Abstract
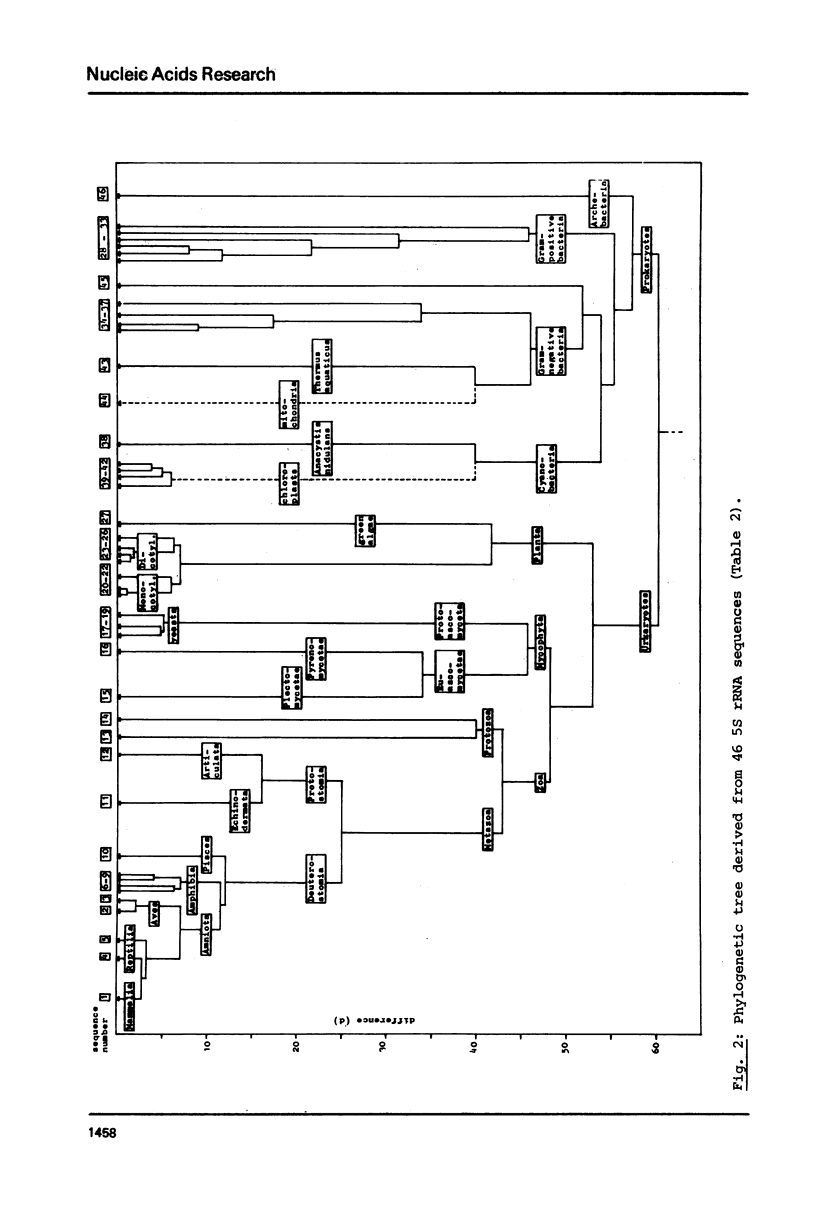
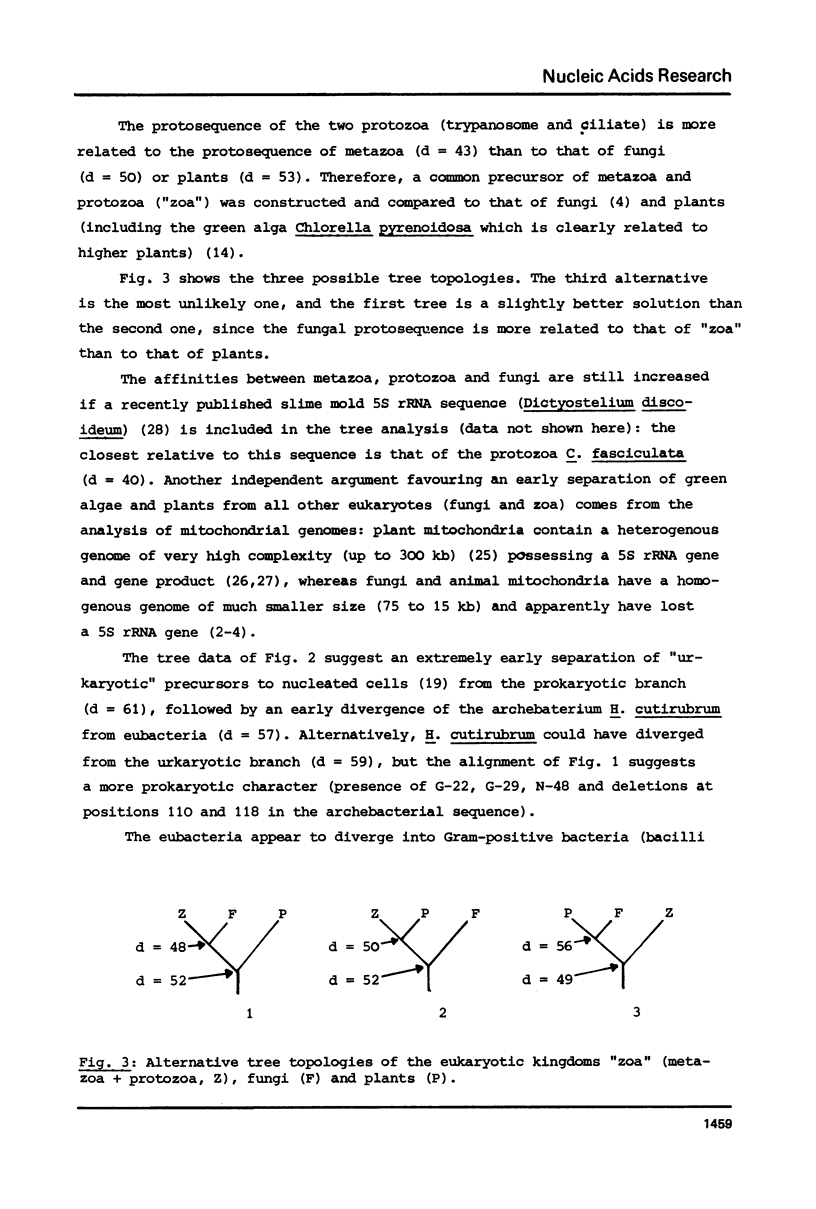
A phylogenetic tree was constructed by computer analysis of 47 completely determined 5S rRNA sequences. The wheat mitochondrial sequence is significantly more related to prokaryotic than to eukaryotic sequences, and its affinity to that of the thermophilic Gram-negative bacterium Thermus aquaticus is comparable to the affinity between Anacystis nidulans and chloroplastic sequences. This strongly supports the idea of an endosymbiotic origin of plant mitochondria. A comparison of the plant cytosol and chloroplast sub-trees suggests a similar rate of nucleotide substitution in nuclear genes and chloroplastic genes. Other features of the tree are a common precursor of protozoa and metazoa, which appears to be more related to the fungal than to the plant protosequence, and an early divergence of the archebacterial sequence (Halobacterium cutirubrum) from the prokaryotic branch.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benhamou J., Jourdan R., Jordan B. R. Sequence of Drosophila 5S RNA synthesized by cultured cells and by the insect at different developmental stages. Homogeneity of the product and homologies with other 5S RNA's at the level of primary and secondary structure. J Mol Evol. 1977 May 13;9(3):279–298. doi: 10.1007/BF01796116. [DOI] [PubMed] [Google Scholar]
- Bonen L., Gray M. W. Organization and expression of the mitochondrial genome of plants I. The genes for wheat mitochondrial ribosomal and transfer RNA: evidence for an unusual arrangement. Nucleic Acids Res. 1980 Jan 25;8(2):319–335. doi: 10.1093/nar/8.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Cedergren R. J., LaRue B., Sankoff D., Lapalme G., Grosjean H. Convergence and minimal mutation criteria for evaluating early events in tRNA evolution. Proc Natl Acad Sci U S A. 1980 May;77(5):2791–2795. doi: 10.1073/pnas.77.5.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham R. S., Bonen L., Doolittle W. F., Gray M. W. Unique species of 5S, 18S, and 26S ribosomal RNA in wheat mitochondria. FEBS Lett. 1976 Oct 15;69(1):116–122. doi: 10.1016/0014-5793(76)80666-7. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., Friend D. A. Comparison of cytoplasmic and mitochondrial 4 S RNA from cultured hamster cells: physical and metabolic properties. J Mol Biol. 1972 Nov 14;71(2):163–175. doi: 10.1016/0022-2836(72)90344-0. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Anderson S., Nierlich D. P. Distinctive sequence of human mitochondrial ribosomal RNA genes. Nature. 1980 Jul 31;286(5772):460–467. doi: 10.1038/286460a0. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Woese C. R. The architecture of 5S rRNA and its relation to function. J Mol Evol. 1975 Oct 3;6(1):61–76. doi: 10.1007/BF01732674. [DOI] [PubMed] [Google Scholar]
- Hori H. Evolution of 5sRNA. J Mol Evol. 1975 Dec 31;7(1):75–86. doi: 10.1007/BF01732181. [DOI] [PubMed] [Google Scholar]
- Hori H. Molecular evolution of 5S RNA. Mol Gen Genet. 1976 May 7;145(2):119–123. doi: 10.1007/BF00269583. [DOI] [PubMed] [Google Scholar]
- Hori H., Osawa S. Evolutionary change in 5S RNA secondary structure and a phylogenic tree of 54 5S RNA species. Proc Natl Acad Sci U S A. 1979 Jan;76(1):381–385. doi: 10.1073/pnas.76.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Osawa S., Iwabuchi M. The nucleotide sequence of 5S rRNA from a cellular slime mold Dictyostelium discoideum. Nucleic Acids Res. 1980 Dec 11;8(23):5535–5539. doi: 10.1093/nar/8.23.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Ohta T. Eukaryotes-prokaryotes divergence estimated by 5S ribosomal RNA sequences. Nat New Biol. 1973 Jun 13;243(128):199–200. doi: 10.1038/newbio243199a0. [DOI] [PubMed] [Google Scholar]
- Leaver C. J., Harmey M. A. Isolation and characterization of mitochondrial ribosomes from higher plants. Biochem J. 1972 Sep;129(3):37P–38P. doi: 10.1042/bj1290037p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizardi P. M., Luck D. J. Absence of a 5S RNA complnent in the mitochondrial ribosomes of Neurospora crassa. Nat New Biol. 1971 Feb 3;229(5):140–142. doi: 10.1038/newbio229140a0. [DOI] [PubMed] [Google Scholar]
- MacKay R. M., Gray M. W., Doolittle W. F. Nucleotide sequence of Crithidia fasciculata cytosol 5S ribosomal ribonucleic acid. Nucleic Acids Res. 1980 Nov 11;8(21):4911–4917. doi: 10.1093/nar/8.21.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechulla B., Hahn U., McLaughlin L. W., Küntzel H. Nucleotide sequence of 5S ribosomal RNA from Aspergillus nidulans and Neurospora crassa. Nucleic Acids Res. 1981 Mar 25;9(6):1445–1450. doi: 10.1093/nar/9.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoff D., Cedergren R. J., Lapalme G. Frequency of insertion-deletion, transversion, and transition in the evolution of 5S ribosomal RNA. J Mol Evol. 1976 Mar 29;7(2):133–149. doi: 10.1007/BF01732471. [DOI] [PubMed] [Google Scholar]
- Schwartz R. M., Dayhoff M. O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science. 1978 Jan 27;199(4327):395–403. doi: 10.1126/science.202030. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Taylor M. W., Brock T. D. Thermal stability of ribosomes and RNA from Thermus aquaticus. Biochim Biophys Acta. 1970 Apr 15;204(2):512–520. doi: 10.1016/0005-2787(70)90171-1. [DOI] [PubMed] [Google Scholar]


