Abstract
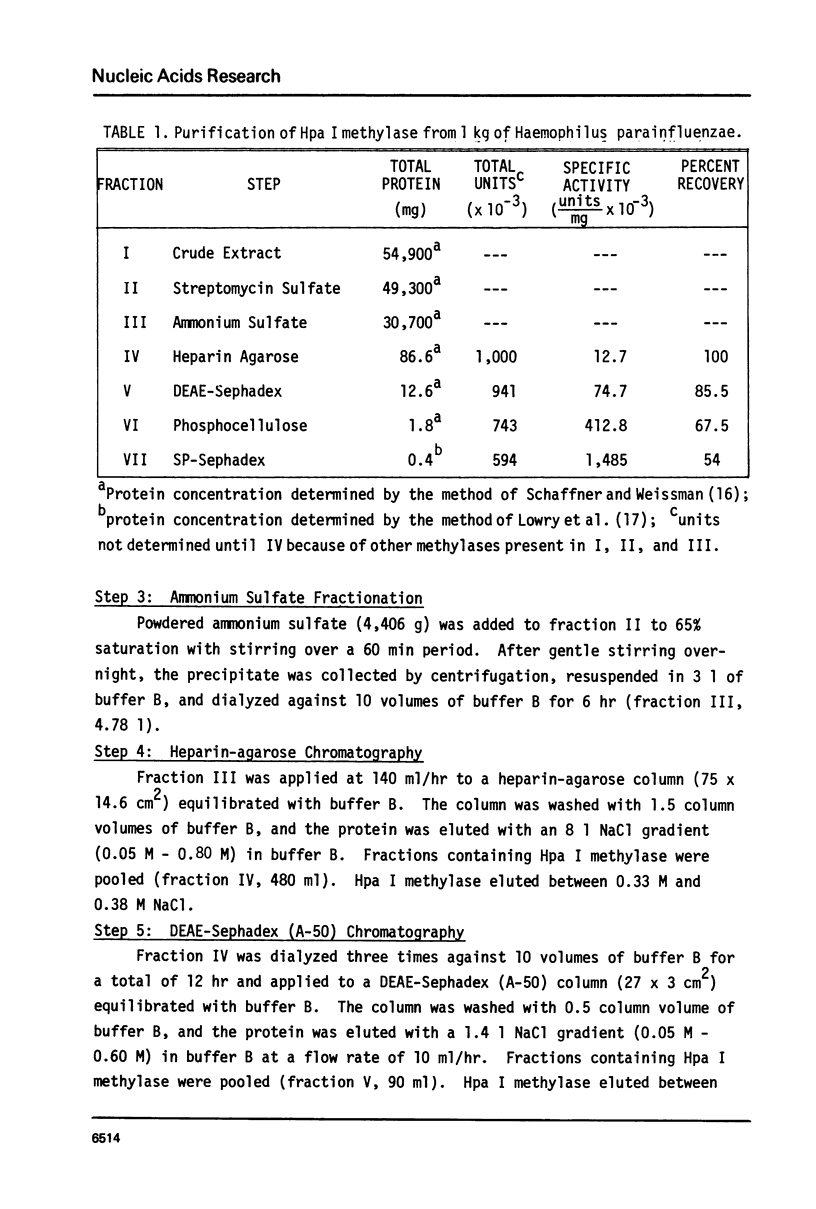
The purification and catalytic properties of the homogeneous Hpa I methylase is described. The enzyme exists as a single polypeptide chain with a molecular weight of 37,000 +/- 2,000 was shown by sedimentation equilibrium and polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. The Hpa I methylase transfers methyl groups of S-adenosylmethionine to adenine present in the recognition sequence d(G-T-T-A-A*-C), A* is the N6 methyl adenosine. An average of 2.1 methyl groups per recognition site are transferred by the Hpa I methylase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Riftina F. Chemical synthesis of a self-complementary octanucleotide, dG-G-T-T-A-A-C-C by a modified triester method. Nucleic Acids Res. 1978 Aug;5(8):2809–2823. doi: 10.1093/nar/5.8.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal K. L., Riftina F. Chemical synthesis of a self-complementary octanucleotide, dG-G-T-T-A-A-C-C by a modified triester method. Nucleic Acids Res. 1978 Aug;5(8):2809–2823. doi: 10.1093/nar/5.8.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mazur B. J., Model P. Regulation of coliphage f1 single-stranded DNA synthesis by a DNA-binding protein. J Mol Biol. 1973 Aug 5;78(2):285–300. doi: 10.1016/0022-2836(73)90117-4. [DOI] [PubMed] [Google Scholar]
- Model P., Zinder N. D. In vitro synthesis of bacteriophage f1 proteins. J Mol Biol. 1974 Feb 25;83(2):231–251. doi: 10.1016/0022-2836(74)90389-1. [DOI] [PubMed] [Google Scholar]
- Razin A., Sedat J. W., Sinsheimer R. L. Structure of the DNA of bacteriophage phiX174. VII. Methylation. J Mol Biol. 1970 Oct 28;53(2):251–259. doi: 10.1016/0022-2836(70)90298-6. [DOI] [PubMed] [Google Scholar]
- Rubin R. A., Modrich P. EcoRI methylase. Physical and catalytic properties of the homogeneous enzyme. J Biol Chem. 1977 Oct 25;252(20):7265–7272. [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Sgaramella V., Khorana H. G. CXII. Total synthesis of the structural gene for an alanine transfer RNA from yeast. Enzymic joining of the chemically synthesized polydeoxynucleotides to form the DNA duplex representing nucleotide sequence 1 to 20. J Mol Biol. 1972 Dec 28;72(2):427–444. doi: 10.1016/0022-2836(72)90155-6. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]