Abstract
A key component of task preparation may be to anticipate the consequences of task-appropriate actions. This task switching study examined whether such type of “intentional” preparatory control relies on the presentation of explicit action effects. Preparatory BOLD activation in a condition with task-specific motion effect feedback was compared to identical task conditions with accuracy feedback only. Switch-related activation was found selectively in the effect feedback condition in the middle mid-frontal gyrus and in the anterior intraparietal sulcus. Consistent with research on attentional control, the posterior superior parietal lobule exhibited switch-related preparatory activation irrespective of feedback type. To conclude, preparatory control can occur via complementary attentional and intentional neural mechanisms depending on whether meaningful task-specific action effects lead to the formation of explicit effect representations.
Descriptors: Action selection, Action effects, Attention, Cognitive control, Task switching
In almost any given situation, there are multiple different possible ways to interact with the environment. Thus, actions have to be selected by choosing one out of the available options. Two control processes appear to govern this selective interaction between agent and environment. First, attentional control processes serve as a perceptual filter that can constrain the selection of actions to those most strongly associated with the currently relevant stimulus dimension or feature. Second, intentional control processes can constrain the selection of actions in terms of the effects that will result from them. In other words, the conceptual distinction between attention and intention made here is tightly related to the fundamental distinction between (a) actions directly specified by the appropriate antecedent stimulus conditions as mediated by stimulus–response associations and (b) actions specified by their anticipated consequences as mediated by response–effect associations (de Wit & Dickinson, 2009; Dickinson, 1985; Hommel, Musseler, Aschersleben, & Prinz, 2001; Waszak et al., 2005). The basic design of the present study rests on previous research using the task switching paradigm, an experimental approach that is particularly well suited to creating consistently high demands on both selective attention and selective intention as defined above and elaborated further below.
Our central objective was to isolate brain areas involved in preparatory intentional control processes that serve to disambiguate actions associated with task-ambiguous “meanings” in the sense that they entail different consequences (i.e., they are used for different purposes) depending on the current task context. We were interested in this particular issue for two reasons. First, although the issue of preparatory intentional control may be central to understanding human goal-directed behavior, it has not yet been examined extensively using neuroimaging methods. Second, and more specifically, existing research suggests that preparatory control during task switching is solely attentional in nature, at least when concrete target stimuli are not yet available (Brass et al., 2003; Meiran, 2000; Ruge et al., 2005; Ruge, Braver, & Meiran, 2009). The current study challenges this general conclusion by postulating that task preparation might also operate at the level of intentional action representations, but only when the involved action effects are made sufficiently salient to engage amore explicit internal representation of task-specific effects.
We examined this hypothesis by use of a modified version of a spatial task switching paradigm. In the original design (Meiran, 1996), participants had to switch between two spatial discrimination tasks regarding a target stimulus that appeared unpredictably in one out of four positions within a 2 × 2 grid. One task required judgment regarding the horizontal position of the target (left or right within the grid) whereas the alternative task required judgment regarding the vertical position of the target (up or down within the grid). In this situation, attentional control is thought to be relevant for selectively activating the stimulus–response associations that are appropriate in the current task. For instance, if the target appears in the upper-left position of the grid and the task is to make a horizontal judgment, spatial attentional mechanisms focus perceptual processing on the horizontal position of the stimulus, so that it is this dimension that triggers the (preexperimentally) associated response, rather than the vertical dimension.
One advantage of this paradigm is that it has been used to not only demonstrate the role of attentional control mechanisms during task-switching but also to show that intentional control mechanisms can play a role in response selection and execution as well. Specifically, the relevance of intentional action representations has been revealed by contrasting two conditions that differed with regard to the presence of task-related ambiguity on the level of response meanings (Meiran, 2000). In the ambiguous condition, the same responses were used in both tasks (i.e., left and down stimulus positions both required a left button press response and right and up stimulus positions both required a right button press response). Such a mapping of four stimulus positions onto two responses implies that a given response, for example, a right button press, is ambiguously associated with two task-dependent intentions: Either it can be used to achieve the goal of indicating “right” or it can be used to achieve the goal of indicating “up.” In contrast, the nonambiguous condition was characterized by a unique one-to-one mapping between the four stimulus positions and four distinct responses. Thus, each response was unambiguously associated with one distinct intention. Importantly, it has been shown that response ambiguity is associated with specific behavioral performance costs (Meiran, 2000) as well as with neuroanatomically specific brain activations within the lateral prefrontal cortex (Brass et al., 2003). Yet, in these previous studies, it was assumed that task-specific disambiguation of response meanings only occurs after presentation of the target stimulus and not during the preparation period (in which the upcoming task is known, but the target has not yet appeared).
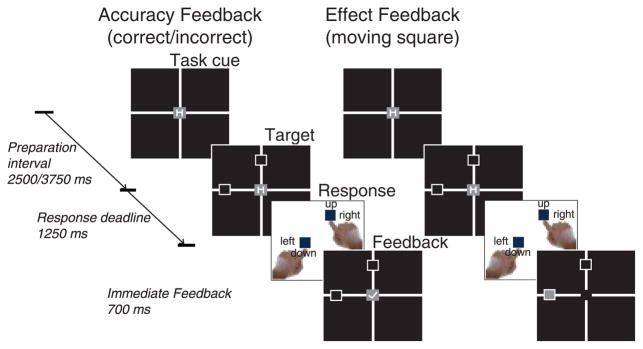
The primary hypothesis of the present study is that task-related disambiguation of response meanings (i.e., intentional control) can occur during preparatory as well as target (imperative) periods. However, intentional action representations might only be engaged for preparatory purposes under conditions in which the meaning of actions are made sufficiently salient. We reasoned that one way to increase salience would be to have task responses result in task-specific and highly plausible perceptual effects. To this end, we modified the task so that correct responses were immediately followed by perceptual motion effects in the direction of the intended target location (cf. Ansorge, 2002; Kiesel & Hoffmann, 2004). We expected that this coupling of responses with a salient and plausible perceptual effect would lead to a stronger and more explicit action–effect associative representation to be formed. Moreover, if action effects were task unique (i.e., upward/downward motions only occurred during the vertical task, and leftward/rightward motions only occurred during the horizontal task), they could be invoked during the preparatory period as a means of reducing interference related to the otherwise task-ambiguous responses (i.e., because the same two responses were used in both tasks). To enable the formation of such unique associations between motion effects and respective tasks, the original spatial target arrangement was slightly modified. For instance, instead of presenting one target square in the upper-left corner of the grid, two target squares were presented, one left and one up (see Figure 1). After a correct response was made, a central red square appeared to “jump” to the task-appropriate square indicated by the response (e.g., for the horizontal task the central square would jump to the left target square position).
Figure 1.
Experimental design. Participants performed two blocked task switching conditions involving either “accuracy feedback” or “effect feedback” after responding. On each trial the currently relevant task was indicated by a centrally displayed task cue (“H” for horizontal discrimination and “V” for vertical discrimination). For further details, see the Methods.
To directly test our hypothesis, we compared preparatory brain activation in the novel “effect feedback” condition with preparatory brain activation in the standard control condition presenting “accuracy feedback” only. Critically, both conditions were physically identical until after response execution. Thus, differences in preparatory brain activation can unambiguously be attributed to strategy differences during task preparation. On the basis of prior research, we expected that two brain regions within lateral prefrontal and parietal cortex—the anterior intra-parietal sulcus (aIPS) and the middle mid-frontal gyrus (mMFG)—would show selective involvement in preparatory intentional control (i.e., increased activity in the effect feedback relative to accuracy feedback conditions). These two regions have been implicated in intentional control processes under accuracy feedback conditions (Brass et al., 2003), but only when concrete target stimuli were present (i.e., when concrete actions can be planned) and not when advance task information was available for preparation (Ruge et al., 2005, 2009). In contrast to these previous results, we hypothesized that under effect feedback conditions, aIPS and mMFG should be engaged even during the preparation period, before actual task implementation (i.e., active prior to target presentation).
Furthermore, we hypothesized that brain regions involved in preparatory attentional control (i.e., selecting the task-appropriate stimulus dimension) should be similarly engaged in both feedback conditions (because there was no difference between conditions with regard to the upcoming target stimuli). Such attentional control regions were expected to be located most prominently in the posterior superior parietal lobule (pSPL; Wager, Jonides, & Reading, 2004).
Finally, we were also interested in evaluating the impact of feedback type on behavioral performance. In particular, the theoretical considerations outlined above directly imply the prediction that advance task preparation involving the usage of task-specific action effect representations in the effect feedback condition should reduce, if not eliminate, residual switch cost as compared to the standard accuracy feedback condition (Meiran, 2000).
Methods
Participants
Eighteen human participants took part in the functional magnetic resonance imaging (fMRI) study (mean age = 22 years; age range: 19–29 years; 12 women, 6 men). An additional 30 participants (mean age = 23 years; age range 20–31 years; 18 women, 12 men) were recruited to perform the behavioral task, but outside of the scanner. All participants gave written informed consent prior to taking part in the experiment.
Experimental Design: fMRI Study
The fMRI experiment consisted of two different blocked task switching conditions, including (a) a standard control condition in which responses were followed by accuracy feedback and (b) a novel condition designed to increase the salience of task-specific response meanings by presenting task-dependent motion effect feedback. The order of blocks was counterbalanced across participants. In each condition, a practice block of 20 trials was performed before the experimental block started. Except for the use of different types of response feedback, the two blocked conditions were identical in terms of performance demands, as described next. On each trial, participants were presented with a task-ambiguous target stimulus comprised of two empty squares, one located on the horizontal axis of a 2 × 2 grid and the other one located on the vertical axis (for an exemplary target, see Figure 1). Participants had to indicate the position of only one of these squares, depending on whether they were instructed to perform a horizontal or a vertical discrimination task. In the horizontal discrimination task participants had to indicate whether the target square located on the horizontal axis appeared to the left or right of center by responding with the left or right index finger, respectively. In the vertical discrimination task participants had to indicate whether the target square located on the vertical axis appeared above or below center by responding with the left or right index finger, respectively. Importantly, within this setup the same two manual responses were involved in both tasks; thus, response meanings (i.e., intentional action representations) were task ambiguous. The currently relevant task was indicated by a task cue displayed at the beginning of each trial (“H” for horizontal task; “V” for vertical task; centrally displayed on a red square). The two tasks occurred in a pseudorandom and unpredictable sequence, constrained so that the number of task switch trials and task repetition trials was equal. Task sequences were generated using the SeqGen2008 algorithm (Remillard, 2008) so that the number of task switch trials and task repetition trials was equal. The preparation interval between task cue and target stimulus (CTI) varied randomly between 2.5 s and 3.75 s. The task cue remained on screen during the entire preparation interval. Participants had to respond within a window of 1.25 s. Following a response, feedback was displayed immediately for 700 ms.
In the accuracy feedback condition, in case of a correct response the central red square turned green, and a check symbol was superimposed. In case of an incorrect or late response, the central red square remained red, and an “X” was superimposed. In the motion effect feedback condition, in case of a correct response, the central red square disappeared and then reappeared in one of the two peripheral target squares according to the currently relevant task (see Figure 1 for an example). Perceptually, these actions gave the appearance of the red square “jumping” to the location indicated by the participant’s response. In case of an incorrect or late response, the central red square remained stationary with an “X” superimposed. Thus, the only difference between the two conditions was the nature of postresponse feedback on correct response trials. The intertrial interval varied between 2.5 s and 12.0 s, with exponentially decreasing probability of longer intervals (Hagberg, Zito, Patria, & Sanes, 2001). The actual trial onset was randomly jittered by TR/2 (i.e., 1.25 s) relative to the start of fMRI acquisition cycles to double the sampling rate of the trial-related BOLD response (Josephs, Turner, & Friston, 1997).
Because the study aimed at comparing preparatory brain activation in the context of accuracy feedback versus motion-effect feedback, we included partial cue-only trials to decorrelate cue-related and target-related BOLD activation components. Thereby, we were able to obtain separate BOLD response estimates for cue-related and target-related activation (Ollinger, Shulman, & Corbetta, 2001; Shulman et al., 1999). Note that the target-related BOLD response estimate also comprises possible activation components elicited by the response or by the feedback. Yet, because we were specifically interested in cue-related preparatory brain activation, disentangling these target-related BOLD response subcomponents was not important, here. Each blocked condition comprised 144 trials, including 96 full cue–target trials and 48 partial cue-only trials. Because responses were only to be made following the target, S1-only trials had no associated task response. The order of condition blocks was counterbalanced across participants.
Experimental Design: Behavioral Pilot Study
Because the fMRI experiment comprised only 18 participants and the expected behavioral effects are rather weak (i.e., residual switch cost following task preparation), we decided to increase statistical power by also including data from an additional 30 participants who performed the behavioral task outside of the scanner. The task design was the same as that for the scanned participants except for the following differences in procedure. First, there was one short CTI of 100 ms and one long CTI of 1500 ms, instead of two long CTIs of 2500 ms and 3750 ms as realized in the fMRI experiment. Thus, different from the fMRI study in which there was always sufficient time to prepare for the upcoming task, the 100-ms CTI condition prevented participants from full advance task preparation. Thus, we used only the trials with the 1500-ms CTI for analysis. Second, there was a constant intertrial interval of only 300 ms instead of a jittered ITI. Third, there were no partial cue-only trials. Fourth, in the standard feedback condition, the correct–incorrect feedback was provided by presenting the written German words for “correct” and “incorrect” in the center of the screen instead of symbols. Fifth, the pilot experiment was controlled by the ERTS software (BeriSoft) instead of Eprime 1.2. The participants completed 170 trials in each feedback condition, and the order of feedback blocks was counterbalanced across subjects.
Imaging Procedure
Whole-brain images were acquired on a Siemens 3 Tesla whole-body Trio System (Erlangen, Germany) with a 16-channel circularly polarized head coil. Headphones dampened scanner noise and enabled communication with the participants. Both structural and functional images were acquired for each participant. Structural images (1.25 mm × 1 mm × 1 mm) were acquired using an MP-RAGE T1-weighted sequence (TR = 9.7 ms, TE = 4 ms, flip = 12°, TI = 300 ms). Functional images were acquired using a gradient echo planar sequence (TR = 2500 ms, TE = 30 ms, flip = 90°, interleaved slice acquisition, slice gap = 0). Each volume contained thirty-two 4.0-mm-thick slices (in-plane resolution 3.0 mm × 3.0 mm).
Participants performed a total of eight functional scanning runs, which were separated into two blocks of four runs of each blocked condition (accuracy feedback, motion effect feedback). Each scanning run consisted of 36 trials (in total 144 trials per blocked condition) and lasted approximately 6 min. The experiment was controlled by Eprime 1.2 software (Psychology Software Tools, Inc., Pittsburgh, PA) running on a Windows-XP PC. Stimuli were projected to participants via Visuastim digital goggles (Resonance Technology, Inc., Northridge, CA) simulating a viewing distance of 100 cm. A fiber-optic, light-sensitive key press was used to record participants’ behavioral responses.
Data Analysis
Preprocessing
The empirical data set was analyzed with SPM5 running under MATLAB 7.1. The preprocessing included slice-time correction, rigid body movement correction (three translation and three rotation parameters), normalization of the functional images by directly registering the mean functional image to the standard MNI EPI template image provided by SPM5 (the resulting interpolated spatial resolution was 3 × 3 × 3 mm), and smoothing of the functional images (Gaussian Kernel, FWHM = 8 mm).
Event-related analysis
The preprocessed imaging data were analyzed using the General Linear Model (GLM) approach as implemented in the SPM5 software package. Model regressors were created by convolving neural input functions for the different event types with the assumed canonical hemodynamic response function used by SPM5, including both derivatives. For each condition block, the GLM included two regressors for cue-related activation separately for task switch and task repetition trials and two regressors for target-related activation separately for task switch and task repetition trials. Thus, a total of eight event-related BOLD responses were to be estimated (plus regressors for the two derivatives). The actual data analysis focused on the four cue-related BOLD estimates (cue–repeat [accuracy feedback], cue–switch [accuracy feedback], cue–repeat [effect feedback], and cue–switch [effect feedback]). These four BOLD estimates were used to compute two whole-brain images that captured the patterns of preparatory brain activation associated with either attentional control or intentional control. Preparatory control demands were expected to be especially high in task switch trials as compared to task repetition trials, reflecting enhanced reconfiguration demands due to proactive interference resulting from implementing the alternative task in the previous trial. Although results from previous fMRI studies on task switching are rather heterogeneous with regard to enhanced switch-related preparatory BOLD activation, we nevertheless focused our primary analysis on this switchrepetition contrast because it allows for a more specific interpretation in terms of task-related preparatory disambiguation processes. Furthermore, from a methodological point of view, by comparing relative BOLD activation differences (switch vs. repeat) across the two blocked conditions (accuracy vs. effect feedback), we could circumvent the potential problem that baseline differences between blocks (resulting from differential activation during the intertrial interval) might cause apparent differences between conditions that are unrelated to the relevant preparatory processes within the trial itself.
Intentional preparatory control, that is, the advance activation of the currently task-relevant response meanings, was hypothesized to be involved specifically in the motion effect feedback condition. We therefore expected enhanced preparatory BOLD activation for switch trials as compared to repetition trials, especially in the effect-feedback condition. Thus, we specifically isolated voxels that exhibited enhanced switch-related preparatory activation in the effect feedback condition more than in the accuracy feedback condition. To this end, we used a two-stage procedure in which voxels were first identified at the group level based on the the switchrepetition contrast for the effect feedback condition with p<.001 and a minimum of 30 contiguous above threshold voxels. Next, voxel clusters were only included for further analysis if switch-related activity in the effect feedback condition was significantly greater than that in the accuracy feedback condition. This constraint was imposed by applying an inclusive mask based on the interaction contrast ([switch – repetition] effect feedback – [switch – repetition] accuracy feedback) with an intermediate threshold of p<.01.
In contrast to intentional preparatory control, preparatory attentional control, that is, the advance activation of the currently task-relevant perceptual dimension, was expected for both feedback conditions. Thus, we specifically isolated voxels that exhibited enhanced switch-related preparatory activation irrespective of the feedback condition. To this end, we again used a two-stage masking procedure. First, voxels were identified based on the switchrepetition contrast collapsed across both feedback conditions with p<.001 and a minimum of 30 contiguous above threshold voxels. Second, voxel clusters were only included for further analysis if there was no effect of feedback condition. This constraint was imposed by applying an exclusive mask based on the same interaction contrast used above. This masking procedure effectively excluded voxels for which the switch – repetition difference was modulated by the type of feedback using a very lenient whole-brain threshold of p<.05, so that voxels showing even subtle effects of feedback type were masked out.
The minimum of 30 contiguous above threshold voxels was chosen arbitrarily, after considering (a) the objectively defined cluster size threshold of 44 contiguous voxels as determined based on Gaussian Random Field theory implemented within SPM5 and (b) the often much more lenient, but rather subjectively defined, cluster sizes found in the literature. In order not to ignore potentially relevant activation clusters comprising less than 44 contiguously activated voxels, we arbitrarily lowered the threshold down to 30. When reporting the fMRI results (Table 1 and Table 2), we explicitly indicate the clusters that did not reach the objectively defined 44 voxel threshold.
Table 1.
Preparatory Intentional Task Control
| Brain region | MNI coordinate
|
Statistics
|
|||
|---|---|---|---|---|---|
| x | y | Z | Switch>repetition for effect feedback
|
||
| z value | Number of voxels | ||||
| L mMFG | −30 | 39 | 30 | 3.50 | 52 |
| L pACC | −6 | 18 | 45 | 4.31 | 48 |
| L dPMC | −39 | −6 | 51 | 5.04 | 58 |
| L aIPS | −51 | −27 | 45 | 4.18 | 159 |
| R aIPS | 48 | −27 | 54 | 4.29 | 284 |
| L OCC | −36 | −63 | −12 | 3.86 | 33a |
| L OCC | −24 | −87 | −9 | 4.38 | 142 |
| R OCC | 39 | −60 | −9 | 4.33 | 338 |
| R OCC | 33 | −75 | 27 | 4.03 | 39a |
Note: a: anterior, IPS: intra-parietal sulcus, L: left, m: mid, OCC occipital cortex, p: posterior, MFG: middle frontal gyrus, PMC: premotor cortex, R: right.
Denotes regions that failed to reach the Gaussian random field cluster size threshold of 44 contiguously activated voxels, but reached a more liberal threshold of 30 contiguous voxels.
Table 2.
Preparatory Attentional Task Control
| Brain region | MNI coordinate
|
Statistics
|
|||
|---|---|---|---|---|---|
| x | y | Z | Switch>repetition irrespective of feedback type
|
||
| z value | Number of voxels | ||||
| L aIFG | −36 | 48 | 12 | 3.96 | 34a |
| L pre-SMA | −9 | 0 | 60 | 3.79 | 32a |
| L/R pSPL | −15 | −72 | 39 | 3.98 | 541 |
| −6 | −60 | 66 | 3.98 | ||
| 24 | −63 | 57 | 3.95 | ||
| L OCC | −6 | −75 | −6 | 4.42 | 48 |
Note: a: anterior, IFG: inferior frontal gyrus, L: left, OCC: occipital cortex, p: posterior, R: right, SMA: supplementary motor area, SPL: superior parietal lobule.
Denotes regions that failed to reach the Gaussian random field cluster size threshold of 44 contiguously activated voxels, but reached a more liberal threshold of 30 contiguous voxels.
Results
Behavioral Performance Data
As described above in the experimental design section, in addition to the behavioral data obtained from the 18 fMRI participants, we also included data from additional 30 subjects who performed the task under unscanned conditions in order to increase statistical power for identifying relevant behavioral effects of feedback type. Mean response times (RT) and error rates of each subject were entered into two separate four-way analyses of variance (ANOVAs). These ANOVAs included the two factors of primary interest, that is, task transition (task repetition vs. task switch) and feedback type (accuracy vs. effect). To check for possible modulatory effects we included the two additional factors response congruency (the two target squares associated with either the same response or different responses depending on task) and response transition (same vs. different response as compared to preceding trial).
The analysis of response times revealed a significant main effect of task transition, F(1,47) = 16.69, p<.001, reflecting the standard residual task-switch cost. More importantly, there was also a significant interaction effect of Task Transition × Feedback Type, F(1,47) = 4.30, p<.05, reflecting slightly larger residual switch cost for the accuracy feedback condition (repeat = 496 ms; switch = 512 ms) relative to the effect feedback condition (repeat = 502 ms; switch = 508 ms). The main effect of feedback type failed to reach significance, F(1,47) = 0.02, n.s.). Follow-up tests revealed that residual switch cost in the accuracy feedback condition were highly significant, F(1,47) = 24.34, p<.001, whereas the residual switch cost in the effect feedback condition failed to reach significance, F(1,47) = 2.40, n.s. This finding confirms the prediction that the presentation of effect feedback would encourage the advance activation of task-specific action effect representations and, thus, reduce residual switch cost. This effect was not significantly modulated by response congruency or response transition. Also, there were no such significant effects regarding error rates (overall error rate was 4%).
Notably, the Task Transition × Feedback Type interaction effect failed to reach significance when evaluated separately for each the two experiments (pilot and fMRI). Yet, importantly, the relevant RT pattern was numerically similar for both experiments. Specifically, in the pilot experiment the residual switch cost was reduced from 18 ms in the accuracy feedback condition to 8 ms in the effect feedback condition. Similarly, in the fMRI experiment, the residual switch cost was reduced from 12 ms in the accuracy feedback condition to 3 ms in the effect feedback condition. These descriptive results indicate that the fMRI-related modifications of the experimental procedure did not alter in a qualitative way the cognitive processes of interest (as reflected by response times).
Imaging Data
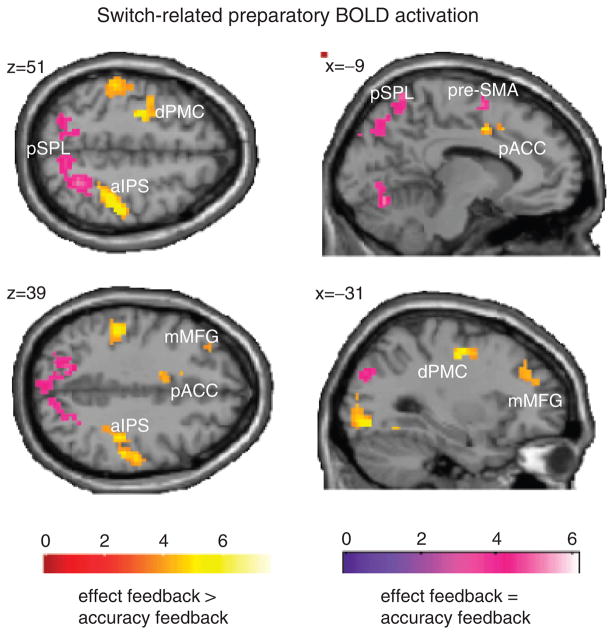
Figure 2 depicts (in yellow) regions exhibiting an activation pattern consistent with intentional preparatory control, that is, stronger switch-related preparatory activation in the effect feedback condition compared to the standard accuracy feedback condition (see Table 1). As predicted, we found activation clusters in the aIPS bilaterally and in the left mMFG. A homologous right mMFG activation cluster comprised only 18 out of the 30 required contiguous above threshold voxels (peak voxel MNI coordinates are 36, 36, and 33; z = 3.42). Additionally, we found activation clusters including the dorsal premotor cortex (dPMC), the posterior portion of the anterior cingulate cortex (pACC), and regions within the occipital cortex.
Figure 2.
Two types of preparatory BOLD activation were identified, associated either with “intentional preparatory control” processes (stronger switch-related activation in the effect feedback condition than in the accuracy feedback condition) colored in red/yellow or with “attentional preparatory control” processes (similar switch-related activation for both feedback types) colored in blue/pink. The depicted brain sections were created with SPM5 within MNI coordinate space.
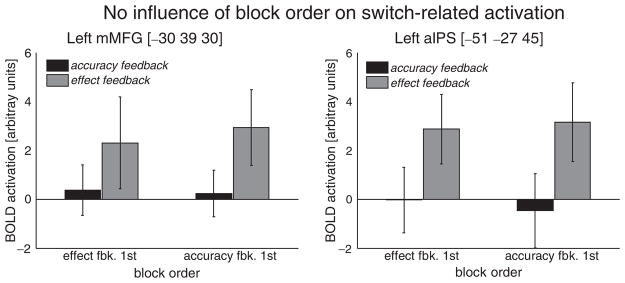
An important follow-up question concerning this effect-feedback-specific activation pattern is whether it is influenced by the order of condition blocks. In particular, one might suspect that participants who performed the accuracy feedback block first might be less inclined to engage in intentional preparation in the subsequent effect feedback block as compared to participants who started with the effect feedback block. Yet, as Figure 3 shows for two representative brain regions, none of the previously indentified intention-related brain regions was modulated by the order of condition blocks.
Figure 3.
Results of a follow-up analysis confirming that effect-feedback-specific switch-related preparatory activation was not influenced by the order of blocks (i.e., effect feedback, then accuracy feedback vs. accuracy feedback, then effect feedback).
We also found voxels (Figure 2, colored in pink) that exhibited an activation pattern consistent with attentional preparatory control, that is, switch-related preparatory activation not affected by feedback type (see Table 2). As predicted, we found activation clusters in the pSPL and in the pre-SMA. Additionally, we found activation in the occipital cortex.
Discussion
The aim of the present study was to identify brain areas specifically involved in intention-based task preparation, that is, preparatory control processes that serve to disambiguate task-ambiguous action effect representations (i.e., “response meanings”) prior to the presentation of concrete target stimuli that trigger response selection and generation processes. We reasoned that intentional preparatory task control is an optional process that may preferentially occur when actions are immediately followed by effects that are task-specific, plausible, and directly perceivable. As explained in detail in the introduction, this is not the case in standard task switching procedures involving only accuracy feedback that is task nonspecific. Thus, we compared this standard control condition with a novel condition in which actions were followed by task-specific motion effects. Importantly, these two conditions were physically identical with regard to the task cue, the preparation interval, and the target stimulus. Thus, differences in preparatory (i.e., cue-related) brain activation could only be due to differences in the mental representation and anticipation of the upcoming response feedback.
Both, the behavioral performance results and the imaging results confirmed our hypothesis. First, the residual switch cost (i.e., switch cost even after ample time to prepare for the current task) was statistically eliminated in the effect feedback condition (6 ms) and was significantly reduced relative to the standard accuracy feedback condition (16 ms). This finding nicely supports a hypothesis suggested earlier (Meiran, 2000), namely, that a substantial portion of the residual switch cost under standard accuracy feedback conditions might indeed be due to insufficiently prepared task-ambiguous response meanings (i.e., representations of task-specific action effects). By contrast, when response meanings are made sufficiently salient as in the present effect feedback condition, task-ambiguous response meanings seem to be disambiguated during the preparation interval, as indicated by much smaller residual switch cost.
Although the performance data seem to reflect the impact of intentional task preparation in terms of reduced residual switch cost, the imaging data might directly reflect the upstream preparatory disambiguation of response meanings, which entails the reduced behavioral switch cost. Several brain regions exhibited switch-related preparatory BOLD activation specifically in the effect feedback condition but not in the standard accuracy feedback condition. Importantly, this included the predicted brain areas within lateral prefrontal and parietal cortex: aIPS and mMFG. Furthermore, our results confirmed the role of other fronto-parietal brain regions in attentional control, notably the pSPL and pre-SMA. In line with such an interpretation, in the present study, these regions exhibited switch-related preparatory activation irrespective of the type of feedback.
Whereas the involvement of mMFG and aIPS in intentional preparatory control was hypothesized, there were three additional brain regions that exhibited the same activation pattern (pACC, dPMC, and occipital cortex) but were not expected from the outset. Yet, from a broader perspective, the involvement of these regions seems quite plausible. First, both pACC and dPMC have been suggested to be related to action-related processes rather than stimulus-directed (i.e., attentional) ones (Passingham, 1995; Picard & Strick, 1996, 2001). Thus, if task preparation in the effect feedback condition really leads to the activation of action codes via their associated effects, it seems plausible that this also implicates the engagement of areas that are involved in action planning processes on a more generic level. Notably, there is evidence that specifically the pACC might be related to the coding of actions with regard their consequences, particularly the incentive values of action effects (Rushworth, Buckley, Behrens, Walton, & Bannerman, 2007; Rushworth, Walton, Kennerley, & Bannerman, 2004), suggesting that the pACC might be involved in motivating the execution of actions. Such a view suggests that the task-specific anticipation of action effects, even when these do not involve explicit incentive value, might nevertheless reflect a motivational drive to prepare the currently task-appropriate response options, that is, coding the task-appropriate set of responses with higher motivational priority than the task-inappropriate set of responses even before the upcoming target stimulus enables the ultimate selection and execution of one specific action.
Finally, the activation of visual cortex might be related to the visual imagery of the anticipated motion effects. This latter aspect suggests an alternative explanation, namely, that what we called “intentional preparatory control” might in fact be nothing but visual imagery of the expected sensory events without any connection to action-related processes. Yet, we believe that the involvement of the other regions discussed above does, in fact, strongly indicate the engagement of truly action-related preparatory processes in the effect feedback condition as compared to the accuracy feedback condition. Also, the finding that intention-based preparation reduced behavioral switch cost speaks against the interpretation that we are solely dealing with an epiphenomenon of pure visual expectation without any relationship to action-related preparation. Otherwise, our experimental manipulation should not have been expressed in the observed modulation of behavioral performance. Of course, this does not exclude the possibility that the activation observed in visual cortex by itself might indeed be solely due to sensory (rather than action) expectation.
It is worth noting that the observed preparatory effects were characterized by increased activity on task-switch relative to task-repeat trials. Such an activation pattern appears highly plausible, as switch trials seem to demand stronger engagement of cognitive control to overcome the task representations established in the preceding trial. This argument holds for both attentional control engagement with regard to ambiguous stimulus representations and intentional control engagement with regard to ambiguous response meanings. In line with such reasoning, enhanced switch-related preparatory activation has consistently been observed in event-related brain-electrical recordings (Karayanidis, Coltheart, Michie, & Murphy, 2003; Kieffaber & Hetrick, 2005; Rushworth, Hadland, Paus, & Sipila, 2002). By contrast, event-related fMRI study results have been rather heterogeneous with regard to preparatory switch-related activation effects (Badre & Wagner, 2006; Brass & von Cramon, 2002; Braver, Reynolds, & Donaldson, 2003; Bunge, Kahn, Wallis, Miller, & Wagner, 2003; Ruge et al., 2005; Wylie, Javitt, & Foxe, 2006; Yeung, Nystrom, Aronson, & Cohen, 2006). Yet, the studies that do report significant switch-related preparatory BOLD activation have most reliably revealed effects in the pSPL overlapping with the parietal cortex region associated with attentional preparatory control in the present study and consistent with the broader literature on flexible attentional control (Wager et al., 2004). As previous task switching studies used designs that seem to rather discourage the engagement of intentional preparatory control (no explicitly perceivable action effects), in the light of the present results it does not seem surprising that those previous studies did not reliably report switch-related preparatory activation in the aIPS and the mMFG (i.e., the regions that we found to be specifically associated with intentional preparatory control).
From a broader perspective, the involvement of aIPS and mMFG in intentional preparatory control during task switching is consistent with results from two conceptually related research fields. First, studies examining action observation and imitation processes, which tap into action planning processes triggered by the observed action effects caused by other agents, typically discuss the aIPS as one important region (Arbib, 2005; Hamilton & Grafton, 2006; Rizzolatti & Craighero, 2004). Second, the significance of the dorsolateral PFC (i.e., mMFG) has been emphasized in various types of paradigms involving nonroutine action planning processes (Genovesio, Brasted, Mitz, & Wise, 2005; Pochon et al., 2001; Rowe, Toni, Josephs, Frackowiak, & Passingham, 2000; Ruge et al., 2009), especially when actions are “freely” determined by participants without external selection criteria (Frith, Friston, Liddle, & Frackowiak, 1991; Jahanshahi & Dirnberger, 1999; Lau, Rogers, Ramnani, & Passingham, 2004).
The present study extends such previous findings by demonstrating that the engagement of these regions (a) can be triggered already during task preparation before the concrete target for action is known, (b) is specifically linked to the availability of explicit action effect information, and (c) preferentially occurs when task-ambiguous action effect representations need to be disambiguated (i.e., under task-switching conditions). Thus, the mMFG and aIPS act together to disambiguate and select the currently appropriate actions based on representations of action goals as defined in the original sense, that is, in terms of the anticipated consequences expected to be achieved by acting in a particular, currently appropriate way. Furthermore, the current study extends and further clarifies the interpretation of results from previous cued task-switching studies involving accuracy feedback only (Brass et al., 2003; Ruge et al., 2009). These studies revealed an enhanced engagement of mMFG, aIPS, or both related to target processing that was even demonstrated to occur in a preparatory manner (Ruge et al., 2009), but when only accuracy feedback was available. These activations related to target presentation were also interpreted as reflecting intention-based as compared to attention-based control. In the light of the present study results, which imply mMFG and aIPS in intention-based preparatory processes specifically in the effect feedback condition, it might appear unclear why these areas should be engaged following target presentation with accuracy feedback only. These apparent contradictions can be resolved when we consider that action effects are implicitly involved also during target processing in these previous studies, but in a relatively implicit and not directly perceivable form. For instance, in Ruge et al. (2009), the implicit effect associated with a left response for the target letter “N” in the “consonant-vowel” task is that the presence of a consonant letter was correctly identified (but not the presence of the concurrently displayed odd digit “3,” as would have been indicated by the same response in case of the “odd-even” task). The implicit nature of such action effects implies that the internal representation of effects would only be (automatically) activated after perceiving the respective target stimulus (e.g., the consonant letter “N”) as a given target becomes associated with its effect irrespective of the level of awareness with regard to this effect. By contrast, a task cue that is not directly associated with a specific response and a corresponding effect would not automatically activate the task-related set of action effects unless the involved effects are more explicitly represented. Consequently, the rationale behind the present study was to encourage the preparatory engagement of intentional action effect representations upon task cue presentation by making action effects more salient and, thereby, more likely be explicitly used during cue-based task preparation. We believe that this present study design—by explicitly manipulating the type of task-related action effects—enables a stronger interpretation of mMFG and aIPS activation as being related to intention-based task preparation. By contrast, the previous studies have confounded intentional and attentional task preparation with target-versus cue-related processing.
Finally, another critical implication of the current results is that they appear to broaden the notion of intentional control. In particular, the term intentional control is often used to refer to basic motor planning processes when a specific action and the respective outcome are known in advance (Andersen & Cui, 2009). By contrast, in the present study, the task cue indicated the dimension, or the set of appropriate effects (e.g., leftward or rightward movement in the horizontal direction), to be achieved from a set of possible actions (left or right button presses), rather than one particular action–effect association. We speculate that the role of the mMFG might be to internally represent these higher level associations between action–effect associative sets, rather than between particular actions and particular outcomes. In this way, the intentional control system might operate hierarchically, along a posterior–anterior axis within the lateral PFC, with posterior regions (i.e., dPMC) representing specific action-effect pairings, whereas more anterior regions (i.e., mMFG) represent action–effect relationships at the set or dimensional level. Thus, the intentional control system might be organized analogously to the types of posterior–anterior hierarchies that have been postulated within attentional control (e.g., Koechlin & Summerfield, 2007).
References
- Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron. 2009;63:568–583. doi: 10.1016/j.neuron.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Ansorge U. Spatial intention-response compatibility. Acta Psychologica. 2002;109:285–299. doi: 10.1016/s0001-6918(01)00062-2. [DOI] [PubMed] [Google Scholar]
- Arbib MA. From monkey-like action recognition to human language: An evolutionary framework for neurolinguistics. Behavioral and Brain Sciences. 2005;28:105–124. doi: 10.1017/s0140525x05000038. discussion 125–167. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Computational and neurobiological mechanisms underlying cognitive flexibility. Proceedings of the National Academy of Sciences, USA. 2006;103:7186–7191. doi: 10.1073/pnas.0509550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Ruge H, Meiran N, Koch I, Rubin O, Prinz W, et al. When the same response has different meanings: Recoding the response meaning in the lateral prefrontal cortex. NeuroImage. 2003;20:1026–1031. doi: 10.1016/S1053-8119(03)00357-4. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cerebral Cortex. 2002;12:908–914. doi: 10.1093/cercor/12.9.908. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. Journal of Neurophysiology. 2003;90:3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- de Wit S, Dickinson A. Associative theories of goal-directed behaviour: A case for animal-human translational models. Psychology Research. 2009;73:463–476. doi: 10.1007/s00426-009-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A. Actions and habits: The development of behavioural autonomy. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1985;308:67–78. [Google Scholar]
- Frith CD, Friston K, Liddle PF, Frackowiak RS. Willed action and the prefrontal cortex in man: A study with PET. Proceedings Biological Sciences. 1991;244:241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- Genovesio A, Brasted PJ, Mitz AR, Wise SP. Prefrontal cortex activity related to abstract response strategies. Neuron. 2005;47:307–320. doi: 10.1016/j.neuron.2005.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg GE, Zito G, Patria F, Sanes JN. Improved detection of event-related functional MRI signals using probability functions. NeuroImage. 2001;14:1193–1205. doi: 10.1006/nimg.2001.0880. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. Goal representation in human anterior intraparietal sulcus. Journal of Neuroscience. 2006;26:1133–1137. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B, Musseler J, Aschersleben G, Prinz W. The theory of event coding (TEC): A framework for perception and action planning. Behavioral and Brain Sciences. 2001;24:849–878. doi: 10.1017/s0140525x01000103. discussion 878–937. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Dirnberger G. The left dorsolateral prefrontal cortex and random generation of responses: Studies with transcranial magnetic stimulation. Neuropsychologia. 1999;37:181–190. doi: 10.1016/s0028-3932(98)00092-x. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston KJ. Event-related fMRI. Human Brain Mapping. 1997;5:243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Karayanidis F, Coltheart M, Michie PT, Murphy K. Electrophysiological correlates of anticipatory and poststimulus components of task switching. Psychophysiology. 2003;40:329–348. doi: 10.1111/1469-8986.00037. [DOI] [PubMed] [Google Scholar]
- Kieffaber PD, Hetrick WP. Event-related potential correlates of task switching and switch costs. Psychophysiology. 2005;42:56–71. doi: 10.1111/j.1469-8986.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- Kiesel A, Hoffmann J. Variable action effects: Response control by context-specific effect anticipations. Psychological Research. 2004;68:155–162. doi: 10.1007/s00426-003-0152-7. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends in Cognitive Sciences. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Ramnani N, Passingham RE. Willed action and attention to the selection of action. NeuroImage. 2004;21:1407–1415. doi: 10.1016/j.neuroimage.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of processing mode prior to task performance. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:1423–1442. [Google Scholar]
- Meiran N. Reconfiguration of stimulus task-sets and response task-sets during task-switching. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 377–400. [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI I. NeuroImage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Passingham RE. The frontal lobes and voluntary action. Oxford, England: Oxford University Press; 1995. [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: A review of their location and functional activation. Cerebral Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Current Opinion in Neurobiology. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Poline JB, Crozier S, Lehericy S, Pillon B, et al. The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: An fMRI study. Cerebral Cortex. 2001;11:260–266. doi: 10.1093/cercor/11.3.260. [DOI] [PubMed] [Google Scholar]
- Remillard G. A program for generating randomized simple and context-sensitive sequences. Behavior Research Methods. 2008;40:484–492. doi: 10.3758/brm.40.2.484. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: Response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Ruge H, Brass M, Koch I, Rubin O, Meiran N, von Cramon DY. Advance preparation and stimulus-induced interference in cued task switching: Further insights from BOLD fMRI. Neuropsychologia. 2005;43:340–355. doi: 10.1016/j.neuropsychologia.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Ruge H, Braver T, Meiran N. Attention, intention, and strategy in preparatory control. Neuropsychologia. 2009;47:1670–1685. doi: 10.1016/j.neuropsychologia.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Current Opinion in Neurobiology. 2007;17:220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: A combined fMRI and TMS study. Journal of Neurophysiology. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends in Cognitive Sciences. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, et al. Areas involved in encoding and applying directional expectations to moving objects. Journal of Neuroscience. 1999;19:9480–9496. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: A meta-analysis. NeuroImage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Waszak F, Wascher E, Keller P, Koch I, Aschersleben G, Rosenbaum DA, et al. Intention-based and stimulus-based mechanisms in action selection. Experimental Brain Research. 2005;162:346–356. doi: 10.1007/s00221-004-2183-8. [DOI] [PubMed] [Google Scholar]
- Wylie G, Javitt DC, Foxe JJ. Jumping the gun: Is effective preparation contingent upon anticipatory activation in task-relevant neural circuitry? Cerebral Cortex. 2006;16:394–404. doi: 10.1093/cercor/bhi118. [DOI] [PubMed] [Google Scholar]
- Yeung N, Nystrom LE, Aronson JA, Cohen JD. Between-task competition and cognitive control in task switching. Journal of Neuroscience. 2006;26:1429–1438. doi: 10.1523/JNEUROSCI.3109-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]