Abstract
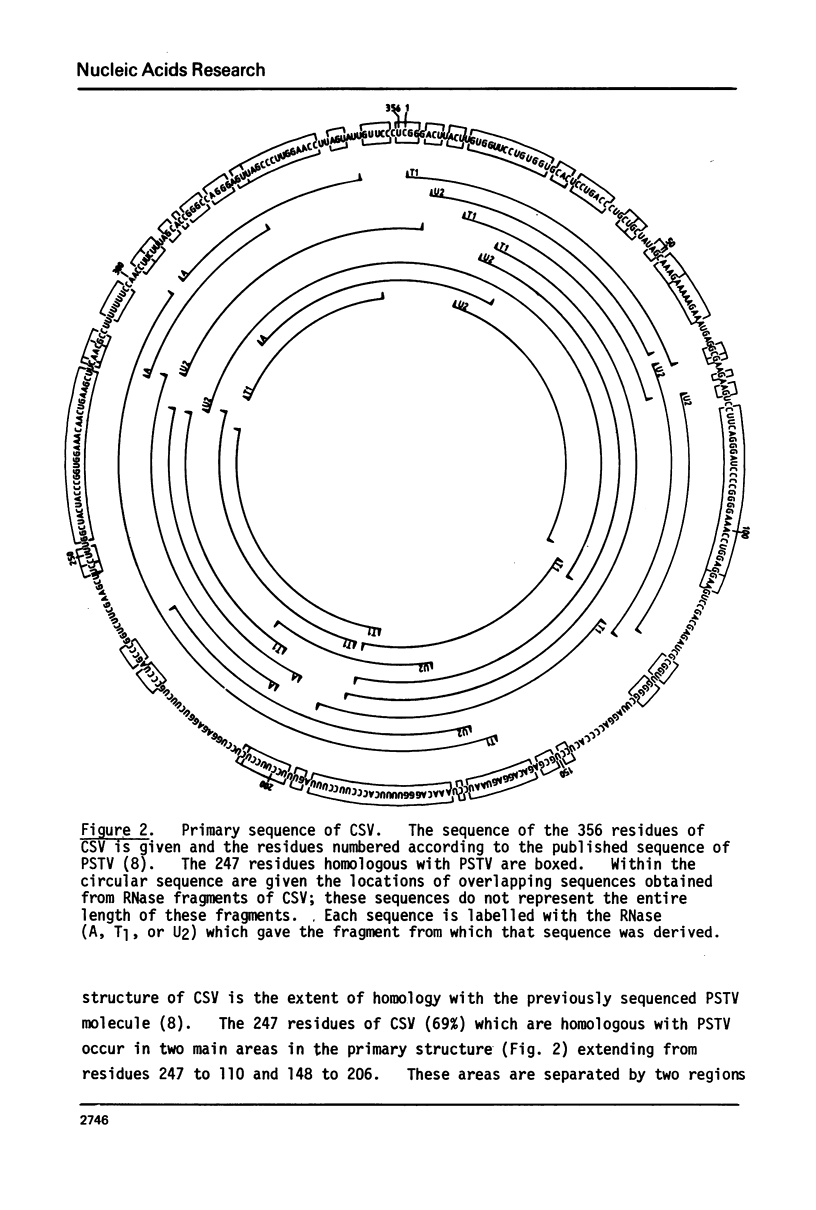
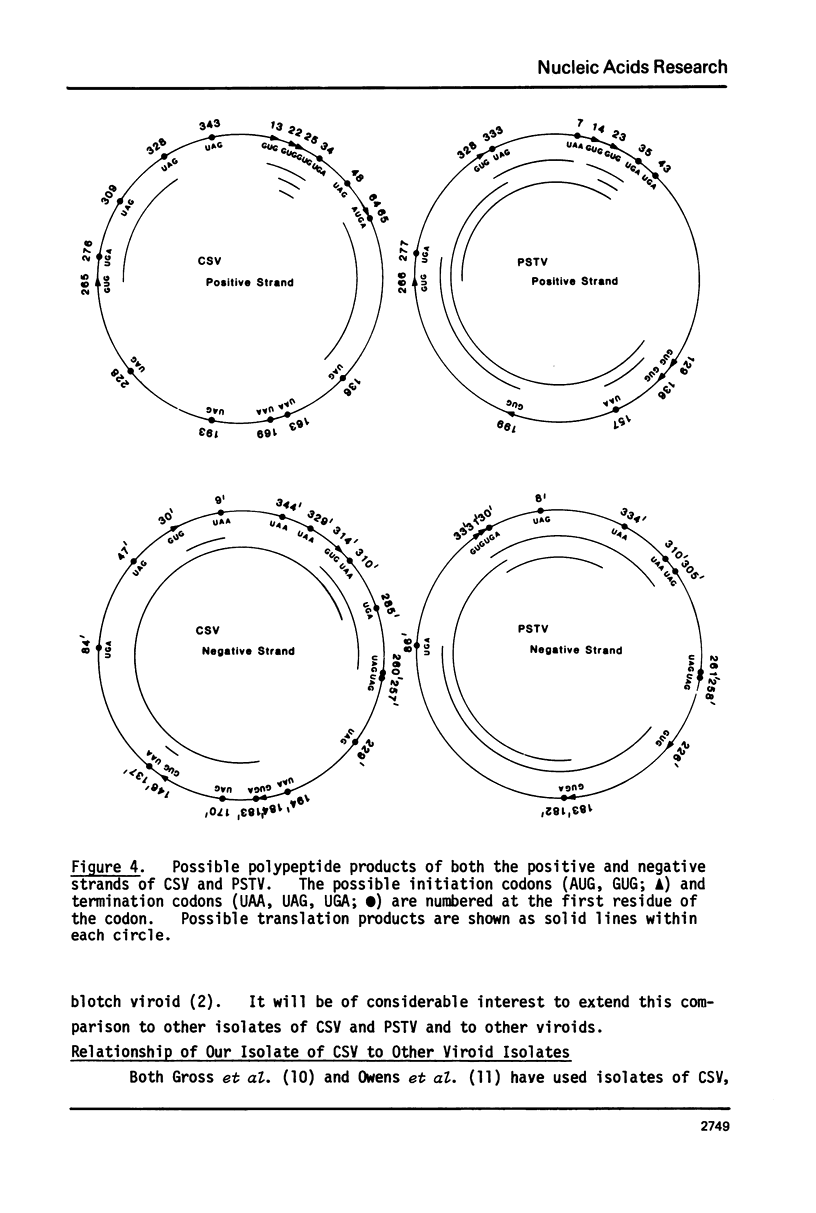
The sequence of the 356 nucleotide residues of chrysanthemum stunt viroid (CSV) has been determined. Overlapping linear viroid fragments were obtained by partial ribonuclease digestion, radiolabelled in vitro at their 5'-ends, and sequenced using partial enzymic cleavage methods. Of the CSV sequence, 69% is contained in the published sequence of potato spindle tuber viroid (PSTV). Differences in the primary sequence of CSV and PSTV suggest that neither the positive nor putative negative strands of these two viroids code for functional polypeptide products. However, the two viroids can form similar secondary structures, implicating a role for viroid structure in replication.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conejero V., Semancik J. S. Exocortis viroid: alteration in the proteins of Gynura aurantiaca accompanying viroid infection. Virology. 1977 Mar;77(1):221–232. doi: 10.1016/0042-6822(77)90420-2. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Kaesberg P., Diener T. O. Potato spindle tuber viroid. XII. An investigation of viroid RNA as a messenger for protein synthesis. Virology. 1974 Sep;61(1):281–286. doi: 10.1016/0042-6822(74)90262-1. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Symons R. H. The use of hybridization analysis with complementary DNA to determine the RNA sequence homology between strains of plant viruses: its application to several strains of cucumoviruses. Virology. 1978 Jul 15;88(2):361–370. doi: 10.1016/0042-6822(78)90292-1. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Sänger H. L. Comparative oligonucleotide fingerprints of three plant viroids. Nucleic Acids Res. 1977 Jun;4(6):2021–2028. doi: 10.1093/nar/4.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer F. R., Mills D. R. RNA sequencing with radioactive chain-terminating ribonucleotides. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5334–5338. doi: 10.1073/pnas.75.11.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp G., Gross H. J. Rapid RNA sequencing: nucleases from Staphylococcus aureus and Neurospora crassa discriminate between uridine and cytidine. Nucleic Acids Res. 1979 Aug 10;6(11):3481–3490. doi: 10.1093/nar/6.11.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Efstratiadis A. Fractionation of low molecular weight DNA or RNA in polyacrylamide gels containing 98% formamide or 7 M urea. Methods Enzymol. 1980;65(1):299–305. doi: 10.1016/s0076-6879(80)65040-x. [DOI] [PubMed] [Google Scholar]
- Riesner D., Henco K., Rokohl U., Klotz G., Kleinschmidt A. K., Domdey H., Jank P., Gross H. J., Sänger H. L. Structure and structure formation of viroids. J Mol Biol. 1979 Sep 5;133(1):85–115. doi: 10.1016/0022-2836(79)90252-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger H. L., Klotz G., Riesner D., Gross H. J., Kleinschmidt A. K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Conejero V., Gerhart J. Citrus exocortis viroid: survey of protein synthesis in Xenopus laevis oocytes following addition of viroid RNA. Virology. 1977 Jul 1;80(1):218–221. doi: 10.1016/0042-6822(77)90395-6. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Uhlenbeck O. C., Levine M. D. Estimation of secondary structure in ribonucleic acids. Nature. 1971 Apr 9;230(5293):362–367. doi: 10.1038/230362a0. [DOI] [PubMed] [Google Scholar]



