Abstract
Background & Aims
The fluid secretion model predicts that intestinal obstruction disorders can be alleviated by promoting epithelial Cl− secretion. The cAMP-activated anion channel CFTR mediates Cl−-dependent fluid secretion in the intestine. Although the role of the ClC-2 channel has not been determined in the intestine, this voltage-gated Cl− channel might compensate for the secretory defects observed in patients with cystic fibrosis and other chronic constipation disorders. We investigated whether mice that lack ClC-2 channels (Clcn2−/−) have defects in intestinal ion transport.
Methods
Immunolocalization and immunoblot analyses were used to determine the cellular localization and the amount of ClC-2 expressed in mouse early (EDC) and late distal colon (LDC). Colon sheets from wildtype and Clcn2−/− littermates were mounted in Ussing chambers to determine transepithelial bioelectrical parameters and Na+, K+ and Cl− flux.
Results
Expression of ClC-2 was higher in the basolateral membrane of surface cells in the EDC, compared to the LDC, with little expression in crypts. Neither cAMP nor Ca2+-induced secretion of Cl− was affected in the EDC or LDC of Clcn2−/− mice, whereas the amiloride-sensitive short circuit current (ISC) was increased approximately 3-fold in Clcn2−/− EDC, compared to that of control littermates. Conversely, electroneutral Na+, K+ and Cl− absorption were dramatically reduced in colons of Clcn2−/− mice.
Conclusions
Basolateral ClC-2 channels are required for colonic electroneutral absorption of NaCl and KCl. The increase in the amiloride-sensitive ISC in Clcn2−/− mice revealed a compensatory mechanism that is activated in the colons of mice that lack the ClC-2 channel.
Keywords: NaCl absorption, KCl absorption, fluid secretory defects, mouse model, intestinal epithelial cells
Introduction
Cystic fibrosis (CF) is the most common genetic disease in Caucasians, occurring in approximately one out of 3,200 live births 1. CF is caused by mutations in the CFTR gene, which encodes an apical cAMP-activated anion channel in epithelial tissues. CF disease has been linked to Cl−, bicarbonate and fluid secretory defects in several organs, such as exocrine glands, gastrointestinal tract and airways 1–3. It has been reasoned that activation of an alternative epithelial Cl− efflux pathway might alleviate the secretion defect in CF. Mouse airway and exocrine glands possess a Ca2+-activated fluid secretory process independent of CFTR 4, 5 that is thought to rely on apical Ca2+-activated Cl− channels (CaCC) to promote Cl− efflux into the luminal space. The CaCC in many epithelia is encoded by the Tmem16A gene, a member of the newly discovered TMEM16 family of Ca2+-activated Cl− channels 6–9. Although it has been suggested that apical Ca2+-activated Cl− channels are directly involved in intestinal Cl− secretion 10, other studies failed to demonstrate a connection between Ca2+-activated Cl− channels and intestinal secretion 11, 12. It appears that activation of the basolateral Ca2+-activated K+ channel KCNN4 during Ca2+-activated intestinal secretion hyperpolarizes intestinal epithelial cells, and consequently, generates a greater driving force for CFTR-mediated Cl− efflux 12. Indeed, if CFTR is not first activated, a rise in intracellular Ca2+ does not cause Cl− secretion 11, 13, 14.
It has been proposed that ClC-2, one of the four plasma membrane-associated Cl− channels in the mammalian CLC family 15, may substitute for CFTR in CF airway cells 16. ClC-2 has also been suggested to mediate Cl− secretion in the mouse small intestine 17, where it was reported to localize to the apical pole and tight junction complex. Conversely, ClC-2 channels are not expressed in the crypt cells of guinea pig distal colon, but in the basolateral membrane of the NaCl absorbing surface epithelium. A Cl− conductance with ClC-2-like rectification and selectivity properties was detected in surface epithelium cells, consistent with the basolateral subcellular distribution of this channel 18–20. It has been argued that transepithelial transport defects are responsible for blindness and male infertility in Clcn2 knockout mice 21, 22. Moreover, double Clcn2 and CFTR knockout mice do not exhibit a worsening of the CF phenotype, indirectly suggesting that ClC-2 does not contribute to fluid secretion 23. This is consistent with the basolateral localization of ClC-2 channels in mouse intestine 24.
NaCl absorption in the intestinal tract occurs by either an electrogenic or electroneutral mechanism depending on the gut segment and species 25. In the mouse colon, the bulk of NaCl absorption occurs across the luminal membrane via the electroneutral Na+/H+ and Cl−/HCO3− exchangers 25. Na+ is subsequently extruded to the serosal side by the basolateral Na+-K+ ATPase, while a basolateral K+ channel recycles K+ ions to the serosa. Finally, a basolateral Cl− efflux pathway completes the molecular machinery involved in electroneutral NaCl absorption. The molecular identity of the basolateral Cl− efflux pathway has not yet been elucidated, but it has been suggested that basolateral ClC-2 channels may mediate this Cl− efflux to the serosal side 19.
The aim of this study was to determine the physiological roles of ClC-2 in mouse colonic epithelium. Immunohistochemical localization and immunoblotting studies found that ClC-2 is differentially expressed along the distal colon, i.e. the amount of plasma membrane ClC-2 channels in surface cells was less in the late than the early distal colon. Consistent with this distinction, mouse distal colon was subdivided into an early (EDC) and late (LDC) distal colon for functional analysis. As noted in a previous report 23, we found that mice lacking ClC-2 channels did not display impaired electrogenic Cl− secretion, consistent with CFTR being the primary, if not the only, channel responsible for Cl− secretion in the colon. Amiloride-sensitive ISC, an index of epithelial Na+ channel ENaC activity, increased ~3 fold in the Clcn2−/− EDC compared to wildtype littermates. In addition to the increased ENaC activity in the Clcn2−/− EDC, unidirectional Na+, K+ and Cl− fluxes studies showed that electroneutral absorption of NaCl and KCl was severely impaired in the Clcn2−/− EDC. Taken together, our results demonstrate that ClC-2 channels are involved in Cl− absorption rather than Cl− secretion in the distal colon. Consequently, ClC-2 specific blockers might be effective agents for treating constipation by reducing NaCl and water absorption in the colon.
Materials and Methods
General procedures
Clcn2−/− mice were generated as previously described 22. Clcn2−/− and wildtype littermates of 2–6 months old were used in experimental procedures approved by the NIDCR Animal Care and Use Committee, University of Rochester Animal Resources Committee and CECs IACUC. Reagents were from Sigma-Aldrich unless otherwise indicated.
Intestinal tissue isolation and Ussing chamber experiments
Mice were anesthetized with CO2 gas and killed by exsanguination via cardiac puncture. A segment of distal colon about 2 cm in length was excised and divided in two pieces. We defined these segments as EDC and LDC, respectively. The EDC and LDC segments (each 1 cm length) were located 1.5 cm and 0.5 cm from the anus, respectively. Segments were rinsed with 0.9% NaCl and cut open lengthwise, and then the muscular layer was partially stripped by scraping with a glass microscope slide to create a mucosal sheet 26. The resulting mucosal sheet was mounted on a tissue-holding slide (0.1 cm2) and placed in an Ussing chamber (Physiologic Instruments). Silicone grease was used to prevent edge damage. The solution bathing the apical and basolateral hemi-chambers consisted of (in mM): 115 NaCl, 5 KCl, 25 NaHCO3, 1 CaCl2 and 2 MgCl2, at pH 7.4. Indomethacin (5 μM) was added to the serosal side to prevent endogenous cAMP production via prostaglandin E2 release 13, 14. Solutions were gassed with 95% O2/5% CO2. The temperature was kept at 37°C using a water jacket system. Current pulses were generated with pClamp 8 software (Molecular Devices) and the voltage and current signals were acquired using a digidata 1320A interface (Molecular Devices). VTE was measured in the current clamp mode using an EVC4000 amplifier (World Precision Instruments). At 8 s intervals, a 10 μA current pulse was injected. RTE was calculated from the voltage deflection created by the current injection (ΔVTE). The equivalent ISC (expressed as μEq*h−1cm−2) was calculated from VTE and RTE according to Ohm’s law.
Na+, K+ and Cl− fluxes in isolated colonic strips
Na+ and Cl− fluxes were measured using 22Na+ and 36Cl− as tracers. Colonic tissue was prepared and mounted in an Ussing chamber as described above. Voltage clamp recordings were acquired using a VCC MC2 amplifier and AD converter (Physiologic Instruments). RTE was calculated by clamping the potential to ±10 mV at 15 s intervals. Radioisotopes were added 30 minutes after equilibration. Mucosal to serosal fluxes were measured 27 by supplementing the bicarbonate buffered solution in the apical side with 2 μCi of 22Na+ (PerkinElmer) and 2 μCi of 36Cl− (American Radiolabeled Chemicals). For serosal to mucosal flux experiments 4 μCi of 22Na+ and 4 μCi of 36Cl− were added to the serosal chamber. An aliquot of the side containing the radioisotopes was taken at the beginning of each experiment, followed by four aliquots from the opposite side at 30 min intervals. To inhibit ENaC-mediated Na+ uptake, 10 μM amiloride was used on the apical side and 1 μM tetrodotoxin on the basolateral side to inhibit any remaining enteric neuronal activity. Radioactivities were measured in a Tri-Carb 2100 TR Liquid Scintillation Analyzer (PerkinElmer) and Cobra Auto-Gamma counter (PerkinElmer). The measured beta counts were corrected for the 22Na+ contribution by calculating and applying a beta spill-over factor calculated with pure 22Na+ isotope standard to obtain 36Cl− activity. 22Na+ radioactivity was taken directly from gamma-counting readings 28.
For mucosa to serosa K+ flux measurements, serosal K+ was replaced by Rb+. For serosa to mucosa K+ flux measurements, mucosal K+ was replaced by Rb+. 250 μL aliquots were taken every 30 minutes, diluted 20 times in a solution containing 500 ppm (mg/L) CsCl and K+ concentrations were measured by atomic absorption spectrometry using a K-Na lamp (AAnalyst 200 Atomic Absorption spectrophotometer; PerkinElmer). Calibrations for potassium were performed by linear regression analysis using 0.25, 0.5 and 1 ppm as standards. To calculate net fluxes, tissues used for mucosa to serosa and serosa to mucosa experiments with similar RTE were paired. Fluxes are expressed as μEq*h−1cm−2.
Tissue processing and immunohistochemistry studies
Immunolocalization studies on EDC and LDC segments were performed as described previously 24. A rabbit polyclonal antibody (1:100 dilution) directed to the C-terminus of ClC-2 (Alomone) was used.
Western blot analysis
EDC and LDC colonic cells were isolated by adding a Ca2+-free solution into the luminal space as described previously 18, except that the incubation step with Ca2+-free solution was increased from 3 minutes to 15 minutes. Colon cells were pooled from wildtype or Clcn2−/− mice (two females and two males per group) prior to biotinylation 29. EDC and LCD cell lysates and plasma membrane fractions (50 μg/lane) were used for analysis as described elsewhere 29 using the same antibody as for immunolocalization studies (1:200 dilution). Densitometric analysis of band intensities was performed using Adobe Photoshop CS3 (Adobe Systems Incorporated).
qPCR studies
Total RNA from wildtype and Clcn2 −/− mice (two females and two males per group) was isolated using RNeasy columns (Qiagen). Two μg of total RNA was transcribed to cDNA using Maxima® First Strand cDNA Synthesis kit (Fermentas). Expression levels of α-ENaC, β-ENaC, γ-ENaC and Clcn2 were evaluated by analysis of relative gene expression using SYBR Green based quantitative PCR (qPCR) by ACGT, Inc. (Wheeling, IL). Expression levels of the target genes were compared to those of three housekeeping genes (GAPDH, β-actin and cyclophilin) in pooled samples. The qPCR primer sets used in this study are provided in supplemental table 1.
Quantification of plasma aldosterone
Mice were anesthetized by intraperitoneal chloral hydrate injection (400 mg/kg) and the blood was collected by cardiac puncture. Blood samples from wildtype and Clcn2 −/− littermate mice (three females and three males per group) were centrifuged at 3000 rpm for 3 minutes and serum samples were stored at −86 °C until further analysis. Aldosterone serum levels were determined from pooled samples by radioimmunoassay by Ani Lytics Incorporated (Gaithersburg, MD).
Results
Differences in electrogenic Na+ absorption along the distal colon of wildtype and Clcn2−/− mice
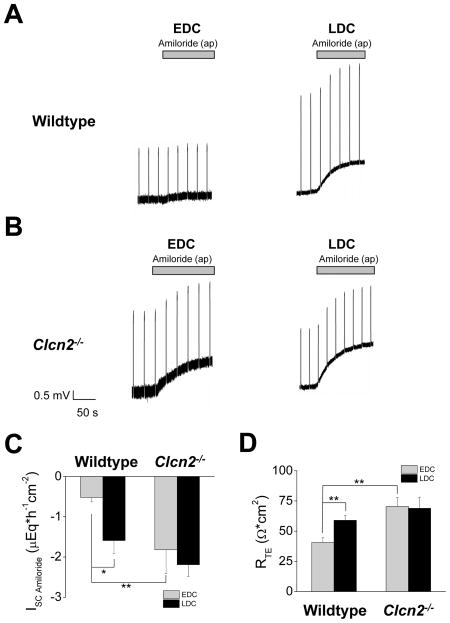
Two segments of distal colon per mouse, EDC and LDC, were mounted in Ussing chambers and their bioelectric properties compared. In the wildtype mouse, LDC displayed a robust amiloride-sensitive ISC, whereas the amiloride-sensitive ISC in the EDC was markedly lower (Figure 1A). Similar measurements using tissues from Clcn2−/− mice suggest that the amiloride-sensitive ISC was enhanced in Clcn2−/− EDC compared to wildtype EDC, whilst that of LDC did not seem changed (Figure 1B). A summary of these results (Figure 1C) shows a statistically significant ~3-fold increase in ISC of EDC from Clcn2−/− animals, but not LDC. Figure 1D shows that the RTE was also less in the EDC compared to the LDC of wildtype animals. The RTE was increased in the EDC of mice lacking ClC-2 compared to their controls littermates (Figure 1D).
Figure 1. Amiloride-sensitive ISC and RTE in EDC and LDC.
Representative current clamp experiments performed in EDC and LDC from wildtype (Panel A) and Clcn2−/− (Panel B) epithelial sheets showing transepithelial potentials before and after addition of 10 μM amiloride to the mucosal side. C. Calculated amiloride-sensitive ISC in EDC and LDC from wildtype and Clcn2−/− mice. D. Transepithelial resistance values for EDC and LDC from wildtype and Clcn2−/− mice before addition of amiloride. Indomethacin (5 μM) was present in the serosal side. Values are given as mean ± SEM from n=10 per experimental condition, except for Clcn2−/− EDC (n=9). (*), p<0.05, t test. (**), p<0.01, t test between different and same genotypes, respectively.
Cl− Secretion is not affected in the mouse distal colon lacking ClC-2 channels
In view of the differences in electrogenic Na+ absorption in the colon of wildtype and Clcn2−/− animals, we designed experiments to test whether wildtype EDC and LDC display differences in electrogenic Cl− secretion, and whether these are affected upon Clcn2 gene ablation. It has been demonstrated that colonic Cl− secretion can be activated by an intracellular increase in cAMP or Ca2+ 25. cAMP and Ca2+ induced Cl− secretion mechanisms are both critically dependent on the presence of active apical CFTR channels. Because CFTR channels are gated by an increase in intracellular cAMP, Ca2+-mediated Cl− secretion requires pre-activation of the CFTR channel by cAMP 13. Considering the dependence of the Ca2+-mediated process on intracellular cAMP, Ussing chambers experiments to examine Cl− secretion in EDC and LDC used a sequential cAMP and then Ca2+ mobilization protocol. cAMP-dependent secretion was induced by addition of 10 μM forskolin and 100 μM IBMX to the serosal side. In order to quantify Ca2+-induced Cl− current, cAMP-activated Cl− secretion was then abolished by blocking the basolateral cAMP-activated KCNQ1/KCNE3 K+ channel with 10 μM serosal chromanol 293B, which does not affect CFTR channels. Ca2+-mediated secretion was then stimulated by serosal addition of 50 μM carbachol.
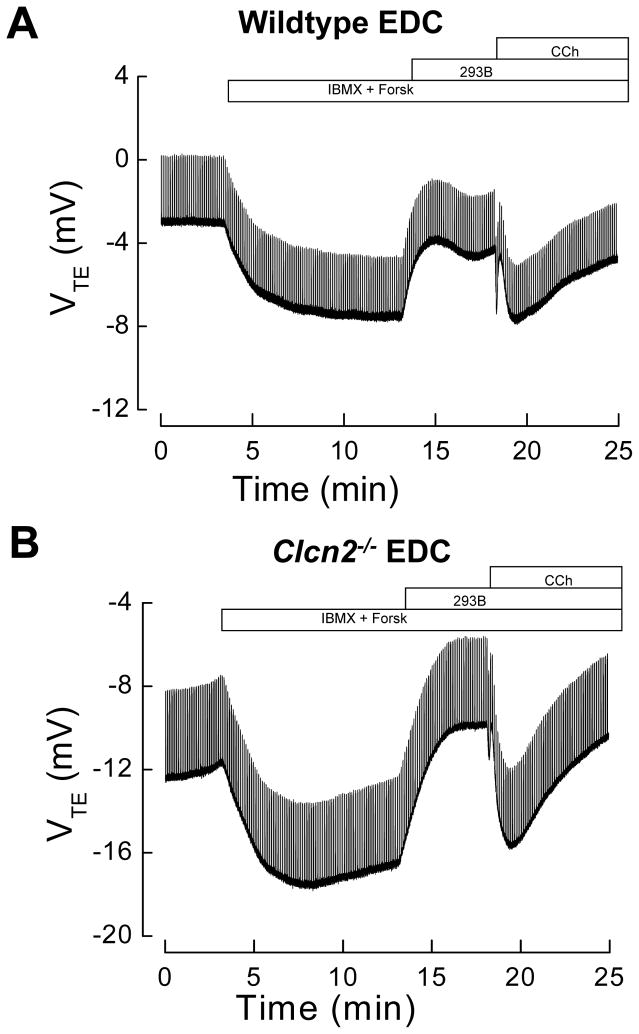
Figure 2 shows the changes in the VTE elicited by addition of secretagogues to the serosal aspect of EDC from wildtype and Clcn2−/− mice. 10 μM amiloride was present in the luminal solution throughout the experiment to block the ENaC-mediated Na+ absorption. The VTE became more negative upon addition of secretagogues (luminal side respect to serosal side), consistent with an increased Cl− secretion into the lumen. As seen in Figures 2A and 2B, EDC from wildtype and Clcn2−/− animals displayed both cAMP- and Ca2+-mediated Cl− secretion. Neither cAMP- nor Ca2+-dependent Cl− secretion was affected in EDC segments in the Clcn2−/− mice compared to wildtype littermates. Table 1 summarizes the values obtained for cAMP- and Ca2+-mediated Cl− colonic secretion in wildtype and Clcn2−/− mice EDC and similar experiments performed in LDC. It was found that cAMP-dependent secretion was significantly higher in LDC than in EDC, but neither cAMP- nor Ca2+-dependent secretion was affected by Clcn2 gene ablation. Similar results were obtained when chromanol 293B was omitted and carbachol was added after stimulation with 10 μM forskolin and 100 μM IBMX (not shown).
Figure 2. cAMP- and Ca2+-activated Cl− secretion in EDC from wildtype and Clcn2−/− mice.
Cl− secretion was elicited by stimulating an increase in cAMP (100 μM IBMX +10 μM Forsk) and Ca2+ (50 μM CCh) in wildtype EDC (Panel A, n=10) and Clcn2−/− EDC (Panel B, n=9). Before addition of CCh, 10 μM chromanol 293B was added to the serosal side to block basolateral cAMP-activated K+ channels, and consequently, cAMP-induced Cl− secretion. Indomethacin (5 μM) was present in the serosal side. Representative experiments are shown in panels A and B.
Table 1.
| Wildtype | Clcn2−/− | |||
|---|---|---|---|---|
| EDC | LDC | EDC | LDC | |
| Basal ISC (μEq*h−1cm−2) | −7.0 ± 1.3 (n=10) | −4.7 ± 0.8 (n=10) | −9.7 ± 0.9 (n=9) | −5.1 ± 1.0** (n=10) |
| Δ IIBMX+Forsk (μEq*h−1cm−2) | −6.0 ± 0.5 (n=10) | −8.7 ± 0.6** (n=10) | −6.2 ± 1.5 (n=9) | −11.6 ± 3.4 (n=10) |
| Δ I293B (μEq*h−1cm−2) | 5.3 ± 0.9 (n=10) | 5.8 ± 0.7 (n=10) | 6.3 ± 1.1 (n=9) | 7.5 ± 2.4 (n=10) |
| Δ ICCh (μEq*h−1cm−2) | −9.5 ± 1.9 (n=10) | −11.2 ± 1.0 (n=10) | −7.1 ± 1.7 (n=9) | −12.3 ± 3.6 (n=10) |
ΔISC values for Ussing chamber experiments. Summary of Ussing chamber experiments performed on EDC and LDC from wildtype and Clcn2−/− mice. Data are means ± SEM (**), p <0.01 between EDC and LDC in the same genotype, respectively (t test).
Serum aldosterone and ENaC expression levels in wildtype and Clcn2−/− mice
ENaC channels are expressed in the apical membrane of absorptive colonocytes. ENaC channels are heteromultimers composed of α, β and γ subunits and their expression is modulated by the steroidal hormone aldosterone 30. Because the amiloride-sensitive ISC was enhanced in the EDC of mice lacking ClC-2, we tested whether the serum aldosterone level was also increased in the Clcn2−/− mice. However, serum aldosterone was unaffected in Clcn2−/− mice (wildtype = 1276 ± 264 pg/mL (n=6); Clcn2−/− = 1031 ± 95 pg/mL (n=6); p> 0.4, t test). Aditionally, qPCR analysis showed that there were no significant differences in the expression levels of α, β and γ ENaC subunit transcripts in the EDC from wildtype and Clcn2−/− mice (Supplementary Table 2).
Electroneutral absorption of NaCl and KCl is markedly decreased in the early distal colon of mice lacking ClC-2 channels
The enhanced amiloride-sensitive ISC in the Clcn2−/− EDC may reveal the activation of an electrogenic mechanism that could compensate for the loss of a ClC-2 channel-dependent electroneutral Cl− absorption. To test the hypothesis that electroneutral Cl− absorption is dependent upon ClC-2 channels, we measured unidirectional Na+ and Cl− fluxes in EDC sheets mounted in Ussing chambers maintained under short circuit conditions (VTE=0 mV). Consistent with ClC-2 channels playing a physiological role in electroneutral Cl− absorption by the EDC, Figure 3 shows that mucosa to serosa fluxes (JMS) for both Na+ and Cl− were significantly decreased in the Clcn2−/− EDC compared to that of wildtype littermates. In contrast, serosa to mucosa fluxes (JSM) for both Na+ and Cl− were enhanced in Clcn2−/− EDC. Consequently, Na+ and Cl− net fluxes (JNet=JMS-JSM) showed that a substantial electroneutral NaCl absorption takes place in wildtype EDC, whereas this absorptive process was essentially abolished in the Clcn2−/− EDC (Figures 3A and 3B).
Figure 3. Unidirectional and net Na+, Cl− and K+− fluxes in wildtype and Clcn2−/− EDC.
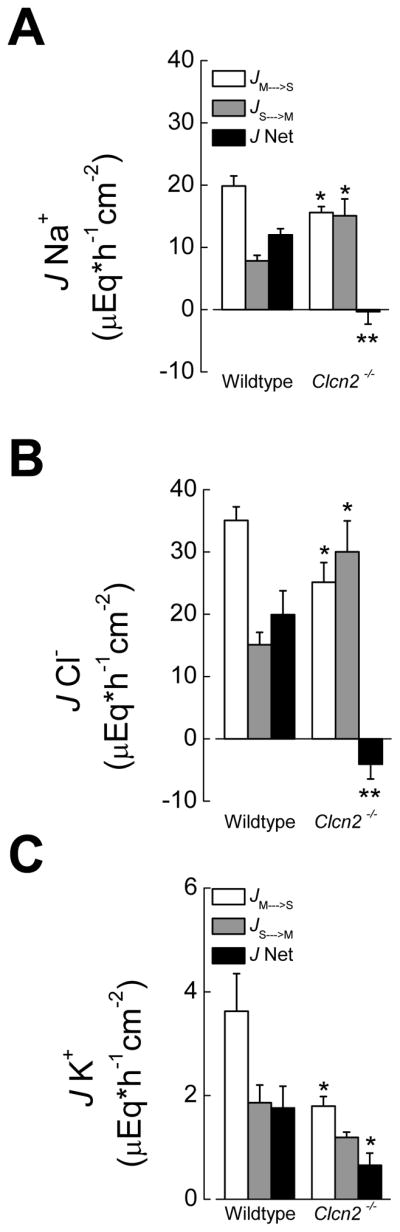
JMS, JSM and Jnet for Na+ (A), Cl− (B) and K+ (C) fluxes studies performed under short circuit conditions. Values are given as mean ± SEM; comparison of the same condition between wildtype and Clcn2−/− mice where (*), p<0.05, and (**), p<0.01, t test. Amiloride (10 μM) was present in the apical side and 1 μM tetrodotoxin in the basolateral side.
As chloride fluxes across the EDC were higher than sodium fluxes we explored the possibility that an additional Na+-independent electroneutral Cl− flux might be present. One such flux could correspond to a K+ absorptive process mediated by apical membrane H+/K+ exchange and basolateral K+ exit through KCl cotransport and/or K+ channels 25, where Cl− efflux through ClC-2 might be involved.
To test this possibility we studied potassium fluxes in the EDC. Our measurements indicate that there is indeed a significant mucosa to serosa movement of K+ across wildtype EDC that was severely decreased in the epithelium from Clcn2−/− animals (Figure 3C). This absorptive K+ flux was Cl−-dependent, since it was significantly lowered when Cl− was reduced from 135 mM to 10 mM by replacement with gluconate in the wildtype EDC. JMS K+ was decreased from 3.6 ± 0.7 μEq*h−1cm−2 (n=5) in control to 2.1 ± 0.3 μEq*h−1cm−2 (n=5) in low Cl− conditions (p<0.05, t test).
ClC-2 expression varies along the mouse distal colon
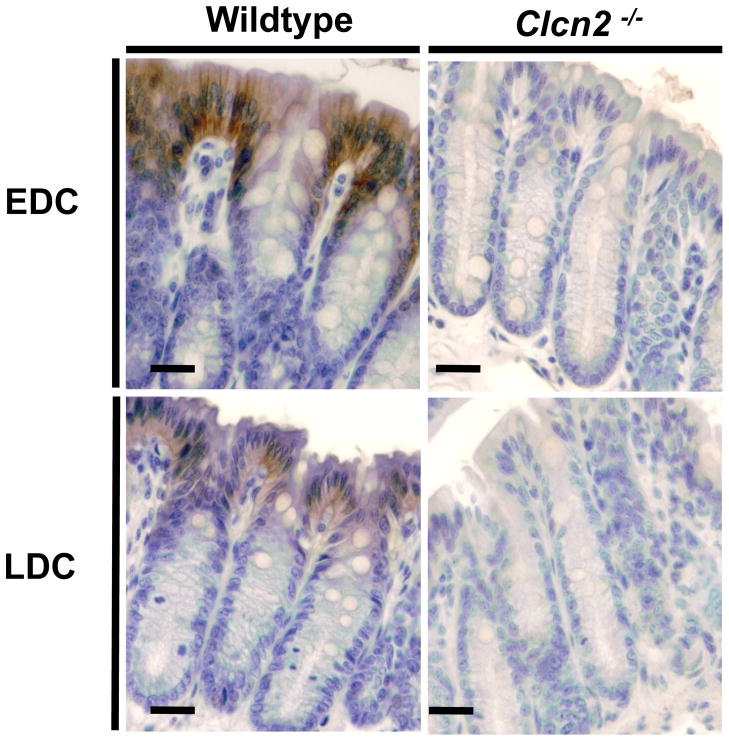
The impairment of electroneutral NaCl and KCl absorption in the Clcn2−/− EDC suggests that ClC-2 channels act as a basolateral Cl− efflux pathway in electroneutral Cl− absorption. It would be reasonable to expect that changes in tissue abundance of ClC-2 might correlate with the observed rates in electroneutral transepithelial Cl− transport. To test this possibility, ClC-2 immunolocalization experiments were performed. Figure 4 shows that the ClC-2-associated immunoreactivity was confined to the surface epithelium of both EDC and LDC (left panels) with no apparent staining in the crypt epithelium.
Figure 4. ClC-2 channels are expressed in the mouse distal colon surface epithelium.
Immunolocalization studies performed in EDC (upper panels) and LDC (lower panels) from wildtype (left panels) and Clcn2−/− (right panels) mice. Bars = 50 μm.
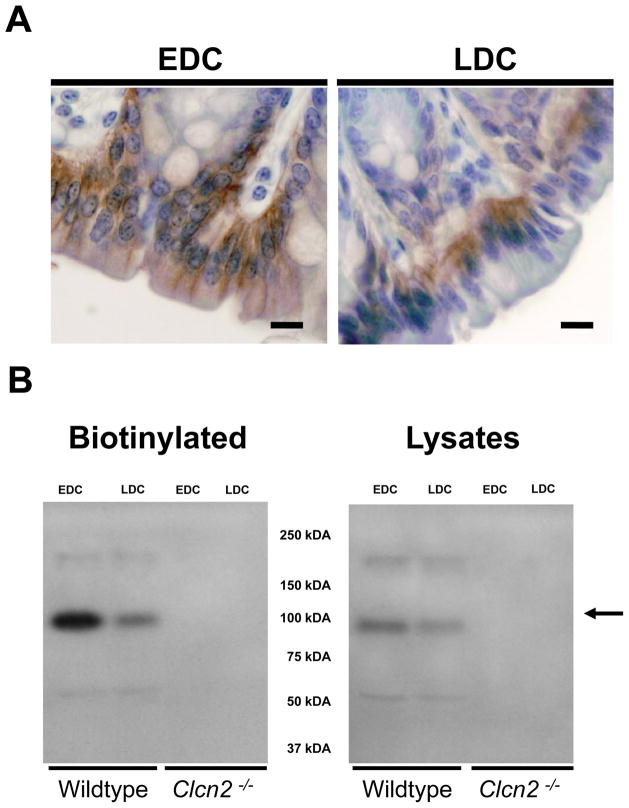
At the subcellular level, we found pronounced differences between the EDC and LDC in the distribution of ClC-2 channels: most of the ClC-2-associated immunostaining was present at the basolateral pole of surface cells in the EDC (left panel, Figure 5A), whereas immunostaining was mostly intracellular in the LDC (right panel, Figure 5A). Further, densitometric analysis of western blot assays performed on biotinylated plasma membrane proteins showed that the amount of ClC-2 protein in EDC was ~2 fold higher than in LDC (left panel, Figure 5B). In contrast, densitometric analysis of western blots from whole cell lysates confirmed that ClC-2 channel protein expression in EDC was comparable to that in the LDC (right panel, Figure 5B). Consistent with this latter observation, qPCR analysis of Clcn2 transcripts showed that there was no difference between EDC and LDC expression (Supplementary table 3) when normalized to three different housekeeping genes (β-actin, GAPDH and cyclophilin).
Figure 5. Differential cellular expression of ClC-2 along the mouse distal colon.
A. Higher magnification of the surface epithelium from wildtype EDC (left panel) and LDC (right panel). Bars = 20 μm. B. Western blot assays for ClC-2 protein abundance using biotinylated plasma membrane (left panel) and whole cell lysates (right panel) in the EDC and LDC from wildtype and Clcn2−/− mice.
There was a strong β-actin immunoreactive signal in whole cell lysates, while no β-actin immunoreactivity was detected in the biotinylated proteins associated with the plasma membranes (Supplemental Figure 1). The lack of immunoreactivity in the biotinylated fraction indicates that there is little contamination of the plasma membrane preparation by intracellular proteins. The specificity of the ClC-2 antibody in both immunolocalization (Figure 4) and western blot (Figure 5) assays was confirmed by the absence of immunoreactivity in tissue and protein samples from the Clcn2−/− colon.
Conclusions
Colonic epithelium displays both absorptive and secretory functions. Transcellular Cl− fluxes are involved in both of these physiological processes. NaCl and water secretion takes place mainly in the crypts in response to secretagogues 31. CFTR Cl− channel activity constitutes the rate-limiting step in intestinal fluid secretion. The importance of the CFTR channel to intestinal fluid secretion can be inferred from the severe intestinal obstructive disorders associated with CF, such as meconium ileus in newborns and intestinal obstruction in CF patients at later ages 31. Variability in the severity of the intestinal phenotype 10 could be due to compensatory changes in the expression of other channels or transporters. In that regard, it has long been postulated that an alternative apical Cl− pathway could compensate for the epithelial hyposecretory defects associated with the CF phenotype. Schwiebert et al. postulated that ClC-2 Cl− channels might compensate for the lack of functional CFTR channels in CF airway epithelium 16. Gyömörey et al. also suggested a secretory role for ClC-2 channels in the mouse small intestine 17. Moreover, it has been postulated that lubiprostone, a prostaglandin E1-derived compound used for chronic constipation treatment, induces Cl− secretion by directly activating ClC-2 channels 32. However, recent work by Bijvelds et al. clearly shows that intestinal Cl− secretion induced by lubiprostone requires CFTR channels 33, supporting previous observations where lubiprostone was linked to activation of prostanoid receptors which increases intracellular cAMP levels 34.
Mice lacking ClC-2 channels do not show apparent intestinal defects, although the role of ClC-2 channels in intestinal ion transport has not been clearly established 21–23. In the present study, we found that neither cAMP- nor Ca2+-mediated Cl− secretion depends on ClC-2 expression in mouse EDC and LDC epithelia. These results are similar to those reported previously in the distal colon of Clcn2−/− mice 23. It is important to note that this last study also characterized Cl− secretion in double mutant mice lacking ClC-2 channels and expressing the ΔF508 CFTR mutation. Surprisingly, the double targeted mice survived better than the CFTRΔF508/ΔF508 mice, apparently by partially reversing the obstructive intestinal defect displayed by the CFTRΔF508/ΔF508 mice 23. This observation is consistent with ClC-2 in the colon playing a major role in NaCl and fluid absorption, but not fluid secretion.
Colonic surface epithelium plays a major role in NaCl and fluid absorption. It is generally accepted that secretion and absorption in the intestine are spatially separated along the surface-crypt axis. Absorptive processes are located in surface epithelial cells, whereas fluid secretion takes place in the crypts 35, although there is evidence that some NaCl absorption might also occur in colonic crypts 36. We found that ClC-2 channels are expressed in the colonic surface epithelium, consistent with ClC-2 playing a role in Cl− absorption. Indeed, our Na+ and Cl− net flux measurements demonstrate that electroneutral NaCl absorption is essentially eliminated in the colon of Clcn2−/− mice. We also found that electroneutral net Na+ absorptive flux is substantially higher than the amiloride-sensitive ISC in the wildtype colon (>10 fold), supporting the concept of electroneutral NaCl absorption as the main mechanism by which Na+ and Cl− ions are reabsorbed in the EDC.
Cl− JMS fluxes were higher than Na+ JMS fluxes, suggesting that there is an additional electroneutral pathway for Cl− entry. Indeed, we found that a Cl−-dependent K+ absorption process takes place in the EDC and is severely affected in the Clcn2−/− mice. Together, it seems that ClC-2 channels act as a basolateral Cl− efflux pathway in the mouse EDC. However, absorptive Na+, K+ and Cl− fluxes (JMS) are not abolished in the mouse lacking ClC-2 channels, suggesting that there is an alternative basolateral Cl−-efflux pathway. Basolateral KCC1 K+-Cl− cotransporters, which are involved in K+ absorption in the rat distal colon 37, may also contribute to Cl− efflux across the basolateral membrane in mouse colonic surface cells.
We also found that Na+ and Cl− JSM fluxes were enhanced in the Clcn2−/− EDC, probably because an alternative Cl− secretion pathway was unmasked upon Clcn2 gene disruption. NaCl absorption and secretion are regulated by signals arising from endocrine, autonomic nervous system and immune system 38. Other regulatory mechanisms may involve protein-protein interactions. For example, CFTR has been linked to the regulation of ENaC 39 and NHE3 40, which are apical membrane proteins that mediate Na+ absorption. Moreover, it has been suggested that KCNN4 K+ channels inhibit KCNMA1 K+ channels by a mechanism involving protein-protein interaction 41. By analogy with these data, one might speculate that the observed increase in Na+ and Cl− JSM fluxes in the Clcn2−/− EDC are related to the removal of a regulatory effect of ClC-2 on cellular or paracellular transport proteins. The higher RTE observed in Clcn2−/− EDC and small intestine 42 and the interaction of ClC-2 with other proteins 43 support this hypothesis. Furthermore, ClC-2 complexes with HSP90 to regulate channel activity 43 and ClC-2 is found in lipid-rich domains where it appears to cluster with other proteins 44.
The enhanced amiloride-sensitive ISC displayed by the EDC lacking ClC-2 resembles the compensatory mechanism observed in the distal colon of mouse deficient in the apical Na+/H+ exchanger NHE3. Nhe3−/− mice displayed more functional ENaC channels in the distal colon, in an apparent attempt to compensate for the absence of the primary apical NHE3-associated Na+ influx pathway 45. The enhanced ENaC activity observed in the EDC of mice lacking ClC-2 did not correlate with an increase in aldosterone levels or expression of ENaC transcripts. Thus, enhanced ENaC activity might be associated with increased targeting of intracellular ENaC to the membrane or to an increase in open probability of the channels already residing there. Whether ClC-2 can substitute for the CFTR channel in CF intestine ultimately depends on the cellular and subcellular location of the ClC-2 channel. If ClC-2 channels play a role in intestinal Cl− secretion, then an apical localization in the secretory crypt cells is expected. In contrast, our studies revealed that the ClC-2-associated immunostaining in the mouse distal colon was confined to the surface epithelium in both EDC and LDC. At the subcellular level, most of the ClC-2-associated immunolabeling was present at the basolateral membrane of the EDC, whereas the immunostaining appears to be mostly intracellular in the LDC. The diffuse intracellular pattern observed in the LDC resembles the intracellular pattern for ClC-2 in human sigmoid colon 20. Western blots of biotinylated plasma membrane proteins from EDC and LDC confirmed that ClC-2 protein in the plasma membrane of EDC was ~2-fold higher compared to LDC. On the other hand, the amount of ClC-2 protein from whole cell lysates was similar between EDC and LDC. Further qPCR analysis of Clcn2 transcripts in EDC and LDC confirmed that there is no difference in the expression levels of Clcn2 between EDC and LDC. We also found that cAMP-activated Cl− secretion and amiloride-sensitive ISC are higher in LDC compared to EDC, demonstrating that secretory and absorptive functions differ along the distal colon.
In summary, our results strongly support an important role for ClC-2 in colonic absorption rather than secretion. The absence of diarrhea in the Clcn2−/− mouse contrasts with the observation in Nhe3−/− animals. This might be due the more restricted localization of ClC-2 along the intestinal tract 24. Controlling ClC-2 function by the use of specific drugs might be of importance for intestinal disorders of fluid transport. On the other hand, the possible role of ClC-2 as regulator of other Cl− and Na+ permeation pathways by direct protein-protein interactions is an open field for future research.
Supplementary Material
Acknowledgments
Grant Support: This study was supported in part by the NIH Intramural Research Program, and research grants from NIH (DE09621) and Takeda Pharmaceuticals North America, Inc to JEM, and Fondecyt (11100408) to CAF. CECs is funded by Conicyt-PFB and Gobierno Regional de Los Ríos.
Abbreviations
- CFTR
cystic fibrosis transmembrane conductance regulator gene
- Clcn2
ClC-2 gene
- EDC
early distal colon
- LDC
late distal colon
- ISC
short circuit current
- RTE
transepithelial resistance
- VTE
transepithelial potential difference
Footnotes
Disclosures: The authors have no conflicts of interest.
Author Contributions: MAC, CAF, FVS and JEM designed the experiments. MAC, CAF, MGB and YZ performed the experiments and analyzed the data. MAC, CAF, FVS and JEM wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quinton PM. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79:S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 2.Knowles MR, Stutts MJ, Spock A, et al. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983;221:1067–70. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- 3.Kopelman H, Corey M, Gaskin K, et al. Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in the cystic fibrosis pancreas. Gastroenterology. 1988;95:349–55. doi: 10.1016/0016-5085(88)90490-8. [DOI] [PubMed] [Google Scholar]
- 4.Anderson MP, Gregory RJ, Thompson S, et al. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–5. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- 5.Clarke LL, Grubb BR, Gabriel SE, et al. Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. Science. 1992;257:1125–8. doi: 10.1126/science.257.5073.1125. [DOI] [PubMed] [Google Scholar]
- 6.Galietta LJ. The TMEM16 protein family: a new class of chloride channels? Biophys J. 2009;97:3047–53. doi: 10.1016/j.bpj.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ousingsawat J, Martins JR, Schreiber R, et al. Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. J Biol Chem. 2009;284:28698–703. doi: 10.1074/jbc.M109.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romanenko VG, Catalan MA, Brown DA, et al. Tmem16A encodes the Ca2+-activated Cl- channel in mouse submandibular salivary gland acinar cells. J Biol Chem. 2010;285:12990–3001. doi: 10.1074/jbc.M109.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreiber R, Uliyakina I, Kongsuphol P, et al. Expression and function of epithelial anoctamins. J Biol Chem. 2010;285:7838–45. doi: 10.1074/jbc.M109.065367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozmahel R, Wilschanski M, Matin A, et al. Modulation of disease severity in cystic fibrosis transmembrane conductance regulator deficient mice by a secondary genetic factor. Nat Genet. 1996;12:280–7. doi: 10.1038/ng0396-280. [DOI] [PubMed] [Google Scholar]
- 11.Strabel D, Diener M. Evidence against direct activation of chloride secretion by carbachol in the rat distal colon. Eur J Pharmacol. 1995;274:181–91. doi: 10.1016/0014-2999(94)00728-p. [DOI] [PubMed] [Google Scholar]
- 12.Flores CA, Melvin JE, Figueroa CD, et al. Abolition of Ca2+-mediated intestinal anion secretion and increased stool dehydration in mice lacking the intermediate conductance Ca2+-dependent K+ channel Kcnn4. J Physiol. 2007;583:705–17. doi: 10.1113/jphysiol.2007.134387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carew MA, Thorn P. Carbachol-stimulated chloride secretion in mouse colon: evidence of a role for autocrine prostaglandin E2 release. Exp Physiol. 2000;85:67–72. [PubMed] [Google Scholar]
- 14.Mall M, Bleich M, Schurlein M, et al. Cholinergic ion secretion in human colon requires coactivation by cAMP. Am J Physiol. 1998;275:G1274–81. doi: 10.1152/ajpgi.1998.275.6.G1274. [DOI] [PubMed] [Google Scholar]
- 15.Thiemann A, Grunder S, Pusch M, et al. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992;356:57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- 16.Schwiebert EM, Cid-Soto LP, Stafford D, et al. Analysis of ClC-2 channels as an alternative pathway for chloride conduction in cystic fibrosis airway cells. Proc Natl Acad Sci U S A. 1998;95:3879–84. doi: 10.1073/pnas.95.7.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gyomorey K, Yeger H, Ackerley C, et al. Expression of the chloride channel ClC-2 in the murine small intestine epithelium. Am J Physiol Cell Physiol. 2000;279:C1787–94. doi: 10.1152/ajpcell.2000.279.6.C1787. [DOI] [PubMed] [Google Scholar]
- 18.Catalan M, Cornejo I, Figueroa CD, et al. ClC-2 in guinea pig colon: mRNA, immunolabeling, and functional evidence for surface epithelium localization. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1004–13. doi: 10.1152/ajpgi.00158.2002. [DOI] [PubMed] [Google Scholar]
- 19.Catalan M, Niemeyer MI, Cid LP, et al. Basolateral ClC-2 chloride channels in surface colon epithelium: regulation by a direct effect of intracellular chloride. Gastroenterology. 2004;126:1104–14. doi: 10.1053/j.gastro.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Lipecka J, Bali M, Thomas A, et al. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol. 2002;282:C805–16. doi: 10.1152/ajpcell.00291.2001. [DOI] [PubMed] [Google Scholar]
- 21.Bosl MR, Stein V, Hubner C, et al. Male germ cells and photoreceptors, both dependent on close cell-cell interactions, degenerate upon ClC-2 Cl(−) channel disruption. Embo J. 2001;20:1289–99. doi: 10.1093/emboj/20.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nehrke K, Arreola J, Nguyen HV, et al. Loss of hyperpolarization-activated Cl(−) current in salivary acinar cells from Clcn2 knockout mice. J Biol Chem. 2002;277:23604–11. doi: 10.1074/jbc.M202900200. [DOI] [PubMed] [Google Scholar]
- 23.Zdebik AA, Cuffe JE, Bertog M, et al. Additional disruption of the ClC-2 Cl(−) channel does not exacerbate the cystic fibrosis phenotype of cystic fibrosis transmembrane conductance regulator mouse models. J Biol Chem. 2004;279:22276–83. doi: 10.1074/jbc.M309899200. [DOI] [PubMed] [Google Scholar]
- 24.Pena-Munzenmayer G, Catalan M, Cornejo I, et al. Basolateral localization of native ClC-2 chloride channels in absorptive intestinal epithelial cells and basolateral sorting encoded by a CBS-2 domain di-leucine motif. J Cell Sci. 2005;118:4243–52. doi: 10.1242/jcs.02525. [DOI] [PubMed] [Google Scholar]
- 25.Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–89. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- 26.Andres H, Rock R, Bridges RJ, et al. Submucosal plexus and electrolyte transport across rat colonic mucosa. J Physiol. 1985;364:301–12. doi: 10.1113/jphysiol.1985.sp015746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Field M, Fromm D, McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971;220:1388–96. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- 28.Rangachari PK, McWade D. Simultaneous measurements of 22Na and 36Cl in aqueous samples: a comparison of three different methods. Am J Physiol. 1987;252:G436–8. doi: 10.1152/ajpgi.1987.252.3.G436. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Begne M, Nakamoto T, Nguyen HV, et al. Enhanced formation of a HCO3- transport metabolon in exocrine cells of Nhe1−/− mice. J Biol Chem. 2007;282:35125–32. doi: 10.1074/jbc.M707266200. [DOI] [PubMed] [Google Scholar]
- 30.Epple HJ, Amasheh S, Mankertz J, et al. Early aldosterone effect in distal colon by transcriptional regulation of ENaC subunits. Am J Physiol Gastrointest Liver Physiol. 2000;278:G718–24. doi: 10.1152/ajpgi.2000.278.5.G718. [DOI] [PubMed] [Google Scholar]
- 31.Greger R. Role of CFTR in the colon. Annu Rev Physiol. 2000;62:467–91. doi: 10.1146/annurev.physiol.62.1.467. [DOI] [PubMed] [Google Scholar]
- 32.Cuppoletti J, Malinowska DH, Tewari KP, et al. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol. 2004;287:C1173–83. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 33.Bijvelds MJ, Bot AG, Escher JC, et al. Activation of intestinal Cl- secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterology. 2009;137:976–85. doi: 10.1053/j.gastro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 34.Bassil AK, Borman RA, Jarvie EM, et al. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol. 2008;154:126–35. doi: 10.1038/bjp.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heintze K, Stewart CP, Frizzell RA. Sodium-dependent chloride secretion across rabbit descending colon. Am J Physiol. 1983;244:G357–65. doi: 10.1152/ajpgi.1983.244.4.G357. [DOI] [PubMed] [Google Scholar]
- 36.Geibel JP, Rajendran VM, Binder HJ. Na(+)-dependent fluid absorption in intact perfused rat colonic crypts. Gastroenterology. 2001;120:144–50. doi: 10.1053/gast.2001.20890. [DOI] [PubMed] [Google Scholar]
- 37.Sangan P, Brill SR, Sangan S, et al. Basolateral K-Cl cotransporter regulates colonic potassium absorption in potassium depletion. J Biol Chem. 2000;275:30813–6. doi: 10.1074/jbc.M003931200. [DOI] [PubMed] [Google Scholar]
- 38.Kato A, Romero MF. Regulation of Electroneutral NaCl Absorption by the Small Intestine. Annu Rev Physiol. 2011 doi: 10.1146/annurev-physiol-012110-142244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stutts MJ, Canessa CM, Olsen JC, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–50. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 40.Casavola V, Turner RJ, Guay-Broder C, et al. CPX, a selective A1-adenosine-receptor antagonist, regulates intracellular pH in cystic fibrosis cells. Am J Physiol. 1995;269:C226–33. doi: 10.1152/ajpcell.1995.269.1.C226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson J, Begenisich T. Membrane-delimited inhibition of maxi-K channel activity by the intermediate conductance Ca2+-activated K channel. J Gen Physiol. 2006;127:159–69. doi: 10.1085/jgp.200509457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nighot PK, Blikslager AT. ClC-2 regulates mucosal barrier function associated with structural changes to the villus and epithelial tight junction. Am J Physiol Gastrointest Liver Physiol. 2010;299:G449–56. doi: 10.1152/ajpgi.00520.2009. [DOI] [PubMed] [Google Scholar]
- 43.Hinzpeter A, Lipecka J, Brouillard F, et al. Association between Hsp90 and the ClC-2 chloride channel upregulates channel function. Am J Physiol Cell Physiol. 2006;290:C45–56. doi: 10.1152/ajpcell.00209.2005. [DOI] [PubMed] [Google Scholar]
- 44.Cornejo I, Niemeyer MI, Zuniga L, et al. Rapid recycling of ClC-2 chloride channels between plasma membrane and endosomes: role of a tyrosine endocytosis motif in surface retrieval. J Cell Physiol. 2009;221:650–7. doi: 10.1002/jcp.21900. [DOI] [PubMed] [Google Scholar]
- 45.Schultheis PJ, Clarke LL, Meneton P, et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–5. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.