Abstract
Objective:
To determine whether clinical and demographic features are associated with prognosis in patients with frontotemporal dementia and motor neuron disease (FTD-MND).
Methods:
This was a case series of FTD-MND categorized according to behavioral- or language-dominant symptoms at presentation and throughout the disease course. Demographic, clinical, imaging, and survival data were analyzed with respect to dominant FTD-MND type. Voxel-based morphometry was used to assess and compare regional patterns of atrophy in behavioral- and language-dominant FTD-MND types.
Results:
Of the 56 patients with FTD-MND who were identified, 31 had dominant behavioral symptoms and 25 had dominant language symptoms; 53 patients had died. A survival difference was present between types, with patients with behavioral-dominant symptoms surviving 506 days longer than patients with language-dominant symptoms (mean 1,397 vs 891 days; p = 0.002). There was also a difference in time from diagnosis to death (p = 0.02) between groups. Patients with language-dominant disease were more likely to have bulbar-onset than limb-onset motor neuron disease (MND) (p = 0.01). There was a similar pattern of frontal and temporal lobe atrophy in both types, although there was some evidence for the behavioral type to have more frontal atrophy and the language type to have more left temporal atrophy.
Conclusions:
In our series of patients with FTD-MND, language-dominant FTD-MND was associated with bulbar-onset MND and a shorter survival. There was also evidence that the dominant FTD-MND type is related to differences in brain atrophy patterns.
Frontotemporal dementia (FTD) encompasses clinical syndromes characterized by progressive and insidious behavioral changes and language deficits.1,2 Clinical symptoms of motor neuron disease (MND) can develop in patients with FTD, whereas patients with MND may manifest behavioral or language symptoms in the disease course.3–6 Beyond the clinical features, a pathologic overlap exists between FTD and MND with the presence of ubiquitin/TAR DNA-binding protein (TDP-43)–immunoreactive inclusions identified in both disorders, suggesting a TDP-43 proteinopathy continuum.7–9
In FTD, behavioral and language deficits are well-characterized with established language syndromes of progressive nonfluent aphasia and semantic dementia.2 Although behavioral and language symptoms often overlap in FTD, the predominant subtype has implications for the disease course with demographic differences existing between behavioral and language groups with respect to gender, age at onset, and survival.10 Distinguishing clinical features in FTD also has implications for pathophysiology because various profiles of tau and TDP-43 deposition have been associated with different clinical syndromes.1,11,12
Despite the evaluation of different subtypes of FTD, no study has evaluated patients with FTD and concomitant motor neuron disease (FTD-MND) with respect to FTD phenotype. This study therefore aimed to identify clinical and imaging features of behavioral- and language-dominant FTD-MND and determine whether a survival difference exists between the different types.
METHODS
Case selection.
A database search of the Mayo Clinic electronic medical record system was used to identify all patients with the diagnosis of both dementia and MND between January 2000 and July 2010. A total of 389 cases were reviewed, of which 66 had the diagnosis of FTD-MND. Of these cases, 10 were excluded due to insufficient clinical data to diagnose either FTD or MND. The dominant FTD type was determined with the aid of consensus criteria for FTD.2 We chose these FTD consensus criteria because they provide clinical symptoms to separate FTD-MND into language- and behavioral-dominant phenotypes.
The patient's presenting symptom, symptom severity, and progression were used along with physical examination data to classify each case as either behavioral- or language-dominant FTD-MND. Those considered to have behavioral-dominant FTD-MND exhibited an early decline in social interpersonal conduct, impairment in regulation of personal conduct, emotional blunting, or loss of insight. Those considered to have language-dominant FTD-MND exhibited either nonfluent spontaneous speech with agrammatism, phonemic paraphasias, or anomia, or features of semantic dementia with progressive, fluent, empty spontaneous speech, loss of word meaning with impaired naming and comprehension, semantic paraphasias, or prosopagnosia.2 In patients with overlapping behavioral and language features, the predominant type was determined according to the consensus criteria; when core behavioral features were met, language and speech features of altered speech output, stereotypy of speech, echolalia, perseveration, and mutism were supportive of the behavioral-dominant type.2 Patients with early preservation of social skills and late characteristic behavior changes who had core language features were classified as having the language-dominant type. MND was diagnosed on the basis of clinical or electromyographic evidence of upper or lower motor neuron dysfunction consistent with the revised El Escorial criteria.13 Patients with solely upper MND were included according to the proposed criteria for primary lateral sclerosis14,15 because FTD-MND includes the primary lateral sclerosis variant.16
Historical data including handedness, gender, state of residency, age at onset, age at diagnosis, and age at death (if available) were obtained from the medical record. The patient's initial diagnosis, family history of a first-degree relative with FTD or amyotrophic lateral sclerosis, behavioral features, language findings, presence of speech or limb apraxia, psychosis, and evidence of parkinsonism were collected. Neuropsychometric data were used to characterize patterns of cognitive deficit and language dysfunction. Information was compiled regarding the onset of motor symptoms, limb, and bulbar involvement and motor examination findings. Speech pathology records and documentation of swallowing function were used to generate speech and swallowing subscores of the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS)17 at presentation. The timing between the onset of cognitive and motor symptoms was calculated in months. Patients were considered to have simultaneous onset if cognitive and motor symptoms appeared concomitantly.
Standard protocol approval and patient consents.
Informed consent was obtained from all patients for participation in the studies, which were approved by the Mayo Clinic institutional review board.
Voxel-based morphometry analysis.
Voxel-based morphometry (VBM)18 was used to determine whether patients with behavioral- and language-dominant FTD-MND would show different patterns of atrophy. Although 55 of the 56 patients with FTD-MND included in the study had a brain MRI, only 25 had a volumetric T1-weighted MRI performed with a standardized protocol suitable for analysis (15 patients with behavioral-dominant FTD-MND and 10 patients with language-dominant FTD-MND). We analyzed all patients with language-dominant FTD-MND matched by age at MRI and disease duration to 10 patients with behavioral-dominant FTD-MND. These 20 patients with FTD-MND were also matched by age and gender to 20 control subjects who were neurologically normal. Subject demographics at time of MRI are shown in table e-1 on the Neurology® Web site at www.neurology.org.
All MRI scans were subjected to preprocessing correction for gradient nonlinearity19 and intensity nonuniformity.20 VBM was implemented using SPM5 as described previously.21 Patterns of gray matter loss were assessed in each FTD-MND group compared with control subjects after correction for multiple comparisons using family-wise error (FWE) at p < 0.05. Patients with behavioral-dominant and language-dominant FTD-MND were also directly compared at both corrected (FWE p < 0.05) and uncorrected (p < 0.001) statistical thresholds. Age and gender were included in all analyses as covariates.
Statistical analysis.
Statistical analyses were performed using JMP software (version 8.0.0; SAS Institute, Cary, NC) with statistical significance set at p < 0.05. Comparisons of gender ratios and other binary variables across behavioral- and language-dominant FTD-MND groups were performed using the χ2 test or the Fisher exact test for analyses in which there were cells with small numbers. Demographic features, for example, age at onset, age at death, time from diagnosis to death, and all other continuous variables, were compared across groups using the Mann-Whitney U test. Survival analyses were performed using Kaplan-Meier estimates. Cox proportional hazard models were used to adjust for bulbar onset.
RESULTS
A total of 56 patients with FTD and MND were identified (table 1). Short Test of Mental Status (STMS) scores22 were available for 43 patients. EMG was performed in 52 of 56 patients. Four patients did not have EMG because of their poor clinical condition or because of definitive evidence of MND findings on examination. In 2 of these 4 patients, a diagnosis of frontotemporal lobar degeneration with TDP-43 immunoreactive inclusions (FTLD-TDP)23 was later confirmed neuropathologically. There were 2 patients with pure upper MND or primary lateral sclerosis, one each from the behavioral- and language-dominant groups.
Table 1.
Demographics of patients with behavioral- and language-dominant FTD-MND
Abbreviations: FTD-MND = frontotemporal dementia with motor neuron disease; STMS = Short Test of Mental Status.
p Value for difference between behavioral and language types.
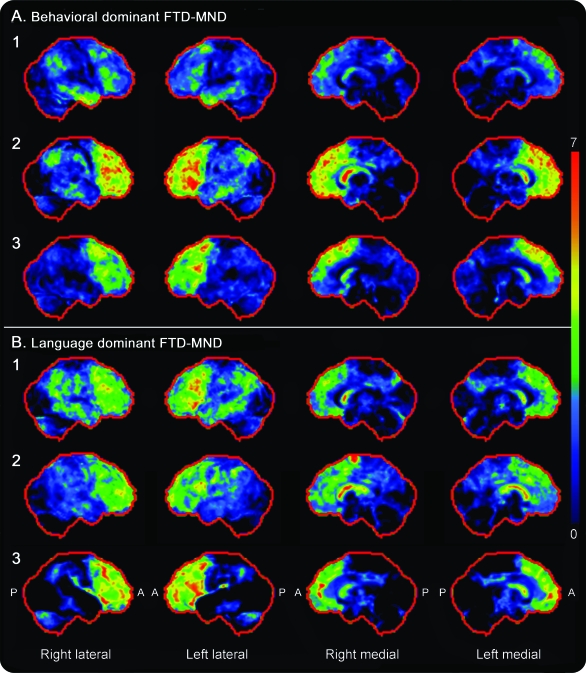
In all 56 patients, MRI findings documented in the medical records by the reviewing neuroradiologist were “focal frontal and temporal lobe atrophy” or “generalized neocortical atrophy.” There was no intracranial lesion pathology accounting for the clinical presentations. Twenty-four patients had completed a functional [18F]fluorodeoxyglucose (FDG) PET or SPECT scan. All 13 patients who underwent FDG-PET scans had frontotemporal decreased uptake (figure 1) with similar findings of hypoperfusion reported in all 11 patients who had a SPECT scan.
Figure 1. Fluorodeoxyglucose (FDG)-PET statistical stereotactic surface projection maps (Cortex ID) showing hypometabolism in 3 patients with behavioral-dominant (A) and 3 patients with language-dominant frontotemporal dementia with motor neuron disease (FTD-MND) (B) compared with that in normal control subjects.
These specific patients are shown because they completed a FDG-PET scan after 2007 when use of Cortex ID was implemented. Variable degrees of frontal and temporal hypometabolism were observed in both groups, without any visually observable differences across groups. A = anterior; P = posterior.
Diagnostic groups.
In this cohort of patients with FTD-MND, 31 had dominant behavioral features of FTD, whereas 25 had dominant language deficits (table 1). There was no significant difference among gender, family history, or STMS mean scores (table 1). There was often overlap of behavioral and language features in an individual's disease course (table 2); behavioral changes were present in 72% of patients with language-dominant FTD-MND at some point in the disease course, and language problems were present at some point in the disease course in 90% of patients with the behavioral-dominant FTD-MND. Behavioral change, psychosis, and abnormal eating behaviors were all significantly more common in the behavioral-dominant FTD-MND group than in the language-dominant FTD-MND group. Limb apraxia and parkinsonism were significantly more common in the language-dominant FTD-MND group. Patients with language-dominant FTD-MND were also more likely to have bulbar onset of MND than patients with behavioral-dominant FTD-MND. Speech problems were more severe in the language-dominant FTD-MND group, although the severity of swallowing problems was similar in both types.
Table 2.
Clinical characteristics of patients with behavioral- and language-dominant FTD-MND
Abbreviations: ALSFRS = Amyotrophic Lateral Sclerosis Functional Rating Scale; FTD-MND = frontotemporal dementia with motor neuron disease.
p Value for difference between behavioral and language types.
There were 2 patients with prosopagnosia (loss of facial recognition) and an additional patient whose central clinical feature was anomia and loss of semantic knowledge. One patient in the language group had marked logopenia as the primary manifestation of language dysfunction. There was no apparent difference on FDG-PET scans between the behavioral- and language-dominant FTD-MND types (figure 1).
Survival.
Fifty-three patients had died and hence had illness duration (table 1). Three patients in the behavioral-dominant group are still alive. Autopsy was performed in 7 patients, all with neuropathologically confirmed FTLD-TDP type 3 pathology,7 i.e., ubiquitin and TDP-43 immunoreactive, predominantly cytoplasm inclusions and evidence of motor neuron degeneration.
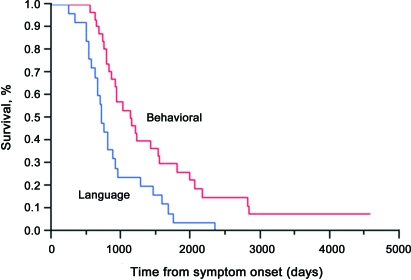
A significant difference in survival was seen between the behavioral- and language-dominant groups, with patients in the behavioral-dominant group living 506 days longer than those in the language-dominant group (mean 1,397 days compared with 891 days; p = 0.002) (table 1). The Kaplan-Meier survival curve is depicted in figure 2. There was no significant difference in the time from onset to diagnosis (p = 0.13) between the groups, but there was a significant difference in the time from diagnosis to death with the behavioral-dominant group living longer after diagnosis (mean 540 days compared with 248 days; p = 0.02). No significant survival difference was seen between women and men. Patients with bulbar onset in the behavioral-dominant group had a shorter survival (p = 0.006) than those in the language-dominant group (p = 0.38). Patients with the behavioral-dominant phenotype with bulbar onset were more likely to have a shorter survival than patients with behavioral-dominant FTD-MND without bulbar onset (hazard ratio 3.4). Those with language-dominant FTD-MND were also more likely to have a shorter survival compared with this reference group, with a hazard ratio of 3.9 for those with bulbar onset and 2.7 for those without.
Figure 2. Kaplan-Meier survival curve of patients with behavioral- or language-dominant frontotemporal dementia and motor neuron disease (FTD-MND) demonstrating shorter survival for language-dominant FTD-MND compared with that for behavioral-dominant FTD-MND (p = 0.002).
A similar number of patients presented with simultaneous onset of cognitive (behavioral or language) and motor symptoms between the behavioral- and language-dominant groups (table 2). In all groups, the majority of patients manifested cognitive symptoms earlier than motor symptoms. The average duration from cognitive to motor symptom onset was 621 days, whereas the duration from motor to cognitive symptoms was 256 days. Patients in the language-dominant group had a more rapid progression to development of secondary symptoms, regardless of whether their initial presentation was characterized by motor or cognitive symptoms (table 2).
VBM analysis.
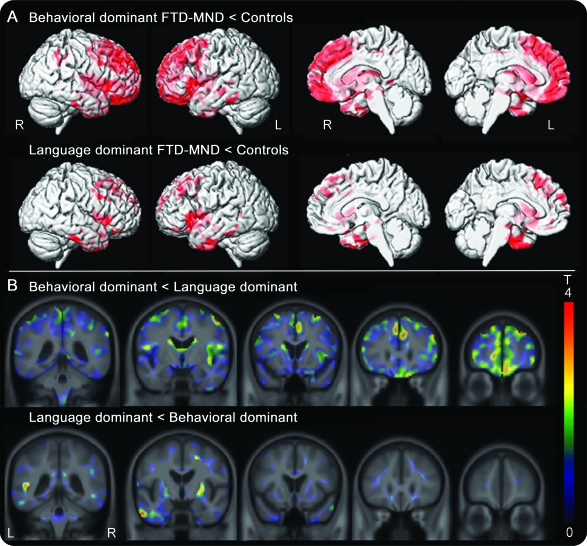
Both the behavioral-dominant and language-dominant FTD-MND groups showed gray matter loss in the frontal and temporal lobes compared with control subjects (figure 3). On direct comparison, with correction for multiple comparisons, no differences were observed between the behavioral- and language-dominant FTD-MND groups. However, using a lenient uncorrected threshold of p < 0.001, we did observe subtle differences across the groups. In particular, the behavioral-dominant group showed greater loss in the frontal lobes than the language-dominant group, and the language-dominant group showed greater loss in the left lateral inferior temporal lobes and right basal ganglia than the behavioral-dominant group (figure 3).
Figure 3. Results of voxel-based morphometry analyses.
(A) Patterns of gray matter loss in the behavioral- and language-dominant frontotemporal dementia with motor neuron disease (FTD-MND) groups compared with those of control subjects (corrected for multiple comparisons using family-wise error, p < 0.05). Both groups show gray matter loss in the frontal and temporal lobes. (B) Unthresholded t statistic effect maps, highlighting differences between the behavioral- and language-dominant FTD-MND groups on direct comparison. The behavioral-dominant group showed greater loss in the frontal lobes than the language-dominant group, whereas the language-dominant group showed greater loss in the left lateral inferior temporal lobe and right putamen than the behavioral-dominant group.
DISCUSSION
In this study we analyzed survival in a large group of patients with clinically diagnosed FTD-MND with respect to demographic and clinical FTD phenotype. The 56 patients with FTD-MND were separated into 31 with behavioral-dominant symptoms and 25 with language-dominant symptoms. Those with the behavioral phenotype had a significantly longer mean survival than those with the language phenotype.
Our overall survival data were similar to data from previous studies in FTD-MND,12,24–26 with an overall survival of less than 3 years, suggesting that our cohort was similar to cohorts reported from other centers. In a recent multicenter study of 87 patients with FTD-MND, the authors identified a long-term survival group that was more likely to have cognitive symptoms at onset.25 Our study has extended this finding by demonstrating that the behavioral-dominant FTD-MND phenotype shows a longer survival time compared with a language-dominant phenotype. When we subdivided overall survival into time from onset to diagnosis and time from diagnosis to death, we found that only time from diagnosis to death was different between groups, suggesting that this time interval is driving the overall survival. This finding is also important because measurement of this time interval does not rely on accurate assessment of onset, which could bias overall results. It should also be noted that both groups were similar in terms of cognitive severity at the time of diagnosis.
A timing difference between the onset of cognitive to motor symptoms and of motor to cognitive symptoms was observed across the groups with a shorter time between onset of these symptoms being observed in the language group. For those with cognitive onset occurring before motor, it is possible that the longer interval observed in the behavioral-dominant group is being driven by earlier recognition of behavioral change because behavioral symptoms are more likely to be disruptive to families than language symptoms. If this hypothesis was correct, however, then we would also expect a shorter time from motor onset to cognitive onset in the behavioral group, which was not observed. Therefore, the most likely explanation for this finding is that language-dominant FTD-MND is a faster progressing disease, regardless of cognitive or motor onset, compared with behavioral-dominant FTD-MND.
There was an association between FTD-MND phenotype and bulbar onset, with bulbar onset of MND being more common in those with language-dominant FTD-MND. This association seems to be clinically meaningful and is contributing to the survival difference observed across the 2 phenotypes. In particular, it appears that patients with behavioral-dominant FTD-MND without bulbar onset have the best survival, whereas those with language-dominant FTD-MND and bulbar onset have the worst survival.
Subtypes of FTD have been well-characterized,1 but work in FTD-MND has primarily focused on behavioral changes associated with MND. However, language-dominant forms of FTD-MND have been established for decades.27 In particular, a progressive nonfluent aphasia subtype with behavioral changes and bulbar onset of MND is recognized.27–30 The majority of our patients with predominant language symptoms had the nonfluent variety. However, semantic dementia, including semantic dementia with pronounced prosopagnosia, was evident in 3 patients, and one patient had logopenic aphasia. MND has been reported to be uncommon in patients with semantic dementia.31
Interestingly, we observed differences in symptoms across both types, with psychosis being more common in behavioral-dominant FTD-MND and parkinsonism and limb apraxia being more common in language-dominant FTD-MND. Likewise, psychosis is associated with the behavioral variant of FTD but not the language variant of FTD,32 and therefore our findings in FTD-MND mirror findings in FTD. Limb apraxia has not been emphasized previously in patients with FTD-MND, but in our study we showed that almost 50% of patients with language-dominant FTD-MND have limb apraxia. Likewise, parkinsonism has not been emphasized in FTD-MND, although parkinsonism has been reported in cases of autopsy-confirmed FTD-MND.33 Using the ALSFRS, we found, not surprisingly, more severe speech problems in those with language-dominant FTD-MND. Interestingly, however, we did not find more severe swallowing problems in this group, suggesting that severity of swallowing problems does not account for the shorter survival in the language-dominant group.
The language- and behavioral-dominant FTD-MND groups showed similar patterns of gray matter loss, involving the frontal and temporal lobes, that were also similar to the patterns reported previously in FTD-MND.34 There were, however, some subtle differences observed across the groups, with a tendency for more severe frontal loss in behavioral-dominant FTD-MND and more severe lateral inferior temporal lobe loss in language-dominant FTD-MND. These differences are not surprising. However, this lateral inferior temporal prominence would be atypical for these language variants of FTD.
There are limitations to this study: it is retrospective and only a few cases were confirmed by autopsy. Despite these limitations, we show in a relatively large cohort that patients with FTD-MND can be categorized according to behavioral or language dominance using clinical features outlined in the consensus criteria and that this categorization has implications for survival in counseling of patients and families.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge all neurologists at Mayo Clinic, Rochester, MN, who cared for these patients, and Dr. Clifford R. Jack, Jr., for the use of his laboratory to perform the VBM analysis.
Supplemental data at www.neurology.org.
- ALSFRS
- Amyotrophic Lateral Sclerosis Functional Rating Scale
- FDG
- fluorodeoxyglucose
- FTD
- frontotemporal dementia
- FTD-MND
- frontotemporal dementia with motor neuron disease
- FTLD-TDP
- frontotemporal lobar degeneration with TDP-43 immunoreactive inclusions
- FWE
- family-wise error
- MND
- motor neuron disease
- STMS
- Short Test of Mental Status
- TDP-43
- TAR DNA-binding protein of 43 kDa
- VBM
- voxel-based morphometry
AUTHOR CONTRIBUTIONS
E.A.C., E.J.S., and K.A.J. initiated the study concept or design. E.A.C., J.L.W., and K.A.J. analyzed or interpreted the data. E.A.C., D.S.K., and K.A.J. acquired the data. K.A.J. performed statistical analysis. E.J.S. and K.A.J. supervised the study. E.A.C., E.J.S., J.L.W., D.S.K., and K.A.J. drafted/revised the manuscript for content. K.A.J. obtained funding.
DISCLOSURE
Dr. Coon reports no disclosures. Dr. Sorenson serves on a scientific advisory board for AriSLA and receives research support from Teva Pharmaceutical Industries Ltd., the CDC, the NIH/NINDS, and the Muscular Dystrophy Association. Dr. Whitwell receives research support from the NIH and the Dana Foundation. Dr. Knopman serves as Deputy Editor of Neurology®; serves on a data safety monitoring board for Eli Lilly and Company; is an investigator in clinical trials sponsored by Elan Corporation, Baxter International Inc., and Forest Laboratories, Inc.; and receives research support from the NIH. Dr. Josephs receives research support from the NIH (NIDCD, NIA) and the Dana Foundation.
REFERENCES
- 1. Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol 2008;64:4–14 [DOI] [PubMed] [Google Scholar]
- 2. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554 [DOI] [PubMed] [Google Scholar]
- 3. Murphy JM, Henry RG, Langmore S, Kramer JH, Miller BL, Lomen-Hoerth C. Continuum of frontal lobe impairment in amyotrophic lateral sclerosis. Arch Neurol 2007;64:530–534 [DOI] [PubMed] [Google Scholar]
- 4. Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol 2007;6:994–1003 [DOI] [PubMed] [Google Scholar]
- 5. Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 2005;65:586–590 [DOI] [PubMed] [Google Scholar]
- 6. Rippon GA, Scarmeas N, Gordon PH, et al. An observational study of cognitive impairment in amyotrophic lateral sclerosis. Arch Neurol 2006;63:345–352 [DOI] [PubMed] [Google Scholar]
- 7. Josephs KA, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW. Clinically undetected motor neuron disease in pathologically proven frontotemporal lobar degeneration with motor neuron disease. Arch Neurol 2006;63:506–512 [DOI] [PubMed] [Google Scholar]
- 8. Mackenzie IR, Baborie A, Pickering-Brown S, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol 2006;112:539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006;314:130–133 [DOI] [PubMed] [Google Scholar]
- 10. Johnson JK, Diehl J, Mendez MF, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol 2005;62:925–930 [DOI] [PubMed] [Google Scholar]
- 11. Grossman M, Libon DJ, Forman MS, et al. Distinct antemortem profiles in patients with pathologically defined frontotemporal dementia. Arch Neurol 2007;64:1601–1609 [DOI] [PubMed] [Google Scholar]
- 12. Josephs KA, Knopman DS, Whitwell JL, et al. Survival in two variants of tau-negative frontotemporal lobar degeneration: FTLD-U vs FTLD-MND. Neurology 2005;65:645–647 [DOI] [PubMed] [Google Scholar]
- 13. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–299 [DOI] [PubMed] [Google Scholar]
- 14. Gordon PH, Cheng B, Katz IB, et al. The natural history of primary lateral sclerosis. Neurology 2006;66:647–653 [DOI] [PubMed] [Google Scholar]
- 15. Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC. Primary lateral sclerosis: clinical features, neuropathology and diagnostic criteria. Brain 1992;115:495–520 [DOI] [PubMed] [Google Scholar]
- 16. Josephs KA, Dickson DW. Frontotemporal lobar degeneration with upper motor neuron disease/primary lateral sclerosis. Neurology 2007;69:1800–1801 [DOI] [PubMed] [Google Scholar]
- 17. The Amyotrophic Lateral Sclerosis Functional Rating Scale: assessment of activities of daily living in patients with amyotrophic lateral sclerosis: the ALS CNTF Treatment Study (ACTS) Phase I–II Study Group. Arch Neurol 1996;53:141–147 [PubMed] [Google Scholar]
- 18. Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage 2000;11:805–821 [DOI] [PubMed] [Google Scholar]
- 19. Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 2006;30:436–444 [DOI] [PubMed] [Google Scholar]
- 20. Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 1998;17:87–97 [DOI] [PubMed] [Google Scholar]
- 21. Whitwell JL, Przybelski SA, Weigand SD, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain 2009;132:2932–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clin Proc 1987;62:281–288 [DOI] [PubMed] [Google Scholar]
- 23. Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 2010;119:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodges JR, Davies R, Xuereb J, Kril J, Halliday G. Survival in frontotemporal dementia. Neurology 2003;61:349–354 [DOI] [PubMed] [Google Scholar]
- 25. Hu WT, Seelaar H, Josephs KA, et al. Survival profiles of patients with frontotemporal dementia and motor neuron disease. Arch Neurol 2009;66:1359–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olney RK, Murphy J, Forshew D, et al. The effects of executive and behavioral dysfunction on the course of ALS. Neurology 2005;65:1774–1777 [DOI] [PubMed] [Google Scholar]
- 27. Caselli RJ, Windebank AJ, Petersen RC, et al. Rapidly progressive aphasic dementia and motor neuron disease. Ann Neurol 1993;33:200–207 [DOI] [PubMed] [Google Scholar]
- 28. Bak TH, Hodges JR. The effects of motor neurone disease on language: further evidence. Brain Lang 2004;89:354–361 [DOI] [PubMed] [Google Scholar]
- 29. Bak TH, O'Donovan DG, Xuereb JH, Boniface S, Hodges JR. Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neurone disease-dementia-aphasia syndrome. Brain 2001;124:103–120 [DOI] [PubMed] [Google Scholar]
- 30. Mitsuyama Y, Takamiya S. Presenile dementia with motor neuron disease in Japan: a new entity? Arch Neurol 1979;36:592–593 [DOI] [PubMed] [Google Scholar]
- 31. Godbolt AK, Josephs KA, Revesz T, et al. Sporadic and familial dementia with ubiquitin-positive tau-negative inclusions: clinical features of one histopathological abnormality underlying frontotemporal lobar degeneration. Arch Neurol 2005;62:1097–1101 [DOI] [PubMed] [Google Scholar]
- 32. Omar R, Sampson EL, Loy CT, et al. Delusions in frontotemporal lobar degeneration. J Neurol 2009;256:600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Claassen DO, Parisi JE, Giannini C, Boeve BF, Dickson DW, Josephs KA. Frontotemporal dementia mimicking dementia with Lewy bodies. Cogn Behav Neurol 2008;21:157–163 [DOI] [PubMed] [Google Scholar]
- 34. Whitwell JL, Jack CR, Jr, Senjem ML, Josephs KA. Patterns of atrophy in pathologically confirmed FTLD with and without motor neuron degeneration. Neurology 2006;66:102–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.