Background: Chloroquine causes serious toxicity by accumulating in lysosomes.
Results: Depletion of α-tocopherol transfer protein caused more severe chloroquine toxicity in both cultured cells and mice.
Conclusion: α-Tocopherol transfer protein plays a role in protecting chloroquine toxicity by preventing chloroquine accumulation in the lysosomes.
Significance: This study describes a novel mechanism for the clearance of lysosomotropic amines.
Keywords: Lipid Binding Protein, Lysosomal Acidification, Lysosomes, Vacuolar Acidification, Vacuolar ATPase, Vitamin E, α-Tocopherol Transfer Protein, Niemann-Pick Type C1, Chloroquine
Abstract
Chloroquine (CQ) is a widely prescribed anti-malarial agent and is also prescribed to treat autoimmune diseases. Clinical treatment with CQ is often accompanied by serious side effects such as hepatitis and retinopathy. As a weak base, CQ accumulates in intracellular acidic organelles, raises the pH, and induces osmotic swelling and permeabilization of acidic organelles, which account for CQ-induced cytotoxicity. We reported previously that CQ treatment caused α-tocopherol transfer protein (α-TTP), a gene product of familial vitamin E deficiency, to change its location from the cytosol to the surface of acidic organelles. Here we show that α-TTP plays a novel role in protecting against CQ toxicity both in vitro and in vivo. In the presence of CQ, rat hepatoma McARH7777 cells, which do not express α-TTP endogenously, showed more severe cytotoxicity, such as larger vacuolation of acidic organelles and caspase activation, than α-TTP transfectant cells. Similarly, α-TTP knockout mice showed more severe CQ toxicity, such as hepatotoxicity and retinopathy, than wild-type mice. These effects were not ameliorated by vitamin E supplementation. In contrast to bafilomycin A1 treatment, which prevents CQ accumulation in cells by raising the pH of acidic organelles, α-TTP expression prevented CQ accumulation without affecting the pH of acidic organelles. Taken together, our data suggest that α-TTP protects against CQ toxicity by preventing CQ accumulation in acidic organelles through a mechanism distinct from vitamin E transport.
Introduction
Chloroquine (CQ)2 is a widely prescribed anti-malarial agent and is also used for treatment of autoimmune diseases including systemic lupus erythematodes and rheumatoid arthritis (1). However, CQ can have serious side effects such as retinopathy, gastrointestinal malaise, pruritus, and visual disturbances (2). Retinal toxicity of CQ has been extensively studied since its first description in 1957 (3). CQ also caused severe acute hepatitis in a patient with autoimmune disease (4) and led to fulminant hepatic failure in patients with chronic rheumatological disorders (5). As a weak base, CQ enters acidic organelles as an uncharged molecule, becoming protonated in the matrix of acidic organelles (6). The loss of protons by protonation of CQ causes the neutralization of the lumenal pH of acidic organelles. Accumulation of protonated CQ in acidic organelles induces osmotic swelling and the release of lysosomal hydrolases (7). Thus, neutralization of acidic organelles and the release of lysosomal hydrolases have been considered as the main causes of CQ-induced cytotoxicity.
α-Tocopherol transfer protein (α-TTP) is a cytosolic protein that binds α-tocopherol (vitamin E) and transfers it between separate cellular organelles. α-TTP is expressed mainly in the liver and regulates plasma α-tocopherol levels. α-TTP gene mutations cause severe vitamin E deficiency in humans, a disease known as familial isolated vitamin E deficiency (8). In addition, α-TTP KO mice show symptoms similar to those of familial isolated vitamin E deficiency patients (9).
We reported previously that CQ treatment causes α-TTP, which is normally distributed homogenously throughout the cytosol, to be translocated to the surface of acidic organelles in hepatocytes (10). Qian et al. (11) showed that α-TTP translocates transiently to the acidic organelles even in the absence of CQ in their α-TTP-transfected McARH7777 cells.
In this study, we found that McARH7777 cells, which do not express α-TTP endogenously, are more vulnerable to CQ toxicity than α-TTP-transfected McARH7777 cells. Consistent with these data, α-TTP KO mice showed more severe CQ toxicity, such as hepatitis and retinopathy, than wild-type mice. Accumulation of CQ in the acidic organelles of McARH7777 cells was suppressed by α-TTP expression, which may account for the protective effect of α-TTP against CQ cytotoxicity. We propose from these results that α-TTP is a novel endogenous determinant of CQ toxicity.
EXPERIMENTAL PROCEDURES
Cell Cultures, Reagents, and Animals
McARH7777, McA-TTP, McA-CT1, and McA-CT6 cells were grown as described previously (12). The mouse anti-rat monoclonal antibody against α-TTP (AT-R1) (13) or anti-myc mAb (9E10) was used for immunofluorescence studies. Specific anti-rat LAMP-1 antibody was a kind gift from Dr. Akasaki (The University of Fukuyama, Fukuyama, Japan). The anti-caspase-3 antibody and anti-NPC1 antibody were purchased from Cell Signaling Technology (Boston, MA) and Novus Biologicals (Littleton, CO), respectively. α-TTP knockout mice were a kind gift from Chugai (Shizuoka, Japan).
Cell Viability
Cell viabilities were quantified by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay and expressed as percentages of non-treated cells.
Immunocytochemistry and Acridine Orange Staining
McARH7777, McA-TTP, McA-CT1, and McA-CT6 were cultured on collagen-coated coverslips and immunostained as described previously (10). The anti-rat antibody against α-TTP (AT-R1), anti-myc antibody, and anti-rat LAMP-1 antibody were used. Acridine orange (AO) staining was performed as described previously (14). Cells were loaded with 5 μg/ml AO for 10 min at 37 °C and observed.
Hepatospecific Serum Markers and Histopathological Studies of Animal Experiments
Starting at 4 weeks of age, mice were fed a control diet (0.002 weight % α-tocopherol) or an α-tocopherol excess diet (0.1 weight % α-tocopherol) for 4 weeks. At 8 weeks of age, wild-type mice and α-TTP KO mice were divided into two groups, a control group (single oral administration of saline, n = 5) and a CQ-treated group (single oral administration of 100 mg/kg CQ, n = 5). Twenty-four hours after treatment, blood and liver were collected. Activity of serum AST was assayed by using the Transaminase C2-test kit (Wako Pure Chemical Industries, Osaka, Japan). Liver tissues were embedded in paraffin. 5-μm sections were stained with hematoxylin-eosin.
Histopathological Studies of Retinal Tissue
Male wild-type mice (n = 10) and male α-TTP KO mice (n = 10) were divided into 2 groups each, a control group (daily oral administration of saline, n = 5) and a CQ-treated group (daily oral administration of 100 mg/kg/day, n = 5). After 14 days of administration, the eyeballs were dissected and embedded in paraffin. 5-μm sections were stained with hematoxylin-eosin. The ganglion cells in the same range (500 μm) of the ganglion cell layer were counted.
Immunohistochemistry of Retinal Tissue
Immunohistochemistry was performed as described previously (15). The anti-mouse α-TTP antibody (A8-F1) or the glutamine synthetase antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were used.
Accumulation of [3H]CQ in McARH7777 and McA-TTP Cells
The levels of [3H]CQ were measured with a liquid scintillation counter (16). The McARH7777 and McA-TTP cells were seeded in 24-well plates. The following day, the medium was removed, and the cells were washed with 2 ml PBS (containing 0.5 mm MgCl2 and 0.9 mm CaCl2) at room temperature. The cells were incubated with 0.4 ml PBS with 10 mm glucose for 15 min. At t = 0 min, 100 μl of [3H]CQ ([3H]CQ = 40 nm, specific radioactivity = 5 Ci/mmol) in PBS with 10 mm glucose was added. Uptake was stopped by adding 2 ml of cold PBS after the indicated periods of time. Then PBS was removed, and cells were solubilized and spun for 10 min at 10,000 × g in a microcentrifuge. Supernatants were counted in a liquid scintillation counter. CQ uptake is expressed as pmol/mg protein.
RNA Interference
A control siRNA and an siRNA directed against the rat Niemann-Pick type C1 gene were obtained from Nippon EGT (Toyama, Japan). The siRNAs were transfected into cells by using Lipofectamine RNAiMAX according to the manufacturer's protocols.
RESULTS
α-TTP Inhibits CQ-induced Vacuole Formation and Cell Death
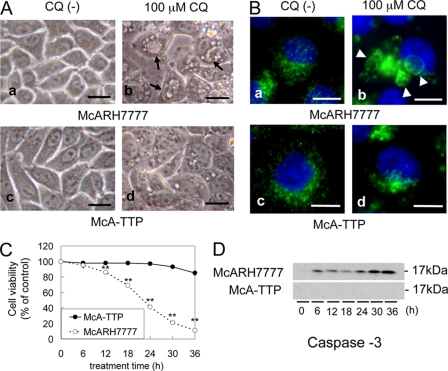
McARH7777 rat hepatoma cells, which do not express α-TTP endogenously (17), showed large vacuoles in their cytoplasm after addition of 100 μm CQ (Fig. 1A, b). Large vacuoles were stained by the antibody against LAMP-1, a major component of the lysosomal membrane, indicating that CQ treatment increased the size of acidic organelles (Fig. 1B, b). In contrast, smaller vacuoles were seen in α-TTP-transfected McARH7777 cells (McA-TTP) after the same treatment (Fig. 1A, d). At 100 μm CQ, the viability of McARH7777 cells decreased with time, dropping to only 11% of non-treatment controls after 36 h (Fig. 1C). In contrast, the viability of McA-TTP cells remained high (84% of non-treatment controls) under the same regimen.
FIGURE 1.
α-TTP inhibits CQ-induced vacuole formation and cell death. A and B, McARH7777 and McA-TTP cells were incubated for 18 h in the absence (a, c) or presence of 100 μm CQ (b, d). Cells were observed by using light-microscope (A) and immunostained with anti-LAMP-1 antibody (green) and DAPI (blue) B, arrows indicate vacuole formation, and arrowheads indicate enlarged acidic organelles. Scale bars = 10 μm. C, viabilities of McARH7777 and McA-TTP cells after 100 μm CQ treatment (mean ± S.D.). A time-dependent decrease of viability was shown for McARH7777 cells. **, p < 0.001 compared with the viability of McA-TTP cells (unpaired Student's t test). D, Western blot analysis of McARH7777 and McA-TTP cells before and after CQ treatment using anti-caspase-3 antibody.
Boya et al. (18) demonstrated that CQ provoked mitochondrial release of cytochrome c and the activation of caspase-3 as detected with an antibody specific for the large subunit of caspase-3 in HeLa cells. Consistent with this study, a 17-kDa activated caspase-3 band was detected in McARH7777 cells exposed to CQ for more than 6 h. This band was not detected in McA-TTP cells (Fig. 1D). From these results, we found that CQ-induced hepatocyte toxicity was greatly prevented by α-TTP expression in the cell.
CQ-induced Hepatotoxicity in the α-TTP Knockout Mice
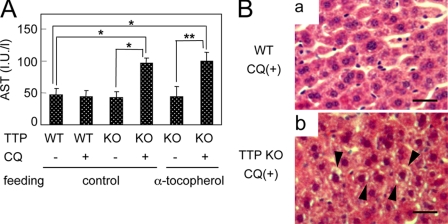
To determine whether α-TTP has the same effect against CQ-induced hepatotoxicity in vivo, we challenged α-TTP KO mice with CQ. No significant changes were evident in the AST activity of wild-type mice treated with 100 mg/kg CQ (Fig. 2A). In contrast, AST activity was significantly greater in CQ-treated α-TTP KO mice than in wild-type mice. In wild-type mice, no changes in liver histology were evident after CQ treatment under the present conditions (Fig. 2B, a). In α-TTP KO mice, however, hepatocyte degeneration and microvesicular formation were observed under the same conditions (Fig. 2B, b). No hepatic changes were detected in α-TTP KO mice not treated with CQ. These histopathological changes in hepatocytes of α-TTP KO mice were comparable with the changes in CQ-treated McARH7777 cells.
FIGURE 2.
α-TTP KO mice are easily affected by CQ hepatotoxicity. Male 8-week-old wild-type (WT) and α-TTP KO (KO) mice that were fed a control or α-tocopherol excess diet starting at 4 weeks of age were divided into two groups, a control group (single oral administration of saline) and a CQ-treated group (single oral administration of 100 mg/kg CQ) as described. A, changes of AST activity in α-TTP KO and WT mice. Significant effects were assessed by one-factor ANOVA (Tukey). *, p < 0.01; **, p < 0.001. B, liver tissues collected from CQ-treated WT mice (a) and α-TTP KO mice (b). Sections were stained with hematoxylin-eosin. Arrowheads indicate hepatocyte degeneration and microvesicular formation. Scale bars = 25 μm.
Enhanced CQ Toxicity in Retinal Tissue of α-TTP KO Mice
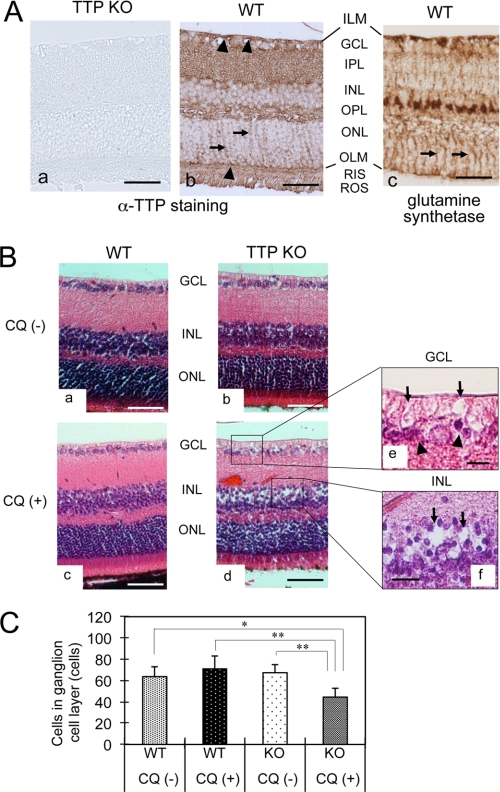
Although α-TTP was first described as a hepatic protein (17), it was subsequently localized in many other tissues, namely in rat brain (19), in pregnant mouse uterus (15), and in the retina (20). Because retinopathy is one of the most serious side effects of clinical treatment with CQ, we compared CQ-induced retinopathy between α-TTP KO mice and wild-type mice. In wild-type mice, immunostaining of α-TTP was observed throughout the retinal layers, including the inner limiting membrane, ganglion cell layer (GCL), inner plexiform layer, outer plexiform layer, outer limiting membrane, rod inner segments, and rod outer segments (Fig. 3A, b), whereas no remarkable immunostaining was observed in retinal sections from α-TTP KO mice (a). The immunostaining pattern of α-TTP was intense in both the inner limiting membrane and outer limiting membrane (arrowheads in Fig. 3A, b). In addition, fibriform vertical staining was observed in the outer nuclear layer (arrows in Fig. 3A, b). This staining pattern corresponds well to the immunostaining pattern of glutamine synthetase (Fig. 3A, c) (21), a specific marker of Müller glial cells, suggesting that α-TTP is expressed in Müller cells. Expressions of α-TTP was also observed in other cell types, including the retinal ganglion cells that correspond to immunostaining in GCL, the photoreceptor cells that correspond to immunostaining in rod inner segments and rod outer segments, and the vascular endothelial or perivascular cells.
FIGURE 3.
Localization of α-TTP in retinal tissue and CQ-induced histopathological changes of α-TTP KO mice. A, immunohistochemistry for α-TTP (a, b) and glutamine synthetase (c) in retinal tissues from α-TTP KO mice (a) and WT mice (b, c). Fibriform vertical staining, a specific staining pattern of Müller cell, is observed in both b and c (arrows). Scale bars = 50 μm. B, representative images of hematoxylin-eosin staining in retinal tissues from CQ-untreated WT (a) and α-TTP KO mice (b), and CQ-treated WT (c) and α-TTP KO mice (d–f). Disarrangement of GCL and INL because of presences of vacuolar spaces (arrows in e and f) and shrinkage of nuclei (arrowheads in e) are observed in retinal sections from CQ-treated α-TTP KO mice. Scale bars = 50 μm (a–d) and 10 μm (e and f). C, quantification of ganglion cell numbers in the GCL (means ± S.D., n = 5 in each group). Significant effects of treatment were assessed by two-factor analysis of variance (Tukey's post hoc test). *, p < 0.01, **, p < 0.001. ILM, inner limiting membrane; IPL, inner plexiform layer; OPL, outer plexiform layer; ONL, outer nuclear layer, OLM, outer limiting membrane; RIS, rod inner segment; ROS, rod outer segment.
The retinal cell layer, when stained with hematoxylin-eosin, was not remarkably changed in CQ-treated wild-type mice (Fig. 3B, c) but was remarkably disarranged in GCL and INL of CQ-treated α-TTP KO mice (Fig. 3B, d). The disarrangement of the retinal cell layer was caused by the formation of vacuolar spaces in the GCL (arrows in Fig. 3B, e) and INL (arrows in f) and by nuclear shrinkage in the GCL (arrowheads in e). The number of nuclei in the GCL was significantly lower in CQ-treated α-TTP KO mice than in the other three groups (Fig. 3C). This result, together with the nuclear shrinkage in the GCL, suggests a selective loss of retinal ganglion cells in the CQ-treated α-TTP KO mice. The localization of the vacuolar spaces observed in the GCL and INL coincide with the localization of cellular bodies of Müller glial cells, which extend to both the GCL and INL, and ganglion cells, which extend to the GCL. The vacuolar spaces in these cell layers correspond well with the microvesicular formations observed in CQ-treated McARH7777 cells. Taken together, these results strongly suggest that the retinal localizations of α-TTP are causally related to both the CQ-induced selective loss of ganglion cells and the specific microvesicular changes in Müller and/or ganglion cells.
Vitamin E Status Is Not Related to CQ-induced Hepatotoxicity
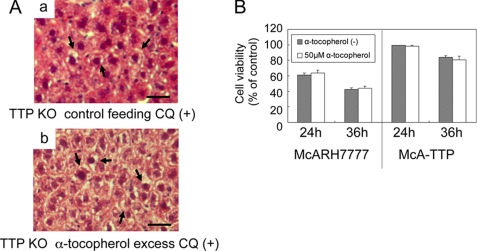
In α-TTP KO mice fed a normal diet (36 mg of α-tocopherol/kg diet), plasma α-tocopherol concentrations were very low (22). It is thus possible to assume that enhanced CQ-induced toxicity in α-TTP KO mice is related to the vitamin E status in the tissues and/or extracellular fluids. Feeding α-TTP KO mice a diet with excess α-tocopherol (567 mg α-tocopherol/kg diet) was found to increase plasma α-tocopherol levels to near the normal range (22) but did not prevent CQ-induced elevation of AST (Fig. 2A) or histopathological changes in the liver (Fig. 4A). Similarly, adding α-tocopherol to the culture media to a concentration of 50 μm did not affect the viability of McARH7777 cells after CQ treatment (Fig. 4B). Fifty micromolar α-tocopherol was found to show significant protection from MeHg-induced neuronal cell death in primary cultured cerebellar granule cells (23). These results imply the unexpected result that vitamin E is not able to protect against CQ-induced hepatotoxicity either in vitro or in vivo.
FIGURE 4.
α-Tocopherol is not able to protect against CQ toxicity. A, α-TTP KO mice were fed a control diet (a) or α-tocopherol excess diet (b). Liver tissues were collected 24 h after single oral administration of 100 mg/kg CQ. Sections were stained with hematoxylin-eosin. The arrows indicate hepatocyte degeneration and microvesicular formation. Scale bars = 25 μm. B, cell viabilities of McARH7777 and McA-TTP cells were measured 24 h and 36 h following treatment with 100 μm CQ with/without the addition 50 μm α-tocopherol.
Accumulation of α-TTP in Acidic Organelles Is Necessary for Inhibition of CQ Cytotoxicity
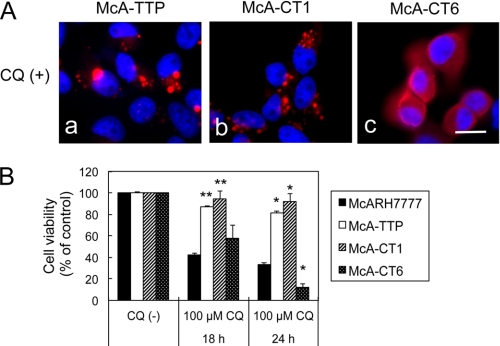
We reported previously that under CQ treatment, α-TTP translocates from the cytosol to the surface of acidic organelles, such as late endosomes and lysosomes, in cultured hepatocytes (10). We then examined whether translocation of α-TTP to acidic organelles is necessary for inhibition of CQ toxicity. By exploiting the fact that cellular retinaldehyde-binding protein (CRALBP), another member of the cytosolic lipid-binding/transfer protein family (Sec14 family), does not change its localization by CQ treatment, we previously constructed α-TTP and CRALBP chimeras to examine which part of α-TTP is necessary for the translocation (10). The CT1 chimera, in which a sequence of 20 amino acids from the N terminus of α-TTP was replaced with a sequence of 64 amino acids from the N-terminal sequence of CRALBP, was able to translocate from the cytosol to acidic organelles upon CQ treatment (Fig. 5A, b) and could prevent the CQ toxicity, resulting in a cell viability similar to that of the wild-type α-TTP (Fig. 5B). In contrast, the CT6 chimera, in which the first 50 amino acids from the N terminus of α-TTP were replaced with the first 94 amino acids from the N terminus of CRALBP, could not translocate upon CQ treatment (Fig. 5A, c) and could not prevent the CQ toxicity (Fig. 5B). As reported previously, both CT1 and CT6 chimeras are able to bind α-tocopherol (10). These results demonstrate that translocation of α-TTP to acidic organelles after CQ treatment was necessary to protect cells against CQ toxicity.
FIGURE 5.
Distribution of two chimeric proteins of α-TTP, CT1 and CT6, after treatment with CQ and the possible protection of McA-CT1 and McA-CT6 cells against CQ treatment. A, localization of α-TTP (a), CT1 (b), and CT6 (c) in the presence of CQ. Cells were incubated with 100 μm CQ for 6 h and immunostained. Scale bar = 10 μm. B, cell viabilities after treatment with 100 μm CQ for 18 and 24 h. Data were expressed as percentages of non-treated controls (mean ± S.D.). *, p < 0.01; **, p < 0.001, as compared with McARH7777 cells treated with same CQ concentrations under the same time periods (analysis of variance, Tukey's post hoc test).
Bafilomycin A1 Inhibits CQ-induced Cell Death
Because CQ has multiple effects on cellular physiology, such as acidic organelle neutralization (6) and sterol synthesis (24), we investigated the mechanism by which CQ causes liver cell death. As CQ inhibits lanosterol synthase, we tested other lanosterol synthase inhibitors such as N,N-dimethyldodecylamine N-oxide, and found no such toxicity in McARH7777 cells. Shacka et al. (25) reported that bafilomycin A1, a specific inhibitor of the vacuolar type H+-ATPase, inhibits CQ-induced cell death of mouse cerebellar granule neurons. Bafilomycin A1 raises the pH of acidic organelles and therefore suppresses the accumulation of CQ in them. Pretreatment of McARH7777 cells with bafilomycin A1 dose-dependently inhibited CQ toxicity (supplemental Fig. S1), as it does in other cell types. Twelve hours after pretreating McARH7777 cells with 100 nm bafilomycin A1, no vacuoles were observed, even in the presence of CQ.
Low pH Is Maintained in the Acidic Compartments of α-TTP-expressing Cells Even in the Presence of CQ
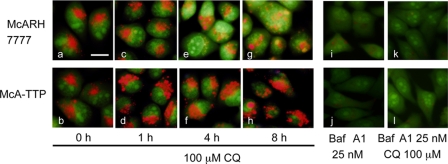
We hypothesized by analogy with the effect of bafilomycin A1 that the pH in the acidic organelles is raised in McA-TTP cells. To examine if α-TTP affects pH-changes in acidic organelles after CQ treatment, we used AO, a fluorescent weak base that accumulates in acidic spaces (14). The increase of lumenal pH induces the AO fluorescence color changes from orange to yellow and, finally, to green. As expected, pretreatment with bafilomycin A1 increased immediately the lumenal pH of acidic compartments, even in the presence of 100 μm CQ (Fig. 6, i, j, k, and l). In McARH7777 cells, the lumenal pH of acidic compartments was increased time-dependently by CQ (Fig. 6, a, c, e, and g). Conversely, in McA-TTP cells, the lumenal pH of acidic compartments was not increased even in the presence of CQ (Fig. 6, b, d, f, and h). These results imply that α-TTP functions to preserve the lumenal low pH against CQ-induced acidic organelle neutralization and that α-TTP protects liver cells from CQ toxicity through a mechanism distinct from the mechanism of bafilomycin A1.
FIGURE 6.
Changes of lumenal pH in CQ-treated McARH7777 and McA-TTP cells. McARH7777 (a, c, e, g, i, and k) and McA-TTP cells (b, d, f, h, j, and l) were stained with acridine orange. Cells were treated with 100 μm CQ for 0 h (a and b), 1 h (c and d), 4 h (e and f), and 8 h (g and h). i and j show cells treated with 25 nm bafilomycin A1 (Baf A1). k and l show cells pre-treated with 25 nm bafilomycin A1 for 45 min before the addition of 100 μm CQ. Scale bar = 10 μm.
CQ Is Not Accumulated in α-TTP-expressing Cells
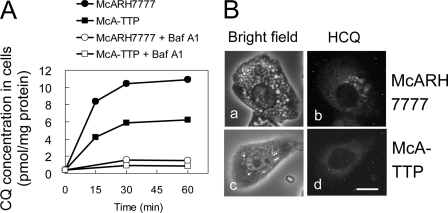
van Es et al. (16) reported that [3H]CQ added to the medium accumulated time-dependently in Chinese hamster ovary cells. Furthermore, the accumulation was abolished by bafilomycin A1, indicating that most of [3H]CQ taken up by the cell is accumulated in the acidic organelles. We performed the same type of experiments using McARH7777 and McA-TTP cells. [3H]CQ accumulated in McARH7777 cells, but the accumulation was strongly inhibited by bafilomycin A1 (Fig. 7A). In contrast, accumulation of [3H]CQ was significantly lower in McA-TTP cells than in McARH7777 cells (Fig. 7A). Hydroxychloroquine (HCQ), another lysosomotropic antimalarial drug, is known to be fluorescent (18). Similarly to CQ, treatment with HCQ induced significant vacuole formation in the cytoplasm of McARH7777 cells but not in the cytoplasm of McA-TTP cells (Fig. 7B, a and c). Intracellular localization of HCQ was determined by fluorescence microscopy. Autofluorescence of HCQ was detected as cytoplasmic granular pattern in McARH7777 cells (Fig. 7B, b), whereas weak autofluorescence was detected in the cytosol of McA-TTP cells (Fig. 7B, d). These results are compatible with the above observation that the lumenal pH of acidic compartments was not increased by CQ in McA-TTP cells and indicate that α-TTP functions to prevent CQ accumulation in acidic organelles.
FIGURE 7.
Accumulation of [3H]CQ and HCQ in McARH7777 cells and McA-TTP cells. A, cells were preincubated without/with 50 nm bafilomycin A1 (Baf A1) before the addition of [3H]CQ. B, subcellular localization of HCQ in McARH7777 (a and b) and McA-TTP cells (c and d). Cells were treated with 100 μm HCQ for 6 h. Autofluorescence of HCQ (excitation 480 ± 40 nm, emission 527 ± 30 nm) was detected as described previously (13). Bright field image (a and c) and fluorescence image of HCQ (b and d) in same cells. Scale bar = 10 μm.
Niemann-Pick Type C1 Protein Is Involved in the Prevention of CQ Accumulation in α-TTP-expressing Cells
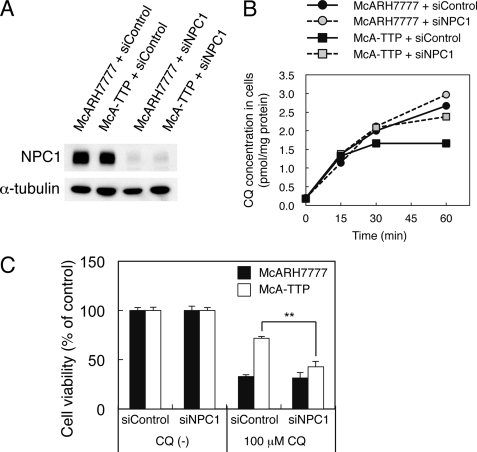
Because Niemann-Pick type C1 (NPC1) protein plays a role in the clearance of lysosomal amines (26–29), we examined whether it is also involved in the prevention of CQ accumulation in α-TTP-expressing cells. Western blotting showed that McA-TTP cells and McARH7777 cells expressed similar levels of NPC1 protein. Transfection of an siRNA against NPC1 efficiently reduced protein levels of NPC1 both in McARH7777 and McA-TTP cells (Fig. 8A). NPC1 knockdown slightly increased the cellular [3H]CQ level in McARH7777 cells. Interestingly, NPC1 knockdown in McA-TTP cells increased the cellular [3H]CQ level to near that in control McARH7777 cells (Fig. 8B). Consistent with this, NPC1 knockdown canceled the protective effect of α-TTP against CQ toxicity (Fig. 8C). These results indicate that NPC1 is involved in α-TTP-mediated prevention of CQ accumulation.
FIGURE 8.
NPC1 is required for the prevention of CQ accumulation in α-TTP-expressing cells. A, a control siRNA (siControl) and NPC1 siRNA (siNPC1) were transfected into McARH777 and McA-TTP cells. After 72 h, cell lysates were prepared and subjected to a Western blot analysis using anti-NPC1 antibody. α-Tubulin expression was used as a loading control. B and C, cells were transfected with the indicated siRNA. At 72 h after transfection, accumulation of [3H]CQ in cells (B) and cell viabilities after treatment with 100 μm CQ for 24 h (C) were measured. **, p < 0.001 (analysis of variance, Tukey's post hoc test).
DISCUSSION
In this study, we showed that hepatotoxicity and retinopathy, prominent side effects of CQ, are more severe in α-TTP KO mice than in wild-type mice. Similarly, rat hepatoma McARH7777 cells, which do not express α-TTP endogenously, are more vulnerable to CQ-induced cytotoxicity than the α-TTP transfectant cells.
Vitamin E concentrations in the plasma of α-TTP KO mice are known to be very low. However, raising their plasma vitamin E concentration to near normal levels by adding vitamin E to the diet did not prevent the toxicity. This indicates that vitamin E deficiency was not a cause of CQ-induced hepatotoxicity and retinopathy in α-TTP KO mice. Adding excess vitamin E to the culture medium of McARH7777 cells also did not prevent CQ toxicity. Together, these data support the idea that vitamin E level is not related to CQ toxicity in α-TTP KO mice.
CQ, a lysosomotropic amine, enters acidic organelles as an uncharged molecule together with anions (e.g. chloride), becoming protonated in the matrix of the organelles (6). The loss of protons by protonation of CQ neutralizes the pH of the acidic organelles. Accumulation of protonated CQ in the acidic organelles induces osmotic swelling of these organelles and permeabilization of their membranes (7). Increased permeabilization should cause a release of lysosomal hydrolases such as cathepsins into the cytoplasm, which may in turn destroy cellular components. Release of lysosomal enzymes into the cytoplasm also induces apoptosis, either by directly activating caspase-11 (30) or by indirectly activating caspase-3 through mitochondrial membrane permeabilization (18). Indeed, CQ treatment in McARH7777 cells resulted in caspase-3 activation. Because bafilomycin A1, which raises the pH of acidic organelles, did not induce cell death but rather inhibited CQ toxicity, osmotic swelling and release of lysosomal enzymes rather than neutralization of lysosomal pH may be the major cause of at least the acute phase CQ toxicity.
Bafilomycin A1 raises the pH of acidic organelles, which in turn prevents protonation of CQ in the luminal space and subsequent osmotic swelling of acidic organelles. In sharp contrast, the expression of α-TTP did not affect the pH in the acidic organelles. The continuous proton-pumping into acidic organelles should cause protonated CQ to accumulate in the organelles, leading to osmotic swelling. However, enlarged acidic organelles were not observed in CQ-treated McA-TTP cells. Moreover, CQ was less concentrated in McA-TTP cells than in McARH7777 cells. Together, these results indicate that α-TTP prevents CQ accumulation in acidic organelles, thus preserving their low pH.
The hydrophobic pocket of α-TTP specifically binds to α-tocopherol (31). In this study, we performed several experiments to determine whether α-TTP can bind CQ as a ligand. Hosomi et al. (13) reported that α-tocopherol that had been incorporated into liposomes could be transferred to recombinant α-TTP and then transferred to other acceptor liposomes. We could not perform the same type of experiment with CQ because CQ cannot be incorporated into liposomes, possibly because it is less hydrophobic than α-tocopherol. We also examined the inhibitory action of CQ on the α-TTP-mediated α-tocopherol transfer and found no significant inhibition, even when CQ was present in excess (supplemental Fig. S2). These results indicate that CQ, unlike α-tocopherol, does not bind to the hydrophobic pocket of α-TTP. This idea is also supported by the observation that excess vitamin E did not affect CQ toxicity either in vitro or in vivo. α-TTP is mainly located in the cytosol under normal conditions but accumulates on the surface of enlarged acidic organelles upon CQ treatment (10). The present data also suggest that the binding of α-TTP to acidic organelles is essential for preventing CQ toxicity. Taken together, our results suggest a novel role for α-TTP. It prevents accumulation of CQ in acidic organelles by association with their surface through a mechanism distinct from vitamin E transport.
Although the mechanistic basis for the clearance of the lysosomal amines has remained unclear, several lines of evidence suggest that NPC1 has a functional role in their clearance. For example, the accumulation of a lysosomotropic drug in lysosomes of fibroblasts from NPC1 patients was greater than from normal subjects (28). Substantial amounts of endogenous hydrophobic amines such as sphingosine are concentrated in the lysosomes of liver from mice with NPC disease (26), and Lloyd-Evans et al. (32) demonstrated that sphingosine accumulation in lysosomes is an initiating factor in NPC1 disease pathogenesis. Krise and co-workers (29) proposed that NPC1 mediates lysosomal amine clearance through vesicular traffic rather than functions as a transmembrane pump (27). The observation that the decrease of the cellular CQ level by α-TTP expression was canceled by NPC1 knockdown (Fig. 8C) indicates that α-TTP-dependent prevention of CQ accumulation is mediated by NPC1. This idea is also supported by the fact that α-TTP suppresses the accumulation of CQ without affecting lysosomal pH. At present, it is unclear how α-TTP activates NPC. Considering that the binding of α-TTP to the surface of acidic organelles is essential for lowering CQ toxicity, it is likely that α-TTP binds to NPC1 on the lysosomal membrane and stimulates the NPC1 activity. However, we could not detect direct binding of α-TTP to NPC1 with immunoprecipitation experiments. Moreover, NPC1 knockdown did not affect the lysosomal association of α-TTP upon CQ treatment. Further studies are needed to clarify how α-TTP participates in NPC1-mediated CQ clearance.
In this study, we also showed that α-TTP is localized in Müller cells of the mouse retina. Müller cells are the principal glial cells of the retina, responsible for many functions carried out by glial cells in the central nervous system (33). Because the photoreceptor outer segment membrane contains unusually high levels of polyunsaturated fatty acids (34), they are easily damaged by oxidation. α-TTP expressed in Müller cells may facilitate the transport of vitamin E from the plasma to neurons. Although patients with familial isolated vitamin E deficiency sometimes show retinitis pigmentosa at relatively old ages, the retinas of 8- to 12-week-old α-TTP KO mice appeared normal. CQ-induced retinopathy in α-TTP KO mice was not rescued by supplementation of excess vitamin E, suggesting that the more severe CQ-induced retinopathy in α-TTP KO mice than in wild-type mice is not due to severe oxidative stress. Our findings of vacuolation in Müller cells in CQ-treated α-TTP KO mice indicate that a mechanism similar to the vacuolation mechanism in hepatocytes is operating in the retina. Dysfunction of Müller cells may affect the viability of other retinal cells, such as neurons. The most prominent side effect of CQ is retinopathy. The American Academy of Ophthalmology has identified several risk factors for CQ retinopathy, including the dosage and duration of use, body habitus, renal or liver function, concomitant retinal disease, and age (35). On the basis of our present studies, α-TTP deficiency is considered as a new risk factor leading to CQ retinopathy.
Supplementary Material
This work was supported by the Core Research for Evolutional Science and Technology, Japan Science and Technology Agency (to H. A.), the Program for Promotion of Basic and Applied Research for Innovations in Bio-Oriented Industry (to H. A.), Grants-in-aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology, and the Japanese Ministry of Health, Labor, and Welfare (to H. A.).

This article contains supplemental Figs. S1 and S2.
- CQ
- chloroquine
- α-TTP
- α-tocopherol transfer protein
- AO
- acridine orange
- AST
- aspartate aminotransferase
- GCL
- ganglion cell layer
- INL
- inner nuclear layer
- CRALBP
- cellular retinaldehyde-binding protein
- LDAO
- N,N-dimethyldodecylamine N-oxide
- HCQ
- hydroxychloroquine
- NPC1
- Niemann-Pick type C1.
REFERENCES
- 1. Wozniacka A., Lesiak A., Narbutt J., Kobos J., Pavel S., Sysa-Jedrzejowska A. (2007) Chloroquine treatment reduces the number of cutaneous HLA-DR+ and CD1a+ cells in patients with systemic lupus erythematosus. Lupus 16, 89–94 [DOI] [PubMed] [Google Scholar]
- 2. Ekpechi O. L., Okoro A. N. (1964) A Pattern of Pruritus due to Chloroquine. Arch. Dermatol. 89, 631–632 [DOI] [PubMed] [Google Scholar]
- 3. Cambiaggi A. (1957) Unusual ocular lesions in a case of systemic lupus erythematosus. AMA Arch. Ophthalmol. 57, 451–453 [DOI] [PubMed] [Google Scholar]
- 4. Giner Galvañ V., Oltra M. R., Rueda D., Esteban M. J., Redón J. (2007) Severe acute hepatitis related to hydroxychloroquine in a woman with mixed connective tissue disease. Clin. Rheumatol. 26, 971–972 [DOI] [PubMed] [Google Scholar]
- 5. Makin A. J., Wendon J., Fitt S., Portmann B. C., Williams R. (1994) Fulminant hepatic failure secondary to hydroxychloroquine. Gut 35, 569–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wibo M., Poole B. (1974) Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J. Cell Biol. 63, 430–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michihara A., Toda K., Kubo T., Fujiwara Y., Akasaki K., Tsuji H. (2005) Disruptive effect of chloroquine on lysosomes in cultured rat hepatocytes. Biol. Pharm. Bull. 28, 947–951 [DOI] [PubMed] [Google Scholar]
- 8. Ouahchi K., Arita M., Kayden H., Hentati F., Ben Hamida M., Sokol R., Arai H., Inoue K., Mandel J. L., Koenig M. (1995) Ataxia with isolated vitamin E deficiency is caused by mutations in the α-tocopherol transfer protein. Nat. Genet. 9, 141–145 [DOI] [PubMed] [Google Scholar]
- 9. Yokota T., Igarashi K., Uchihara T., Jishage K., Tomita H., Inaba A., Li Y., Arita M., Suzuki H., Mizusawa H., Arai H. (2001) Delayed-onset ataxia in mice lacking α-tocopherol transfer protein. Model for neuronal degeneration caused by chronic oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 98, 15185–15190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horiguchi M., Arita M., Kaempf-Rotzoll D. E., Tsujimoto M., Inoue K., Arai H. (2003) pH-dependent translocation of α-tocopherol transfer protein (α-TTP) between hepatic cytosol and late endosomes. Genes Cells 8, 789–800 [DOI] [PubMed] [Google Scholar]
- 11. Qian J., Morley S., Wilson K., Nava P., Atkinson J., Manor D. (2005) Intracellular trafficking of vitamin E in hepatocytes. The role of tocopherol transfer protein. J. Lipid Res. 46, 2072–2082 [DOI] [PubMed] [Google Scholar]
- 12. Arita M., Nomura K., Arai H., Inoue K. (1997) α-Tocopherol transfer protein stimulates the secretion of α-tocopherol from a cultured liver cell line through a brefeldin A-insensitive pathway. Proc. Natl. Acad. Sci. U.S.A. 94, 12437–12441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hosomi A., Arita M., Sato Y., Kiyose C., Ueda T., Igarashi O., Arai H., Inoue K. (1997) Affinity for α-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 409, 105–108 [DOI] [PubMed] [Google Scholar]
- 14. Soyombo A. A., Tjon-Kon-Sang S., Rbaibi Y., Bashllari E., Bisceglia J., Muallem S., Kiselyov K. (2006) TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J. Biol. Chem. 281, 7294–7301 [DOI] [PubMed] [Google Scholar]
- 15. Kaempf-Rotzoll D. E., Igarashi K., Aoki J., Jishage K., Suzuki H., Tamai H., Linderkamp O., Arai H. (2002) α-Tocopherol transfer protein is specifically localized at the implantation site of pregnant mouse uterus. Biol. Reprod. 67, 599–604 [DOI] [PubMed] [Google Scholar]
- 16. van Es H. H., Renkema H., Aerts H., Schurr E. (1994) Enhanced lysosomal acidification leads to increased chloroquine accumulation in CHO cells expressing the pfmdr1 gene. Mol. Biochem. Parasitol. 68, 209–219 [DOI] [PubMed] [Google Scholar]
- 17. Sato Y., Arai H., Miyata A., Tokita S., Yamamoto K., Tanabe T., Inoue K. (1993) Primary structure of α-tocopherol transfer protein from rat liver. Homology with cellular retinaldehyde-binding protein. J. Biol. Chem. 268, 17705–17710 [PubMed] [Google Scholar]
- 18. Boya P., Gonzalez-Polo R. A., Poncet D., Andreau K., Vieira H. L., Roumier T., Perfettini J. L., Kroemer G. (2003) Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 22, 3927–3936 [DOI] [PubMed] [Google Scholar]
- 19. Hosomi A., Goto K., Kondo H., Iwatsubo T., Yokota T., Ogawa M., Arita M., Aoki J., Arai H., Inoue K. (1998) Localization of α-tocopherol transfer protein in rat brain. Neurosci. Lett. 256, 159–162 [DOI] [PubMed] [Google Scholar]
- 20. Yokota T., Shiojiri T., Gotoda T., Arita M., Arai H., Ohga T., Kanda T., Suzuki J., Imai T., Matsumoto H., Harino S., Kiyosawa M., Mizusawa H., Inoue K. (1997) Friedreich-like ataxia with retinitis pigmentosa caused by the His101Gln mutation of the α-tocopherol transfer protein gene. Ann. Neurol. 41, 826–832 [DOI] [PubMed] [Google Scholar]
- 21. Linser P. J., Sorrentino M., Moscona A. A. (1984) Cellular compartmentalization of carbonic anhydrase-C and glutamine synthetase in developing and mature mouse neural retina. Brain Res. 315, 65–71 [DOI] [PubMed] [Google Scholar]
- 22. Jishage K., Arita M., Igarashi K., Iwata T., Watanabe M., Ogawa M., Ueda O., Kamada N., Inoue K., Arai H., Suzuki H. (2001) α-Tocopherol transfer protein is important for the normal development of placental labyrinthine trophoblasts in mice. J. Biol. Chem. 276, 1669–1672 [DOI] [PubMed] [Google Scholar]
- 23. Shichiri M., Takanezawa Y., Uchida K., Tamai H., Arai H. (2007) Protection of cerebellar granule cells by tocopherols and tocotrienols against methylmercury toxicity. Brain Res. 1182, 106–115 [DOI] [PubMed] [Google Scholar]
- 24. Chen H. W., Leonard D. A. (1984) Chloroquine inhibits cyclization of squalene oxide to lanosterol in mammalian cells. J. Biol. Chem. 259, 8156–8162 [PubMed] [Google Scholar]
- 25. Shacka J. J., Klocke B. J., Shibata M., Uchiyama Y., Datta G., Schmidt R. E., Roth K. A. (2006) Bafilomycin A1 inhibits chloroquine-induced death of cerebellar granule neurons. Mol. Pharmacol. 69, 1125–1136 [DOI] [PubMed] [Google Scholar]
- 26. Goldin E., Roff C. F., Miller S. P., Rodriguez-Lafrasse C., Vanier M. T., Brady R. O., Pentchev P. G. (1992) Type C Niemann-Pick disease. A murine model of the lysosomal cholesterol lipidosis accumulates sphingosine and sphinganine in liver. Biochim. Biophys. Acta 1127, 303–311 [DOI] [PubMed] [Google Scholar]
- 27. Davies J. P., Chen F. W., Ioannou Y. A. (2000) Transmembrane molecular pump activity of Niemann-Pick C1 protein. Science 290, 2295–2298 [DOI] [PubMed] [Google Scholar]
- 28. Gong Y., Duvvuri M., Duncan M. B., Liu J., Krise J. P. (2006) Niemann-Pick C1 protein facilitates the efflux of the anticancer drug daunorubicin from cells according to a novel vesicle-mediated pathway. J. Pharmacol. Exp. Ther. 316, 242–247 [DOI] [PubMed] [Google Scholar]
- 29. Kaufmann A. M., Krise J. P. (2008) Niemann-Pick C1 functions in regulating lysosomal amine content. J. Biol. Chem. 283, 24584–24593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schotte P., Van Criekinge W., Van de Craen M., Van Loo G., Desmedt M., Grooten J., Cornelissen M., De Ridder L., Vandekerckhove J., Fiers W., Vandenabeele P., Beyaert R. (1998) Cathepsin B-mediated activation of the proinflammatory caspase-11. Biochem. Biophys. Res. Commun. 251, 379–387 [DOI] [PubMed] [Google Scholar]
- 31. Min K. C., Kovall R. A., Hendrickson W. A. (2003) Crystal structure of human α-tocopherol transfer protein bound to its ligand. Implications for ataxia with vitamin E deficiency. Proc. Natl. Acad. Sci. U.S.A. 100, 14713–14718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lloyd-Evans E., Morgan A. J., He X., Smith D. A., Elliot-Smith E., Sillence D. J., Churchill G. C., Schuchman E. H., Galione A., Platt F. M. (2008) Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 14, 1247–1255 [DOI] [PubMed] [Google Scholar]
- 33. Newman E., Reichenbach A. (1996) The Müller cell. A functional element of the retina. Trends Neurosci. 19, 307–312 [DOI] [PubMed] [Google Scholar]
- 34. Neuringer M., Anderson G. J., Connor W. E. (1988) The essentiality of n-3 fatty acids for the development and function of the retina and brain. Annu. Rev. Nutr. 8, 517–541 [DOI] [PubMed] [Google Scholar]
- 35. Marmor M. F., Carr R. E., Easterbrook M., Farjo A. A., Mieler W. F. (2002) Recommendations on screening for chloroquine and hydroxychloroquine retinopathy. A report by the American Academy of Ophthalmology. Ophthalmology 109, 1377–1382 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.