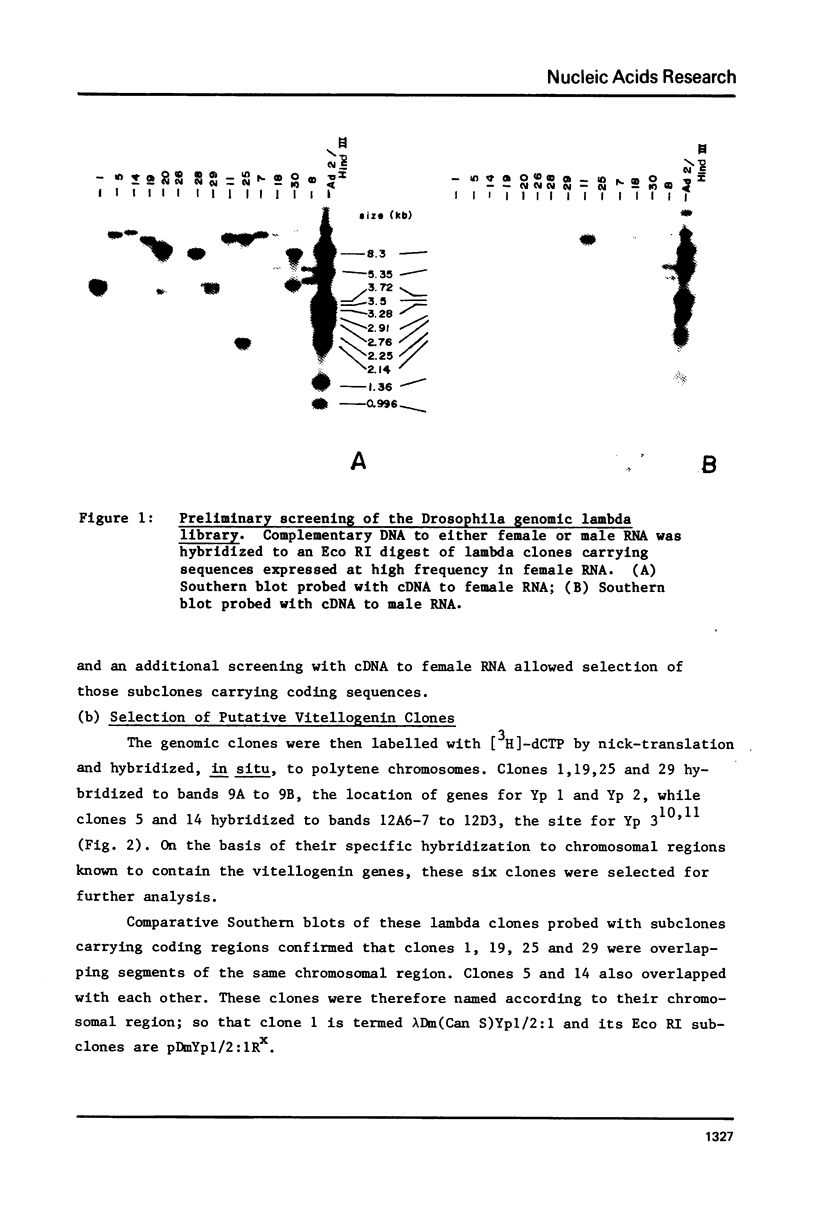

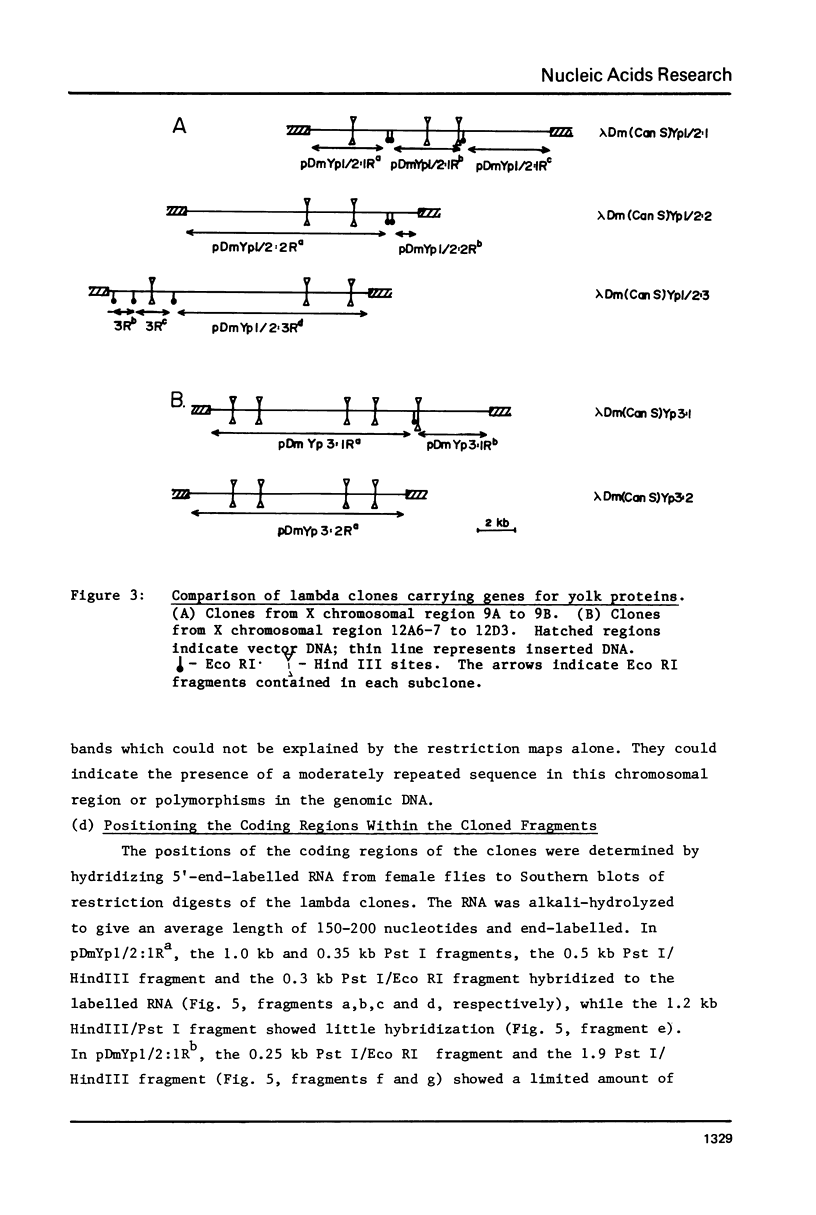
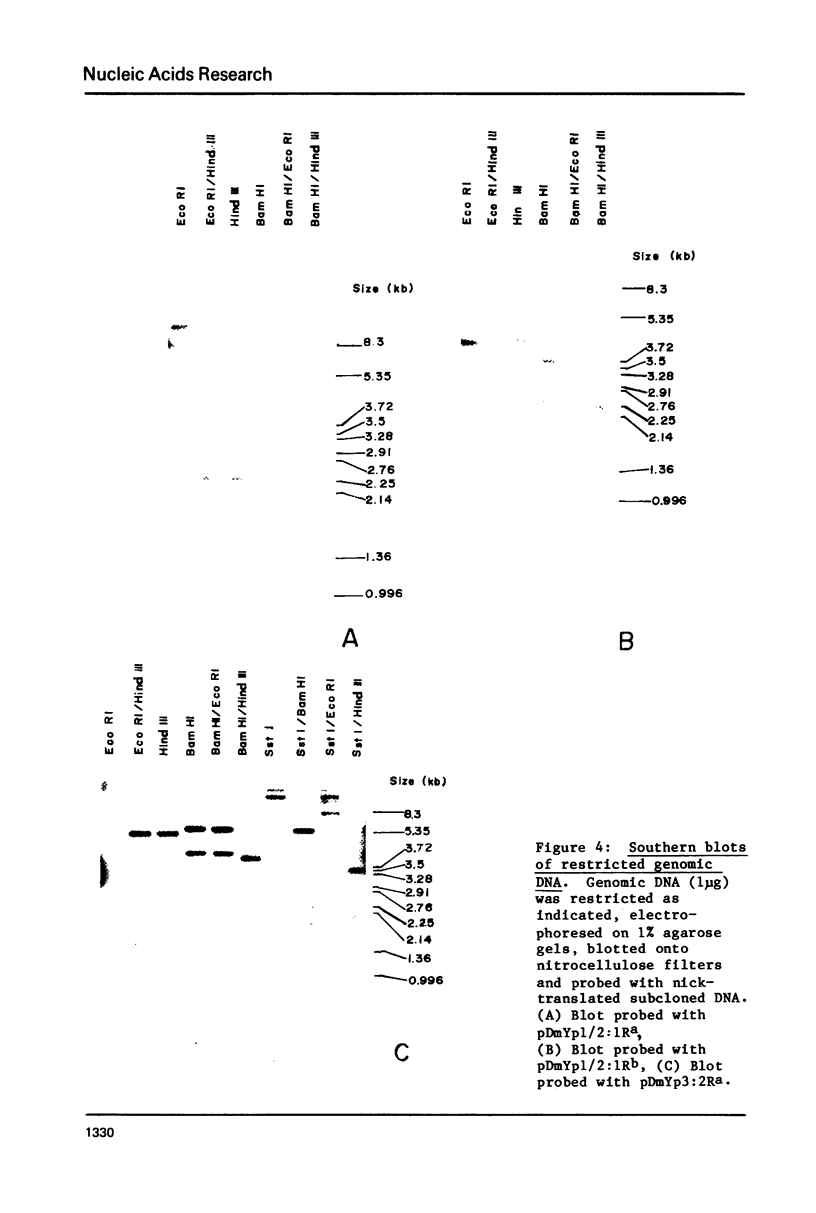
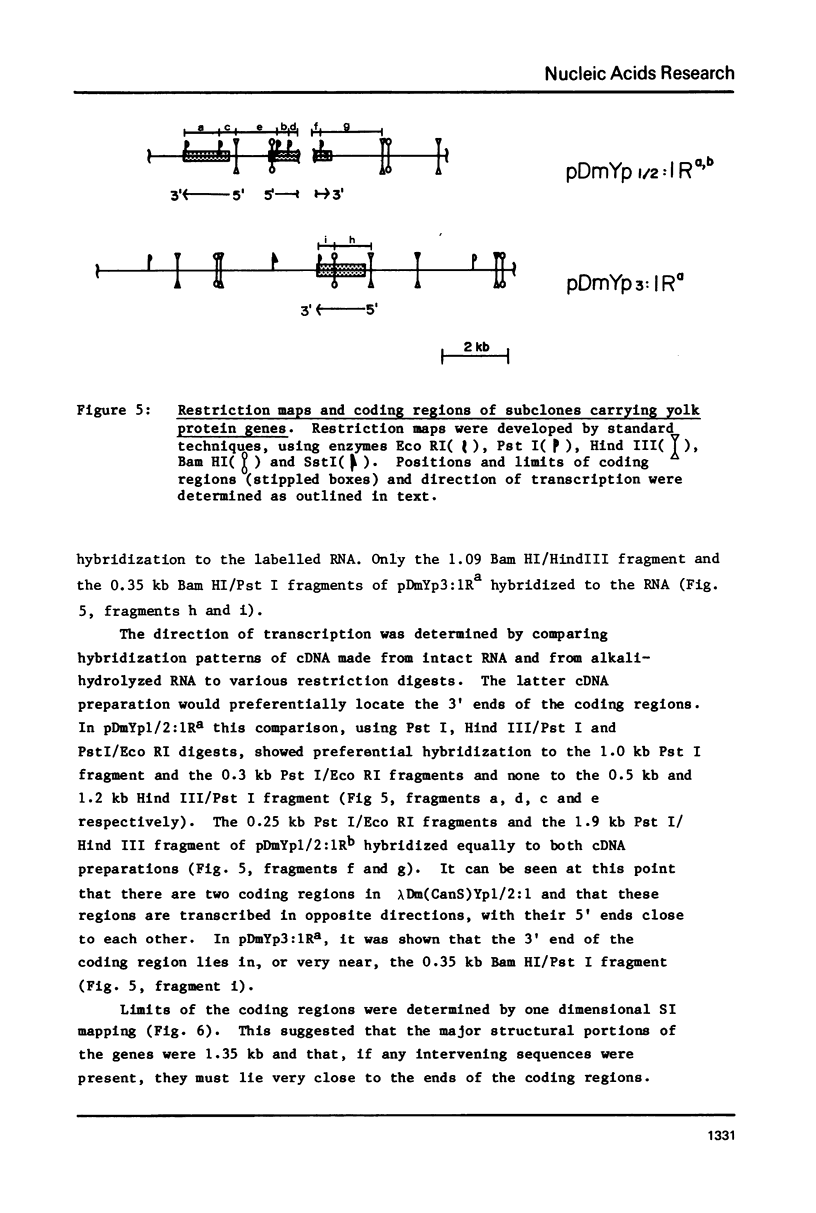




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Barnett T., Pachl C., Gergen J. P., Wensink P. C. The isolation and characterization of Drosophila yolk protein genes. Cell. 1980 Oct;21(3):729–738. doi: 10.1016/0092-8674(80)90436-5. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bownes M., Hames B. D. Accumulation and degradation of three major yolk proteins in Drosophila melanogaster. J Exp Zool. 1977 Apr;200(1):149–156. doi: 10.1002/jez.1402000118. [DOI] [PubMed] [Google Scholar]
- Bownes M., Hames B. D. Analysis of the yolk proteins in Drosophila melanogaster. Translation in a cell free system and peptide analysis. FEBS Lett. 1978 Dec 15;96(2):327–330. doi: 10.1016/0014-5793(78)80428-1. [DOI] [PubMed] [Google Scholar]
- Bownes M. The use of yolk protein variations in Drosophila species to analyse the control of vitellogenesis. Differentiation. 1980 Apr;16(2):109–116. doi: 10.1111/j.1432-0436.1980.tb01065.x. [DOI] [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Payvar F., Schimke R. T. Synthesis of full length cDNAs from four partially purified oviduct mRNAs. J Biol Chem. 1978 Apr 10;253(7):2471–2482. [PubMed] [Google Scholar]
- Chen T. T. Vitellogenin in locusts (Locusta migratoria): translation of vitellogenin mRNA in Xenopus oocytes and analysis of the polypeptide products. Arch Biochem Biophys. 1980 Apr 15;201(1):266–276. doi: 10.1016/0003-9861(80)90511-1. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. I., Burns A. T., Christmann J. L., Deeley R. G. Cloning of a double-stranded cDNA that codes for a portion of chicken preproalbumin. A general method for isolating a specific DNA sequence from partially purified mRNA. J Biol Chem. 1978 Dec 10;253(23):8629–8639. [PubMed] [Google Scholar]
- Ish-Horowicz D., Pinchin S. M. Genomic organization of the 87A7 and 87Cl heat-induced loci of Drosophila melanogaster. J Mol Biol. 1980 Sep 15;142(2):231–245. doi: 10.1016/0022-2836(80)90047-9. [DOI] [PubMed] [Google Scholar]
- Kidd S. J., Glover D. M. A DNA segment from D. melanogaster which contains five tandemly repeating units homologous to the major rDNA insertion. Cell. 1980 Jan;19(1):103–119. doi: 10.1016/0092-8674(80)90392-x. [DOI] [PubMed] [Google Scholar]
- Kunkel J. G., Pan M. L. Selectivity of yolk protein uptake: comparison of vitellogenins of two insects. J Insect Physiol. 1976;22(6):809–818. doi: 10.1016/0022-1910(76)90248-1. [DOI] [PubMed] [Google Scholar]
- Lifton R. P., Goldberg M. L., Karp R. W., Hogness D. S. The organization of the histone genes in Drosophila melanogaster: functional and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1047–1051. doi: 10.1101/sqb.1978.042.01.105. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Nucleic acid hybridization to the DNA of cytological preparations. Methods Cell Biol. 1975;10:1–16. doi: 10.1016/s0091-679x(08)60727-x. [DOI] [PubMed] [Google Scholar]
- Postlethwait J. H., Jowett T. Genetic analysis of the hormonally regulated yolk polypeptide genes in D. melanogaster. Cell. 1980 Jul;20(3):671–678. doi: 10.1016/0092-8674(80)90313-x. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Searle P. F., Williams J. G. Characterisation of bacterial clones containing DNA sequences derived from Xenopus laevis vitellogenin mRNA. Nucleic Acids Res. 1979 Feb;6(2):487–506. doi: 10.1093/nar/6.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Warren T. G., Mahowald A. P. Isolation and partial chemical characterization of the three major yolk polypeptides from Drosophila melanogaster. Dev Biol. 1979 Jan;68(1):130–139. doi: 10.1016/0012-1606(79)90248-3. [DOI] [PubMed] [Google Scholar]
- White R. L., Hogness D. S. R loop mapping of the 18S and 28S sequences in the long and short repeating units of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):177–192. doi: 10.1016/0092-8674(77)90213-6. [DOI] [PubMed] [Google Scholar]