Abstract
Objective To quantify the separate contributions of menopausal hormone treatment and mammography screening activities on trends in incidence of invasive breast cancer between 1987 and 2008.
Design Population study using aggregated data analysed by an extended age-period-cohort model.
Setting Norway.
Population Norwegian women aged 30-90 between 1987 and 2008, including 50 102 newly diagnosed cases of invasive breast cancer.
Main outcomes measures Attributable proportions of mammography screening and hormone treatment to recent incidence of invasive breast cancer, and the remaining variation in incidence after adjustment for mammography screening and hormone treatment.
Results The incidence of invasive breast cancer in Norway increased steadily until 2002, levelled off, and then declined from 2006. All non-linear changes in incidence were explained by use of hormone treatment and mammography screening activities, with about similar contributions of each factor. In 2002, when the incidence among women aged 50-69 was highest, an estimated 23% of the cases in that age group could be attributed to mammography screening and 27% to use of hormone treatment.
Conclusions Changes in incidence trends of invasive breast cancer since the early 1990s may be fully attributed to mammography screening and hormone treatment, with about similar contributions of each factor.
Introduction
In most countries the incidence of invasive breast cancer has increased steadily for several decades, but recently the incidence has levelled off or declined in many developed countries.1 2 3 4 It is well known that use of both hormone treatment and mammography screening increases the risk of breast cancer,5 6 and it has been suggested that the recently observed decrease in incidence may reflect the reduction in hormone treatment use during recent years.1 2 4 7 8 9 10 A relative decrease in incidence could also be expected after the first wave of public mammography screening, since the initial screening may detect a larger number of preclinical cases compared with subsequent screening rounds.5 In several countries, systematic mammography screening was introduced around the time when the use of hormone treatment was at its highest, and both factors involve roughly the same age groups of women.5 It is therefore a challenge to separate the contributions of hormone treatment and mammography screening on trends in incidence of breast cancer.
Norway has a high quality nationwide cancer registry,11 county specific information on sales of hormone treatment, and a public mammography screening programme that was gradually introduced between 1995 and 2004. This makes Norway well suited for the study of how hormone treatment and mammography screening could have contributed to the observed patterns in breast cancer. Using Norwegian data we separated the contributions of hormone treatment use and mammography screening on recent trends in incidence of invasive breast cancer.
Methods
We used data from different sources to separate the contributions of mammography screening and hormone treatment use on recent incidence trends in invasive breast cancer, including data on incident cases of breast cancer, population figures, sales figures for hormone treatment, and data on public mammography screening patterns (see web extra figure on bmj.com).
The clinical treatment of ductal carcinoma in situ and invasive breast cancer is similar, but their recent trends in incidence differ and the detection of ductal carcinoma in situ seems to be considerably more strongly associated with mammography screening than with invasive disease.12 13 Therefore we restricted the end point of this study to invasive breast cancer, to achieve a clear and more uniform analysis where the results are easier to interpret. Overall, the proportion of cases of ductal carcinoma in situ has never exceeded 10% of breast malignancies in Norway. However, in the overall evaluation of the effects of mammography screening, ductal carcinoma in situ should be included in the analysis, as a large proportion of cases may represent over-diagnoses.14 15
For each of the 19 Norwegian counties we aggregated annual population data and data on invasive breast cancer. We collected data by one year age groups for each calendar year, and included all women between 30 and 90 years of age and living in Norway during the study period, from 1987 to 2008. This age range was chosen to strengthen contrasts between cohorts with different levels of hormone treatment and mammography screening, and simultaneously to avoid age groups with a low number of breast cancer cases that would yield imprecise estimates. Reporting of cancer to Norway’s cancer registry is mandatory, and information is obtained separately from clinicians, pathologists, and death certificates. Only 0.2% of cancer cases are ascertained from death certificates only.11 Norwegian citizens are identified by a unique 11 digit personal identification number that includes date of birth. The authorities keep a record of place of residence, and vital status is carefully monitored. Thus the population at risk of breast cancer is clearly defined.16
In 1995 the Norwegian breast cancer screening programme was initiated by the Norwegian government. It was first established in four counties in 1995/6, then gradually extended to the remaining 15 counties, until the programme reached national coverage in 2004/5.13 17 Biannually, all women aged between 50 and 69 years receive a written invitation to the screening programme, and two view mammograms are independently evaluated by two readers. Attendance to the programme has been about 78%.18 For administrative purposes, almost all women are invited by birth cohort. This results in a small departure from the official screening age, but for all practical purposes we assumed that screening took place between ages 50 and 69, when the screening variables were calculated.
Before and also after the introduction of public screening, private clinics have offered mammography screening. The exact volume of the private activity is not known, but a questionnaire survey at the first public screening revealed that a substantial proportion of women had attended mammography screening before entering the public screening programme.18 Preliminary inquiries at the Norwegian cancer registry, however, indicate that screening at private clinics only contributes to a small proportion of the total number of screening examinations after the public screening programme was introduced.
All sales of hormone treatment in Norway are recorded in the wholesales drug statistics database at the Norwegian Institute of Public Health. Total sales statistics have been available in electronic form since 1987, with separate statistics for preparations containing only oestrogens (G03C according to the Anatomical Therapeutic Chemical classification, www.whocc.no/) and combined preparations of progestogens and oestrogens (G03F). Since 1999, county specific data on sales of hormone treatment have been available in electronic form from all 19 counties. Between 1992 and 1998, county specific data were available in electronic form only in four counties. For the other counties, and before 1992, county specific data on sales of hormone treatment had to be collected from paper lists and adjusted according to newer Anatomical Therapeutic Classification and defined daily dose definitions. Therefore we collected county specific data for 1987, 1991, and 1995 to use in the estimation of the distribution of sales in different counties before 1999 (see web extra table on bmj.com for an overview of data).
The statistics on sales do not include the age distribution of users, but in 2004 the Norwegian prescription database was established to include all prescribed drugs in Norway. Thus prescription drugs are now registered on a personal identification form, and we used the age of the registered users to relate hormone treatment to the women’s age. Information on age was collected separately for total use, use of oestrogens alone, and use of oestrogen-progestogen combinations.
Statistical methods and modelling approach
Choice of a basic breast cancer incidence model
Age-period-cohort models may separate the effects of age from the effects of risk factors related to calendar period and birth cohort.19 20 The incidence of breast cancer increases with age,5 21 and there is a substantial birth cohort effect owing to variation in age at menarche and variation in parity patterns.5 22 We therefore included separate variables for age, cohort, and period effects, and gradually extended the model to include information on mammography screening and use of hormone treatment.5 23 Thus we could evaluate the association of hormone treatment and mammography screening with trends in incidence and assess the remaining period effects that could not be accounted for by age, cohort, hormone treatment, and mammography screening.
Screening variables
We modelled the potential effect of screening as suggested previously,5 using three variables that cover different phases of the mammography screening programme. One variable covered the first screening round, with the expected increase in incidence of breast cancer owing to the detection of prevalent but not yet detected cases. A second variable covered the subsequent screening rounds. A third variable modelled a potential drop in incidence for women who had already been screened, since some breast cancers were probably detected already as part of earlier mammography screening. Motivated by previously estimated mammography screening lead time,24 25 we designed a third variable to cover the first five years after leaving the screening programme. All screening variables were modelled as continuous proportions calculated for each age, period, and county combination.
Hormone treatment variables
For each combination of age, period, and county we estimated the proportion of current users of hormone treatment. For years with limited information on county specific sales of hormone treatment (see web extra table), we distributed the use of hormone treatment between counties using linear interpolation between the previously observed county specific hormone sales and the next county specific hormone sales. Although information on age at hormone treatment was provided by the Norwegian prescription database, this only dates back to 2004, so we used the age distribution in 2004 as an approximation for the age distribution of use before 2004. The data showed that a small proportion of hormone treatment was prescribed to men. In calculating the hormone treatment variable, we accounted for the use among men and scaled the hormone treatment variable according to reported daily doses. As a time lag probably exists between use of hormone treatment and the potential influence on trends in incidence of breast cancer, we examined the effects of adding different lag times to the hormone treatment data when calculating the hormone treatment variable.
For the period covered by our study, we estimated that among women aged 30-90 years hormone treatment was used by 3% in 1987, 15% in 2000, and by 6% in 2008. In the 50-69 year age group, the corresponding proportions were 7%, 33%, and 12%.
Statistical methods and modelling age, period, and cohort effects
Based on county specific one year age-period tables, we modelled the incidence of breast cancer using log-linear Poisson regression.19 20 The full model could be written as:
Ra,p=exp(Aa+Cc+Pp+S1×scr1×scr2+S3×scr3+H×ht)
where Ra,p is the incidence rate in age group a in calendar period p, Aa is the age component for age group a, Cc is the cohort component for birth cohort c, Ppis the period component for year p, and S1, S2, and S3 are regression coefficients (parameters) for the screening variables scr1, scr2, and scr3, the initial screening variable scr1, the subsequent screening variable, scr2, and the first five years after leaving the screening programme variable, scr3, respectively, and H is the regression coefficient for the hormone treatment variable ht.
In practice, the cohort variable will model effects related to changes in fertility patterns (for example, age at first birth, number of children) and other known risk factors related to birth cohort. Age is another key variable, owing to the strong age related increase in incidence.5 As for period effects, we tested their statistical contribution and removed effects that were not statistically significant from the model. For model comparisons, we evaluated the differences in model deviance and the related P values from goodness of fit tests at a 5% significance level using the difference in the observed deviance and the degrees of freedom between a set of nested models. To evaluate overall model fit, we studied over-dispersion using methods of quasi likelihood, and tested for any statistically significant deviations from the random variation of the assumed model.
To assess statistical uncertainty, we calculated 95% confidence intervals for the estimated regression coefficients. In plots, we estimated smoothed curves using the R statistical package “supsmu” implementation of Jerome Friedman super smoother algorithm (where the degree of smoothing is chosen by leave-one-out cross validation).26 Since the evaluation of time related changes of incidence curves is not suited to orderly confidence intervals, we used bootstrap replications to guide the evaluation of estimated curves of age and cohort components (not shown). All calculations, simulations, and plots were done using the R statistical package.26
Results
Incidence trends of invasive breast cancer in Norway, hormone treatment use, and screening
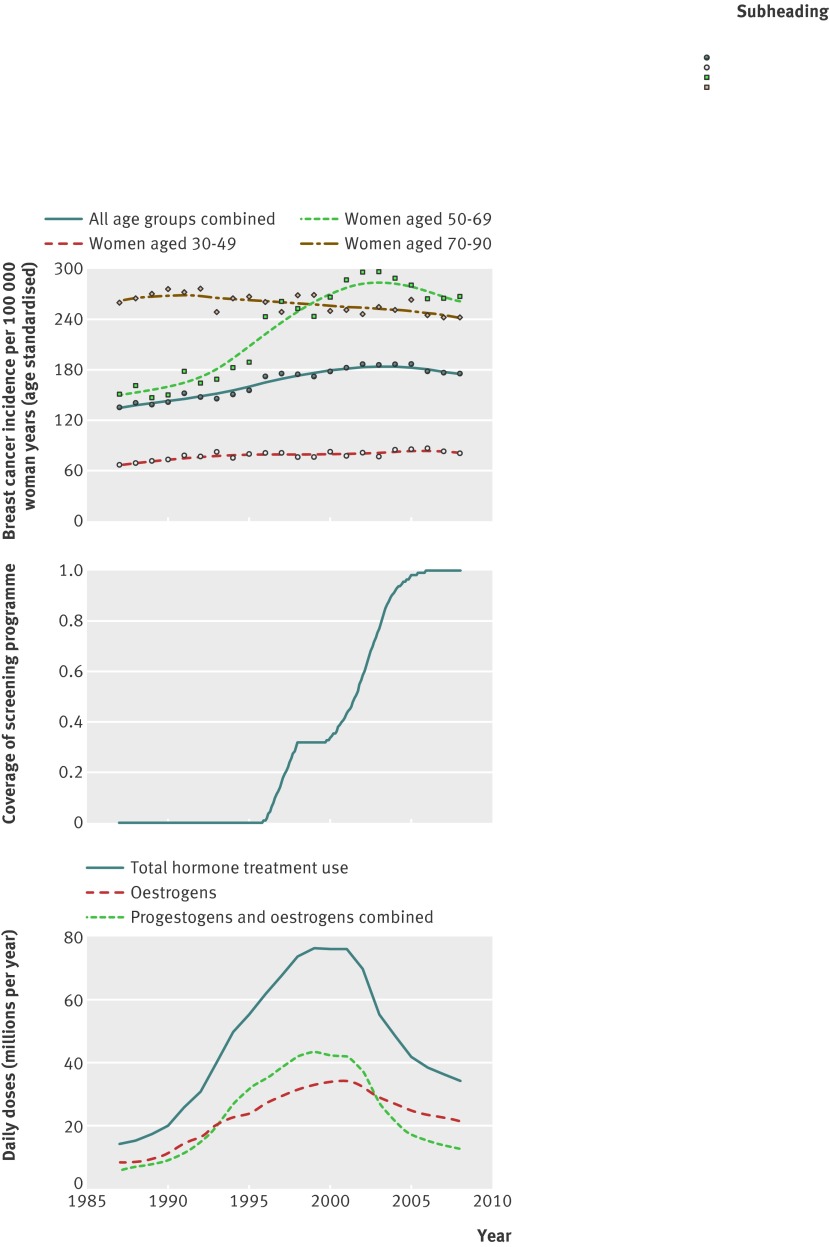
The age adjusted incidence of invasive breast cancer has increased steadily in Norway from the 1950s until 2002,27 after which the incidence levelled off, followed by a modest decline (fig 1). By studying age specific incidence and grouping women in age groups (30-49, 50-69, and 70-90 years), the large variation that explains the pronounced changes since the 1990s is among women aged 50-69 years. In this age group the incidence steeply increased during the late 1990s, before a gradual but modest decline from around 2003. During this period changes were only moderate in younger (30-49 years) and older (70-90 years) women, with a slight increase in the younger age group and a moderate decline in the older age group.
Fig 1 (Top) Incidence of invasive breast cancer among Norwegian women, 1987-2008. Curves smoothed with Friedman’s super smoother as implemented in statistical program R.26 Points and curves are all age standardised relative to year 2000. (Middle) Overview of Norwegian screening programme’s gradual introduction (calculated as approximated proportion of woman in target group invited). (Bottom) Sales of hormone treatment in Norway, 1987-2008
Sales of hormone treatment increased sharply in Norway during the 1990s and declined shortly after 2000, following similar patterns in all Norwegian counties (data not shown). These large changes occurred around the time that public mammography screening was introduced (fig 1), and correspond with the sharp increase in breast cancer incidence in the late 1990s. Public mammography screening was offered to women aged 50-69, and prescription data on hormone treatment show that treatment was mainly used among women in the age group 50-69 years, with the peak consumption between ages 55 and 60 (data not shown). For the 19 Norwegian counties combined, a strong increase in hormone treatment overlapped with the introduction of mammography screening, both for age groups and birth cohorts. Although the time trends were similar between counties, the amount of hormone treatment varied (data not shown). The mammography screening programme was also gradually introduced by county, and therefore hormone treatment sales peaked at different phases of the programme.
Model fitting
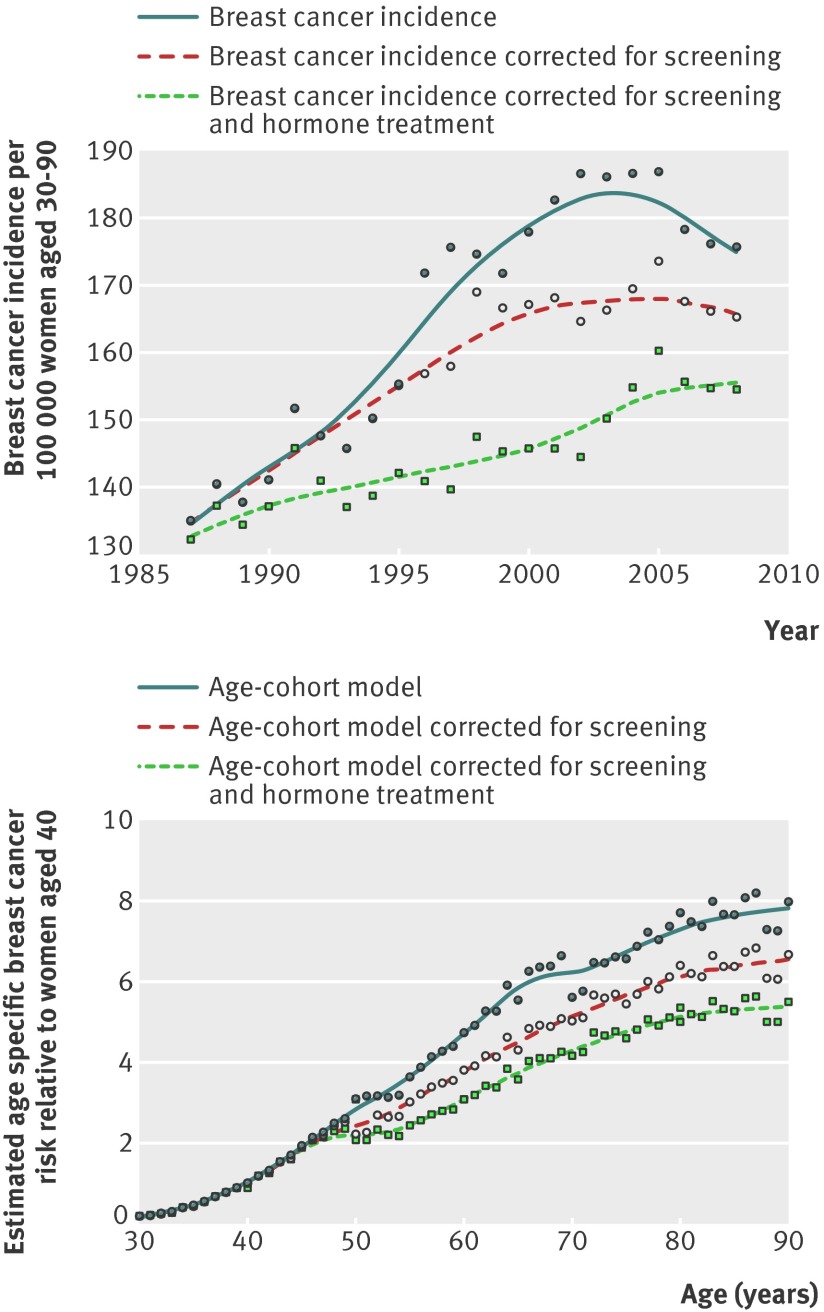
A classic age-period-cohort model showed considerably better model fit, with a large and significant decline in model deviance (−20 078 with 178 fewer degrees of freedom, P<0.001, table 1) compared with using a combined breast cancer rate for all age groups, cohorts, and periods. Significant non-linear cohort effects were still observed (P<0.001). By including screening variables the model fit was further improved (model deviance −467 with three fewer degrees of freedom, P<0.001), but considerable and significant non-linear period effects remained that could not be explained by age, cohort, or mammography screening variables (P<0.001, fig 2). By further extending the model to include sales of hormone treatment, the model fit improved further (model deviance −68 with one less degrees of freedom, P<0.001), whereas the non-linear period effects were strongly reduced, leaving only small and non-significant non-linear period effects (model deviance 30 with 20 more degrees of freedom, P=0.07, fig 2). Since all significant non-linear period effects were explained by mammography screening and use of hormone treatment, an age-cohort model with screening and hormone treatment variables was chosen as the final model, without any period variables. After taking hormone treatment and screening into account, no statistically significant non-explained variation in incidence remained (the P value for potential statistical over-dispersion was 0.08 for the final model).
Table 1.
Model fit for alternative models: basic age-period-cohort model, then model extended with screening variables and with screening variables and hormone treatment using either shared or separate modelling of hormone treatment types
| Model | Deviance | Degrees of freedom | P values | ||
|---|---|---|---|---|---|
| Last variables added | Over-dispersion | Non-linear period effects | |||
| Age-period-cohort | 27 994 | 25 319 | <0.001 | <0.001 | <0.001 |
| Age-period-cohort+screening variables | 27 527 | 25 316 | <0.001 | 0.035 | <0.001 |
| Age-period-cohort+screening variables+hormone treatment | 27 459 | 25 315 | <0.001 | 0.082 | 0.074 |
| Age-period-cohort+screening variables+hormone treatment including types* | 27 459 | 25 314 | 0.647 | 0.082 | 0.140 |
*Grouped as oestrogens alone or oestrogen-progestogen combinations.
Fig 2 Changes in invasive breast cancer incidence over study period and estimated age curves with and without correction for either screening or combined screening and hormone treatment use. Calculated using age-cohort model with one year lag added to hormone treatment variable
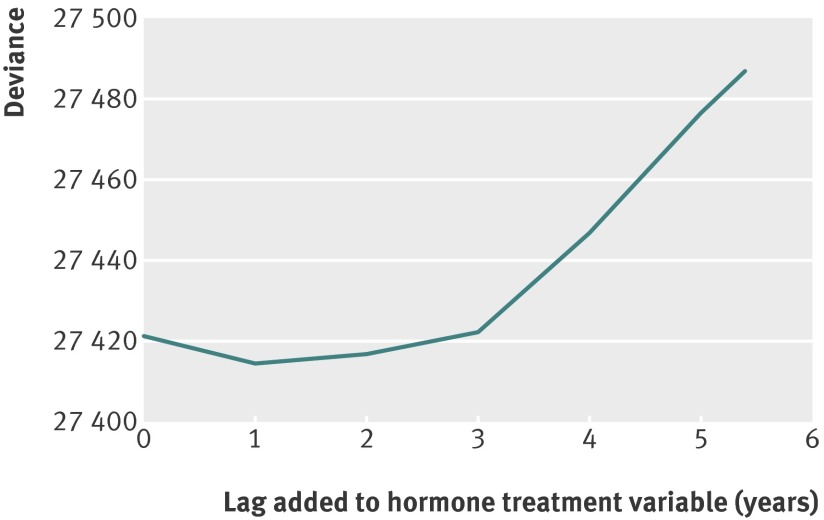
To account for a possible induction period related to the association of hormone treatment with changes in incidence of breast cancer, changes in model deviance were examined after applying different time lags when calculating the hormone treatment variable (fig 3). A time lag of one year gave the best fit and was used in the final analysis, but all time lags from zero to three years yielded similar model fit. The analysis was also extended to estimate the separate contributions of using oestrogen alone and combinations of oestrogen and progestogen, and applied different combinations of time lags for each variable. For oestrogen alone, the best model fit was observed using a one year time lag, whereas for the combined hormone treatment formulations, a time lag of two years provided the best fit. However, the overall model fit was not improved by extending the model to distinguish between the two main types of hormone treatment, and the associations of the two treatment variants with risk of breast cancer did not differ substantially (table 2).
Fig 3 Model fit, measured by model deviance, for final age-cohort model, with screening variables and different time lags added to hormone treatment variable. Lower model deviance implies better model fit, implying better accordance between observed data and assumed model. Without information on hormone treatment, age-cohort model has a deviance of 27 505
Table 2.
Estimated effects of hormone treatment and of being in target age group for breast cancer screening programme
| Variables | Age-cohort model with screening variables | |
|---|---|---|
| Use of hormone treatment* | Use of hormone treatment subgroups | |
| Scr1†: effect of starting screening programme | 1.59 (1.52 to 1.67) | 1.57 (1.50 to 1.65) |
| Scr2‡: effect of being in screening programme | 1.16 (1.11 to 1.22) | 1.14 (1.09 to 1.20) |
| Scr3§: effect of leaving screening programme | 0.86 (0.79 to 0.93) | 0.85 (0.78 to 0.92) |
| Effect of hormone treatment with 1 year lag*¶ | 2.17 (1.85 to 2.54) | — |
| Effect of oestrogens alone with 1 year lag¶ | — | 2.18 (0.83 to 5.71) |
| Effect of oestrogen-progestogen combinations with 2 year lag¶ | — | 2.22 (1.44 to 3.44) |
Hormone treatment variable is scaled to estimate risk for ongoing use of hormone treatment.
*Shared estimate for oestrogens alone and oestrogen-progestogen combinations.
†Initial screening variable, defined as first two years covered by screening programme, since Norwegian programme uses biannual screening.
‡Subsequent screening variable, defined as two years plus period covered by screening programme.
§Variable covering first five years after leaving screening programme.
¶Applied lag of hormone treatment use variables where chosen by minimising model deviance in univariate age-cohort model with screening variables.
Estimated effects of screening and hormone treatment
Overall, there was an estimated increase in invasive breast cancer incidence of 59% (95% confidence interval 52% to 67%) associated with being invited to the initial screening (table 2), and for the subsequent screening there was an increase of 16% (11% to 21%) associated with being in the target age group. During the first five years after leaving the screening programme’s target age group, incidence decreased by an estimated 14% (7% to 21%). The relative risk of breast cancer associated with use of hormone treatment was estimated to be 2.2 (1.9 to 2.5; table 3). Colinearity between the estimates for hormone treatment and mammography screening was not considerable, thus the negative correlation between the hormone treatment estimate and the three screening related estimates was only moderate (−0.14 for initial screening, −0.07 for subsequent screening, and −0.06 for leaving the screening programme’s target group).
Table 3.
Estimated fractions of breast cancer cases attributable to hormone treatment and screening mammography
| Age group | 1987 | 2002 (peak incidence) | 2008 |
|---|---|---|---|
| 50-69: | |||
| Hormone treatment attributable proportion | 0.07 | 0.27 | 0.13 |
| Screening attributable proportion | 0.00 | 0.23 | 0.17 |
| All studied age groups*: | |||
| Hormone treatment attributable proportion | 0.04 | 0.19 | 0.09 |
| Screening attributable proportion | 0.00 | 0.12 | 0.08 |
*Age 30-90 years.
The estimated relative risks related to use of hormone treatment based on oestrogen alone compared with combinations of oestrogen and progestogen did not differ substantially (table 2): relative risk 2.2 (95% confidence interval 0.8 to 5.7) and 2.2 (1.4 to 3.4).
Age and cohort effects
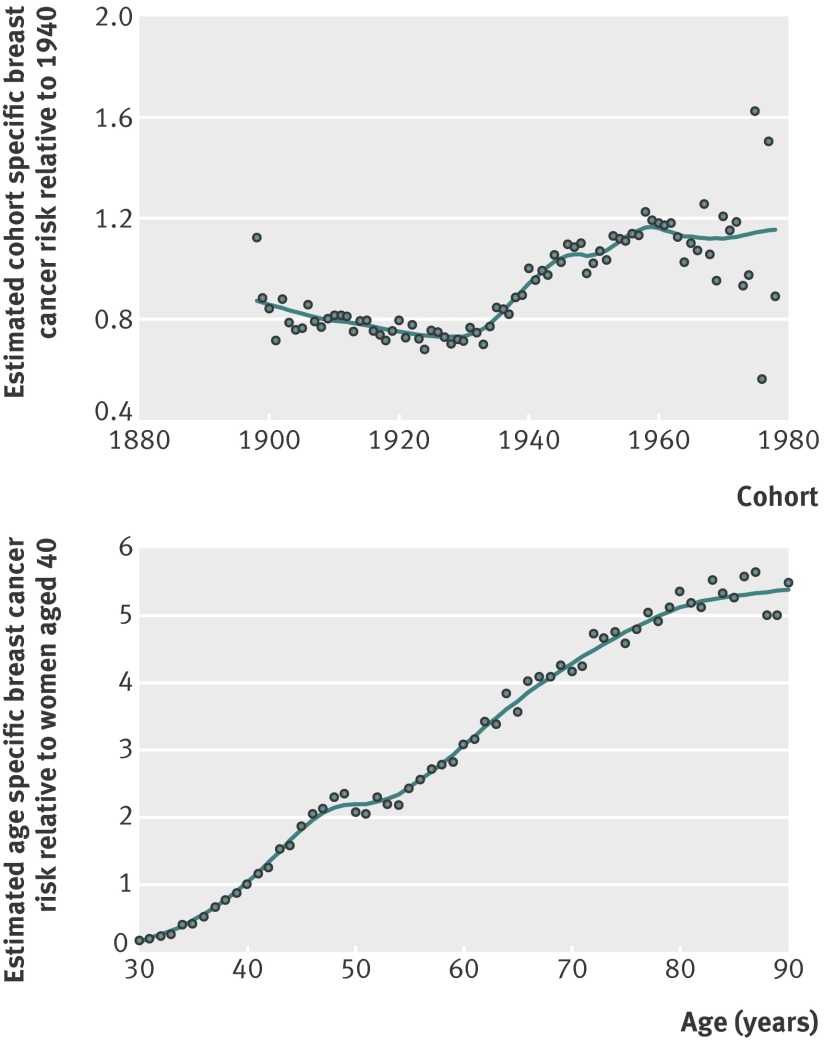
The reduction in incidence of invasive breast cancer for birth cohorts born between 1900 and 1930 was modest (fig 4). In subsequent birth cohorts, a sharp increase was observed until the cohorts born around 1960. After 1960, the number of cases was still low but the incidence may have stabilised (fig 4).
Fig 4 Estimated cohort and age curves for incidence of invasive breast cancer from age-cohort model, with correction for screening and hormone treatment use (one year lag)
After taking birth cohort, mammography screening, and hormone treatment into account, the age specific incidence of breast cancer steadily increased except for a fairly stable plateau at around age 50 (figs 3 and 4). One hundred bootstrap replications were visually examined to assess the significance of the slower increase in incidence around age 50, and in all replications the incidence levelled off at around age 50 (data not shown). This confirms the well known plateau in incidence around the age of menopause,21 which has not been apparent in recent decades in Norway.3
Estimated influence of hormone treatment use and screening on overall trends in incidence
After taking hormone treatment and mammography screening into account, the strong increase in incidence of invasive breast cancer in the 1990s disappeared, as did the subsequent decline from around 2006 (fig 4). The contribution of each factor in explaining the recent variation in trends in incidence of invasive breast cancer was similar, with a slightly stronger estimated effect of mammography screening than of hormone treatment (table 4 and fig 4). For most periods, however, the proportion of invasive breast cancer that may be attributed to hormone treatment seemed to be slightly larger than the attributable proportion of mammography screening (table 4 and fig 4). In 2002, when breast cancer incidence was at its highest among women aged 50-69, 23% of cases in that age group may be attributed to mammography screening and 27% to hormone treatment (table 3). The results indicate that among women in their 50s, as many as one in three cases of breast cancer that occurred around 2000 may be attributed to hormone treatment (table 4).
Table 4.
Estimated proportion of cases attributable to hormone treatment for selected age groups, based on age-cohort model with screening variables and one year lag time after hormone treatment
| Age group | 1988 | 1990 | 1992 | 1994 | 1996 | 1998 | 2000 | 2002 | 2004 | 2006 | 2008 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 47 years | 0.03 | 0.04 | 0.07 | 0.10 | 0.12 | 0.13 | 0.13 | 0.12 | 0.08 | 0.06 | 0.05 |
| 52 years | 0.09 | 0.12 | 0.17 | 0.25 | 0.28 | 0.31 | 0.31 | 0.29 | 0.21 | 0.16 | 0.13 |
| 57 years | 0.11 | 0.13 | 0.19 | 0.27 | 0.31 | 0.34 | 0.34 | 0.32 | 0.24 | 0.18 | 0.16 |
| 62 years | 0.09 | 0.11 | 0.16 | 0.24 | 0.27 | 0.30 | 0.30 | 0.28 | 0.20 | 0.16 | 0.14 |
Discussion
Prominent and statistically significant associations were found for both hormone treatment and mammography screening that could fully explain recent trends in breast cancer incidence in Norway. In the analysis, we extended the classic age-period-cohort model to include information on the use of hormone treatment and patterns of organised national mammography screening. The information on hormone treatment and mammography screening improved the model substantially, with no remaining statistically significant over-dispersion or non-linear period effects and no substantial departures from the historical gradual increase in incidence of invasive breast cancer. The results suggest that the variation in incidence during the past 20 years may be caused by the use of hormone treatment and mammography screening activities.
A common challenge in age-period-cohort models is that the linear components of period and cohort effects cannot be separated, and the choice of contrast is therefore decisive for the results.19 20 23 This problem does not apply to our results, because the period components did not contribute significantly to the model and were excluded from the final analysis.
Without access to individual data we could not study effects of different durations of hormone treatment, or estimate separate effects of when treatment started or finished. Without randomisation of study participants it is also difficult to exclude the possibility that confounding by other factors could play a part. Given the high quality data on breast cancer11 and the good model fit, however, the observed estimates are probably reliable. We used imputation for some data points related to hormone treatment, but the time related changes in hormone treatment were gradual and the total use for each calendar year is known. Therefore the impact of these imputations was probably limited. We also had to make imputations based on assumptions of the age distribution of hormone treatment use, since this information was not available before 2004 and not available for the quarter of hormone treatment that does not require a prescription. The assumption that the age distribution of hormone treatment up to 2004 was similar to the age distribution after 2004, and that the age distribution of users of over the counter drugs and prescribed drugs was similar, probably adds some uncertainty to the estimates. The good model fit is, however, an indication that the effect of this potential attenuation may be limited.
The age-incidence curve after taking hormone treatment use and mammography screening into account, corresponded well with historical curves on age related incidence of invasive breast cancer,21 and includes the classic plateau around menopause.21 Any departures from the targeted screening age have probably had little impact on the given estimates, indicated by the smooth estimates for age specific incidence around the assumed screening age (fig 4).
A substantial proportion of women in Norway attended screening at private clinics before the official programme was established,18 and some women may have continued to do so. This could have attenuated the estimated contributions of both the initial public screening and the women who left the programme after age 69. It is also possible that some doctors recommended additional mammography among women who used hormone treatment, which may have inflated the estimated association of hormone treatment. For private screening to bias the results substantially, however, we would have expected to see non-linear period effects or significant over-dispersion introduced by variations in private screening by period. The lack of such observations suggests that non-registered private screening has not undermined the validity of our results.
We also carried out a separate analysis to distinguish between the use of oestrogens alone and combined preparations of oestrogen and progestogen, but found no evidence for any difference in the estimated effects. However, plots of the simultaneous confidence region for the two estimates indicated internal colinearity (figure not shown). Both preparations had similar curves for period and age, which make it difficult to estimate any separate effects using aggregated data.
Comparison with other studies
It seems clear that both the initial and the subsequent screenings substantially increased the incidence of invasive breast cancer, and that a reduction in incidence occurred after leaving the screening programme. These observations correspond with the findings of others.5 28 Our estimated effects were moderate compared with reported estimates of Swedish data.5 The Swedish study found that being invited to initial screening was associated with more than a doubling of the incidence and that being invited to subsequent screening was associated with an increase in incidence of 34%. The researchers also reported that leaving the programme’s target group was associated with a reduction in incidence of 32%. In comparison with that study,5 we had access to more detailed information, including county specific data on sales of hormone treatment, which may provide more precise estimates.
In the Women’s Health Initiative randomised trial, the relative risk of breast cancer associated with current use of hormone treatment was 1.266; our corresponding estimate was 2.17. In the Norwegian prescription database the number of hormone treatment users is high compared with the number of prescribed daily doses (data not shown), suggesting that many women use hormone treatment for a short period only. If breast cancer risk is only moderately increased by short term use, our estimates would be attenuated by the large number of short term users. Therefore duration of hormone treatment use is not likely to explain the stronger association of hormone treatment in our study compared with that of the Women’s Health Initiative randomised trial. In addition, we found that use of hormone treatment was high in the mammography screening age group, and the interaction between hormone treatment and mammography screening could have further biased the association of hormone treatment towards the null since the treatment is known to decrease the sensitivity of the screening test.29 Therefore, the association with breast cancer risk is a possible indication for a genuinely stronger effect of hormone treatment use in Norway than expected from the Women’s Health Initiative trial.
Other studies suggest that the combined hormone treatment preparations typically used in Europe may be more strongly associated with breast cancer risk than preparations typically used in the United States.29 30 31 32 In the Million Women Study in the United Kingdom, the relative risk of breast cancer associated with all current use of hormone treatment was 1.66,30 and the relative risk associated with use of combined hormone treatment preparations was 2.0. Similarly, in a Norwegian cohort study with individual data, the relative risk of current hormone treatment use was estimated to be 2.1.33 Hormone treatment preparations in Norway differ from those in the United States, and combined preparations with a testosterone derived progestogen are preferred. These provide a higher dose of progestogens than do other preparations.31 Differences in risk estimates attributed to use of hormone treatment may therefore be caused by differences in dose or drug properties between preparations. Our results confirm the short lag time that others have reported from use of hormone treatment until a change in breast cancer incidence is observed,30 33 suggesting that hormone treatment may promote the growth of an already initiated tumour. Whether tumours that may be attributed to hormone treatment are less aggressive than other tumours is still not resolved.34 35
We observed a steep increase in incidence of invasive breast cancer among cohorts born from the 1930s until the late 1950s, whereas in cohorts born before 1930 and after 1960 the changes were moderate. Judged from the literature, it is difficult to identify cohort specific factors that may explain these differences, suggesting that a combination of several changing factors may be important.22 36
Implications of the current study
Recently the frequency of pre-invasive ductal carcinomas in situ have increased sharply. Ductal carcinoma in situ was not included in the present study because trends in incidence for this condition differ substantially from those of invasive breast cancer. Effects of mammography screening are also likely to differ, and hormone treatment use and other risk factors may have a different influence on ductal carcinoma in situ compared with invasive disease. Based on the findings of previous studies, including ductal carcinoma in situ in our study would probably have increased the fraction of breast malignancies that may be attributed to mammography screening.13 However, it is likely that the effect would be limited, as the proportion of ductal carcinoma in situ of Norwegian breast malignancies never exceeded 10% during the observation period.
Over-diagnosis associated with mammography screening,28 37 38 and subsequent overtreatment, may represent one of the most pressing challenges of breast cancer screening. We found a strong positive association of mammography screening with recent incidence of invasive breast cancer; however, our study was not designed to assess the proportion of cancers detected by mammography that may represent over-diagnosis. That would require a more detailed analysis of the potential reduction in incidence after screening and also an analysis of trends in incidence of ductal carcinoma in situ, as a substantial proportion of these cases most likely represent over-diagnosis of breast cancer.39 None the less, an evaluation of over-diagnosis requires reliable estimates of the expected incidence of breast cancer in the absence of screening. Therefore our study may be an appropriate basis for future studies of over-diagnosis.
In summary, our results suggest that the recent changes in trends in incidence of invasive breast cancer may be explained by a combination of mammography screening patterns and hormone treatment use, with similar contributions of each factor.
What is already known on this topic
After decades of a consistent increase, the incidence of invasive breast cancer has recently declined in many developed countries
Reduced use of hormone therapy and completion of the first wave of mammography screening have been suggested as explanatory factors
The importance of each factor’s contribution is, however, uncertain
What this study adds
The changes in incidence of invasive breast cancer observed in many developed countries may be attributed to mammography screening and hormone treatment use, with about similar contributions of each factor
We thank Solveig Sakshaug (Norwegian Institute of Public Health) for providing the sales data on hormone treatment, and Solveig Hofvind and Jan Husebye (Norwegian cancer registry) for sharing their knowledge about the Norwegian breast cancer screening programme.
Contributors: HW-F conceived the study, analysed the data, and wrote the paper. KB contributed insight on the use of hormone treatment. KB, LV, and ST interpreted the results and wrote the paper. All authors had full access to all of the data and analysis results, and can take responsibility for the integrity of the data and the accuracy of the data analysis. ST is the guarantor.
Funding: This work was supported by the Norwegian Cancer Society through a postdoctoral scholarship to HW-F (PK01-2008-0080).
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: The data used are available for research projects from the cancer registry of Norway and the Norwegian Institute of Public Health, who are the legal administrators of the data. The data can, however, not be shared directly with other researchers.
Cite this as: BMJ 2012;344:e299
Web Extra. Extra material supplied by the author
Overview of data sources
Observation periods and summary of applied data
References
- 1.Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 2007;356:1670-4. [DOI] [PubMed] [Google Scholar]
- 2.Lambe M, Wigertz A, Holmqvist M, Adolfsson J, Bardage C, Fornander T, et al. Reductions in use of hormone replacement therapy: effects on Swedish breast cancer incidence trends only seen after several years. Breast Cancer Res Treat 2010;121:679-83. [DOI] [PubMed] [Google Scholar]
- 3.NORDCAN. Association of Nordic Cancer Registries. 2010. www.ancr.nu/nordcan.asp.
- 4.Katalinic A, Rawal R. Decline in breast cancer incidence after decrease in utilisation of hormone replacement therapy. Breast Cancer Res Treat 2008;107:427-30. [DOI] [PubMed] [Google Scholar]
- 5.Moller B, Weedon-Fekjaer H, Hakulinen T, Tryggvadottir L, Storm HH, Talback M, et al. The influence of mammographic screening on national trends in breast cancer incidence. Eur J Cancer Prev 2005;14:117-28. [DOI] [PubMed] [Google Scholar]
- 6.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among US women. Breast Cancer Res 2007;9:R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glass AG, Lacey JV Jr, Carreon JD, Hoover RN. Breast cancer incidence, 1980-2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst 2007;99:1152-61. [DOI] [PubMed] [Google Scholar]
- 9.Kerlikowske K, Miglioretti DL, Buist DS, Walker R, Carney PA. Declines in invasive breast cancer and use of postmenopausal hormone therapy in a screening mammography population. J Natl Cancer Inst 2007;99:1335-9. [DOI] [PubMed] [Google Scholar]
- 10.Hemminki E, Kyyronen P, Pukkala E. Postmenopausal hormone drugs and breast and colon cancer: Nordic countries 1995-2005. Maturitas 2008;61:299-304. [DOI] [PubMed] [Google Scholar]
- 11.Larsen IK, Smastuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009;45:1218-31. [DOI] [PubMed] [Google Scholar]
- 12.Virnig BA, Wang SY, Shamilyan T, Kane RL, Tuttle TM. Ductal carcinoma in situ: risk factors and impact of screening. J Natl Cancer Inst Monogr 2010;41:113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofvind S, Vacek PM, Skelly J, Weaver DL, Geller BM. Comparing screening mammography for early breast cancer detection in Vermont and Norway. J Natl Cancer Inst 2008;100:1082-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen M, Jensen J, Andersen J. Precancerous and cancerous breast lesions during lifetime and at autopsy. A study of 83 women. Cancer 1984;54:612-5. [DOI] [PubMed] [Google Scholar]
- 15.Welch HG, Black WC. Using autopsy series to estimate the disease “reservoir” for ductal carcinoma in situ of the breast: how much more breast cancer can we find? Ann Intern Med 1997;127:1023-8. [DOI] [PubMed] [Google Scholar]
- 16.Statistics Norway. Statistisk sentralbyra. 2010. www.ssb.no.
- 17.Hofvind S. The Norwegian Breast Cancer Screening Program: selected process indicators and their utilization in epidemiological research. University of Oslo, 2005.
- 18.Weedon-Fekjaer H, Lindqvist BH, Vatten LJ, Aalen OO, Tretli S. Estimating mean sojourn time and screening sensitivity using questionnaire data on time since previous screening. J Med Screen 2008;15:83-90. [DOI] [PubMed] [Google Scholar]
- 19.Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: age-period and age-cohort models. Stat Med 1987;6:449-67. [DOI] [PubMed] [Google Scholar]
- 20.Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: age-period-cohort models. Stat Med 1987;6:469-81. [DOI] [PubMed] [Google Scholar]
- 21.Clemmesen J. Carcinoma of the breast; results from statistical research. Br J Radiol 1948;21:583-90. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson S, Husebye E. Breast cancer. In: Adami HO, Hunter D, Trichopoulos D, eds. Textbook of cancer epidemiology. Oxford University Press, 2002:3001-339.
- 23.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics 1983;39:311-24. [PubMed] [Google Scholar]
- 24.Paci E, Duffy SW. Modelling the analysis of breast cancer screening programmes: sensitivity, lead time and predictive value in the Florence District Programme (1975-1986). Int J Epidemiol 1991;20:852-8. [DOI] [PubMed] [Google Scholar]
- 25.Weedon-Fekjaer H, Vatten LJ, Aalen OO, Lindqvist B, Tretli S. Estimating mean sojourn time and screening test sensitivity in breast cancer mammography screening; new results. J Med Screen 2005;12:172-8. [DOI] [PubMed] [Google Scholar]
- 26.R core group. R. 2003. www.r-project.org.
- 27.Moller B, Fekjaer H, Hakulinen T, Tryggvadottir L, Storm HH, Talback M, et al. Prediction of cancer incidence in the Nordic countries up to the year 2020. Eur J Cancer Prev 2002;11(suppl 1):S1-96. [PubMed] [Google Scholar]
- 28.De Gelder R, Heijnsdijk EA, van Ravesteyn NT, Fracheboud J, Draisma G, de Koning HJ. Interpreting overdiagnosis estimates in population-based mammography screening. Epidemiol Rev 2011;33:111-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofvind S, Moller B, Thoresen S, Ursin G. Use of hormone therapy and risk of breast cancer detected at screening and between mammographic screens. Int J Cancer 2006;118:3112-7. [DOI] [PubMed] [Google Scholar]
- 30.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003;362:419-27. [DOI] [PubMed] [Google Scholar]
- 31.Bakken K, Fournier A, Lund E, Waaseth M, Dumeaux V, Clavel-Chapelon F, et al. Menopausal hormone therapy and breast cancer risk: impact of different treatments. The European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2011;128:144-56. [DOI] [PubMed] [Google Scholar]
- 32.Calle EE, Feigelson HS, Hildebrand JS, Teras LR, Thun MJ, Rodriguez C. Postmenopausal hormone use and breast cancer associations differ by hormone regimen and histologic subtype. Cancer 2009;115:936-45. [DOI] [PubMed] [Google Scholar]
- 33.Bakken K, Alsaker E, Eggen AE, Lund E. Hormone replacement therapy and incidence of hormone-dependent cancers in the Norwegian Women and Cancer study. Int J Cancer 2004;112:130-4. [DOI] [PubMed] [Google Scholar]
- 34.Gapstur SM, Morrow M, Sellers TA. Hormone replacement therapy and risk of breast cancer with a favorable histology: results of the Iowa Women’s Health Study. JAMA 1999;281:2091-7. [DOI] [PubMed] [Google Scholar]
- 35.Chlebowski RT, Anderson GL, Gass M, Lane DS, Aragaki AK, Kuller LH, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010;304:1684-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tretli S, Gaard M. Lifestyle changes during adolescence and risk of breast cancer: an ecologic study of the effect of World War II in Norway. Cancer Causes Control 1996;7:507-12. [DOI] [PubMed] [Google Scholar]
- 37.Zackrisson S, Andersson I, Janzon L, Manjer J, Garne JP. Rate of over-diagnosis of breast cancer 15 years after end of Malmo mammographic screening trial: follow-up study. BMJ 2006;332:689-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jorgensen KJ, Gotzsche PC. Overdiagnosis in publicly organised mammography screening programmes: systematic review of incidence trends. BMJ 2009;339:b2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ernster VL, Barclay J. Increases in ductal carcinoma in situ (DCIS) of the breast in relation to mammography: a dilemma. J Natl Cancer Inst Monogr 1997;22:151-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of data sources
Observation periods and summary of applied data