Abstract
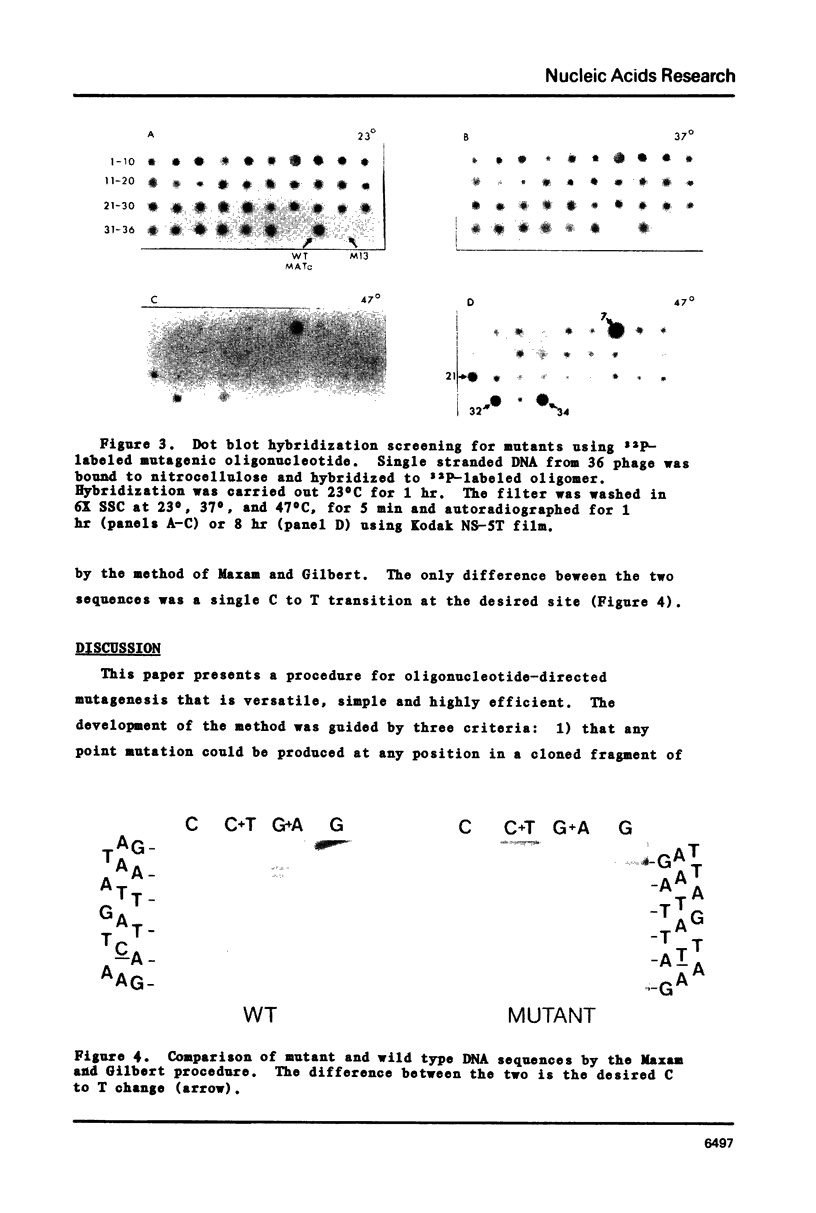
This paper presents a versatile and efficient procedure for the construction of oligodeoxyribonucleotide directed site-specific mutations in DNA fragments cloned into M13 derived vectors. As an example, production of a transition mutation in a clone of the yeast MATa1 gene is described. The oligonucleotide is hybridized to the template DNA and covalently closed closed double stranded molecules are generated by extension of the oligonucleotide primer with E. coli DNA polymerase (large fragment) and ligation with T4 DNA ligase. The resulting double stranded closed circular DNA (CC-DNA) is separated from unligated and incompletely extended molecules by alkaline sucrose gradient centrifugation. This purification is essential for production of mutants at high efficiency. Competent E. coli JM101 cells are transformed with the CC-DNA fraction and single stranded DNA is isolated from individual plaques. The recombinants are screened for mutant molecules by 1) restriction endonuclease screening for the loss of the Hinf I site in the target region, and 2) by dot blot hybridization using the mutagenic oligonucleotide as probe. Double stranded DNA is isolated from the sequencing. Efficiency of mutant production is in the range of 10-45% and no precautions to prevent mismatch repair are required.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Ahlstrom-Jonasson L., Smith M., Tatchell K., Nasmyth K. A., Hall B. D. The sequence of the DNAs coding for the mating-type loci of Saccharomyces cerevisiae. Cell. 1981 Nov;27(1 Pt 2):15–23. doi: 10.1016/0092-8674(81)90356-1. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Doel M. T., Jahnke P. A., Smith M. Further studies on the properties of oligonucleotide cellulose columns. Biochemistry. 1973 Dec 4;12(25):5068–5074. doi: 10.1021/bi00749a007. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Smith M. Synthesis and properties of oligonucleotide--cellulose columns. Biochemistry. 1972 Oct 24;11(22):4114–4120. doi: 10.1021/bi00772a014. [DOI] [PubMed] [Google Scholar]
- Astell C., Smith M. Thermal elution of complementary sequences of nucleic acids from cellulose columns with covalently attached oligonucleotides of known length and sequence. J Biol Chem. 1971 Mar 25;246(6):1944–1946. [PubMed] [Google Scholar]
- Baas P. D., Teertstra W. R., van Mansfeld A. D., Jansz H. S., van der Marel G. A., Veeneman G. H., van Boom J. H. Construction of viable and lethal mutations in the origin of bacteriophage 'phi' X174 using synthetic oligodeoxyribonucleotides. J Mol Biol. 1981 Nov 15;152(4):615–639. doi: 10.1016/0022-2836(81)90120-0. [DOI] [PubMed] [Google Scholar]
- Carmichael G. G., Schaffhausen B. S., Dorsky D. I., Oliver D. B., Benjamin T. L. Carboxy terminus of polyoma middle-sized tumor antigen is required for attachment to membranes, associated protein kinase activities, and cell transformation. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3579–3583. doi: 10.1073/pnas.79.11.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaconas G., van de Sande J. H. 5'-32P labeling of RNA and DNA restriction fragments. Methods Enzymol. 1980;65(1):75–85. doi: 10.1016/s0076-6879(80)65012-5. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Delaney A. D. A DNA sequence handling program. Nucleic Acids Res. 1982 Jan 11;10(1):61–67. doi: 10.1093/nar/10.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillam S., Smith M. Site-specific mutagenesis using synthetic oligodeoxyribonucleotide primers: I. Optimum conditions and minimum ologodeoxyribonucleotide length. Gene. 1979 Dec;8(1):81–97. doi: 10.1016/0378-1119(79)90009-x. [DOI] [PubMed] [Google Scholar]
- Gillam S., Waterman K., Smith M. The base-pairing specificity of cellulose-pdT9. Nucleic Acids Res. 1975 May;2(5):625–634. doi: 10.1093/nar/2.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman B. W., Radman M. Escherichia coli mutator mutants deficient in methylation-instructed DNA mismatch correction. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1063–1067. doi: 10.1073/pnas.77.2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M., Goulian S. H., Codd E. E., Blumenfield A. Z. Properties of oligodeoxynucleotides that determine priming activity with Escherichia coli deoxyribonucleic acid polymerase I. Biochemistry. 1973 Jul 17;12(15):2893–2901. doi: 10.1021/bi00739a019. [DOI] [PubMed] [Google Scholar]
- Goulian M., Kornberg A., Sinsheimer R. L. Enzymatic synthesis of DNA, XXIV. Synthesis of infectious phage phi-X174 DNA. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2321–2328. doi: 10.1073/pnas.58.6.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Edgell M. H. Genetic assay for small fragments of bacteriophage phi X174 deoxyribonucleic acid. J Virol. 1971 Aug;8(2):181–189. doi: 10.1128/jvi.8.2.181-189.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Phillips S., Edgell M. H., Gillam S., Jahnke P., Smith M. Mutagenesis at a specific position in a DNA sequence. J Biol Chem. 1978 Sep 25;253(18):6551–6560. [PubMed] [Google Scholar]
- Kudo I., Leineweber M., RajBhandary U. L. Site-specific mutagenesis on cloned DNAs: generation of a mutant of Escherichia coli tyrosine suppressor tRNA in which the sequence G-T-T-C corresponding to the universal G-T-pseudouracil-C sequence of tRNAs is changed to G-A-T-C. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4753–4757. doi: 10.1073/pnas.78.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miyada C. G., Soberón X., Itakura K., Wilcox G. The use of synthetic oligodeoxyribonucleotides to produce specific deletions in the araBAD promoter of Escherichia coli B/r. Gene. 1982 Feb;17(2):167–177. doi: 10.1016/0378-1119(82)90070-1. [DOI] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature. 1982 Feb 4;295(5848):380–384. doi: 10.1038/295380a0. [DOI] [PubMed] [Google Scholar]
- Razin A., Hirose T., Itakura K., Riggs A. D. Efficient correction of a mutation by use of chemically synthesized DNA. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4268–4270. doi: 10.1073/pnas.75.9.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons G. F., Veeneman G. H., Konings R. N., van Boom J. H., Schoemakers J. G. Oligonucleotide-directed mutagenesis of gene IX of bacteriophage M13. Nucleic Acids Res. 1982 Feb 11;10(3):821–832. doi: 10.1093/nar/10.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple G. F., Dozy A. M., Roy K. L., Kan Y. W. Construction of a functional human suppressor tRNA gene: an approach to gene therapy for beta-thalassaemia. Nature. 1982 Apr 8;296(5857):537–540. doi: 10.1038/296537a0. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Johnson P. F., Tanaka S., Schöld M., Itakura K., Abelson J. Directed deletion of a yeast transfer RNA intervening sequence. Science. 1980 Sep 19;209(4463):1396–1400. doi: 10.1126/science.6997991. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Schold M., Johnson M. J., Dembek P., Itakura K. Oligonucleotide directed mutagenesis of the human beta-globin gene: a general method for producing specific point mutations in cloned DNA. Nucleic Acids Res. 1981 Aug 11;9(15):3647–3656. doi: 10.1093/nar/9.15.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Derbyshire R., Guy A., Molko D., Roget A., Téoule R., Chambon P. Specific in vitro transcription of conalbumin gene is drastically decreased by single-point mutation in T-A-T-A box homology sequence. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7024–7028. doi: 10.1073/pnas.77.12.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbeek P. J., van de Pol J. H. Bological activity of phi X174 replicative form DNA fragments. Biochim Biophys Acta. 1970 Dec 14;224(2):328–338. [PubMed] [Google Scholar]
- Winter G., Fields S. Cloning of influenza cDNA ino M13: the sequence of the RNA segment encoding the A/PR/8/34 matrix protein. Nucleic Acids Res. 1980 May 10;8(9):1965–1974. doi: 10.1093/nar/8.9.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]




