Abstract
Yellow fever virus (YFV), a mosquito-borne virus endemic to tropical Africa and South America, is capable of causing large urban outbreaks of human disease. With the ease of international travel, urban outbreaks could lead to the rapid spread and subsequent transmission of YFV in distant locations. We designed a stochastic metapopulation model with spatiotemporally explicit transmissibility scenarios to simulate the global spread of YFV from a single urban outbreak by infected airline travelers. In simulations of a 2008 outbreak in Asunción, Paraguay, local outbreaks occurred in 12.8% of simulations and international spread in 2.0%. Using simple probabilistic models, we found that local incidence, travel rates, and basic transmission parameters are sufficient to assess the probability of introduction and autochthonous transmission events. These models could be used to assess the risk of YFV spread during an urban outbreak and identify locations at risk for YFV introduction and subsequent autochthonous transmission.
Introduction
Yellow fever virus (YFV) is endemic to sub-Saharan Africa and tropical South America, where it is maintained in nature by transmission between nonhuman primates and sylvatic mosquito species.1 Humans become infected when they enter jungle areas and are fed on by infectious mosquitoes. As infected humans move, they can transport the virus from one region to another, serving as a source of infection for naïve mosquitoes in distant locations. Although the vast majority of yellow fever occurs in remote, rural areas, urban outbreaks can occur in areas infested by the anthropophilic mosquito Aedes aegypti, a highly efficient vector of YFV. In 2008, an outbreak of urban yellow fever was identified in metropolitan Asunción, Paraguay.2 This was the first urban yellow fever outbreak documented in South America since 1942 and raised concerns of the potential spread of the virus to non-endemic areas with vectors capable of transmitting the virus, such as the Caribbean, Central America, and North America.
In the Americas, the scale of yellow fever outbreaks over the last one-half century has been limited by large-scale Ae. aegypti control efforts3 and the use of YFV vaccine.4 However, problems with vector control program sustainability,5,6 vaccine supply,7–9 and adverse events associated with vaccination10–12 threaten primary prevention efforts. Recognition of the outbreak in Asunción was quickly followed by intensive vector control efforts in over 25,000 households and administration of more than 1 million doses of YFV vaccine.2 Because of either interventions or natural abatement, the Asunción outbreak was limited to only nine confirmed cases. With a more hospitable environment and less control effort, this small outbreak could have led to a larger, possibly international epidemic.
Previous work has quantified the continuing risk of introduction of YFV into urban environments13 but has not addressed the risk of further spread. With the convenience and speed of modern airline travel, travelers infected with YFV may quickly arrive in nearby or distant international locations. Given the high densities of competent vector mosquitoes in many tropical and sub-tropical areas of the world and the low vaccine coverage rates outside of endemic regions, YFV-infected travelers could present a major risk to many populations where suitable conditions for transmission are present. The challenge that we confront is to estimate the magnitude of that risk. To simulate the global spread of YFV from a single urban outbreak by infected airline travelers, we developed a metapopulation model to quantify critical measures of global spread and estimated the risk of spread associated with the Asunción outbreak. We then used probabilistic models to estimate the probabilities of spread based solely on simplified estimates of the most critical components.
Materials and Methods
Stochastic metapopulation model
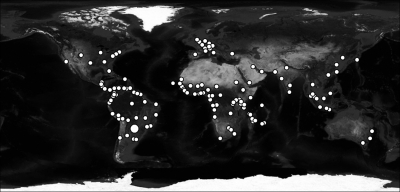
A full description of the model and parameterization can be found in the Supplemental Information. Briefly, we included 141 cities (Figure 1) based on their importance to international travel, proximity to yellow fever endemic areas, or involvement in the recent spread of chikungunya virus (another arthropod-borne virus transmitted by Aedes mosquitoes). Each city was given a local human population consisting of susceptible, incubating, infectious, and immune individuals, any of whom can engage in temporary travel to other cities.14 Climate data for all cities were extracted from long-term climate models created by the Climate Research Unit of East Anglia University, United Kingdom.15 Cities where at least 6 months of a typical year have an average temperature of less than 10°C or no rainfall were considered unsuitable for Ae. aegypti habitation.16 Each suitable city was given an Ae. aegypti mosquito population that varies depending on local, daily, climate-dependent mortality rates determined using a spline-smoothed version of the climate data. The mosquito populations included susceptible, incubating, and infectious mosquitoes. Mosquitoes may be infected by feeding on viremic humans, at which point they undergo an incubation period before becoming infectious. Humans, in turn, may be infected by infectious mosquitoes and then undergo an incubation period followed by a viremic phase and then recovery, at which point they gain immunity to YFV. We incorporated two vaccination scenarios in the model: no vaccination and previous vaccination based on the latest available country-specific vaccine coverage estimates from the World Health Organization.17 Previously vaccinated individuals were considered immune.
Figure 1.
The 141 cities included in the analysis. Asunción is indicated by the largest dot.
Travel (including connecting travel) between each city pair was estimated using city and network characteristics in a regression model based on US sampled itinerary data (US Department of Transportation; www.transtats.bts.gov/Tables.asp?DB_ID=125) and global airline data (Official Airline Guide; www.oagaviation.com/Solutions/AnalysisTools/Traffic/t100inet.html).
Incubation periods were modeled based on historical YFV data.18 Temperature- and humidity-dependent Ae. aegypti mortality was derived from previous work by Focks and others.19
Published data on the human infectious period, vector density, vector biting rate, efficiency of human to vector transmission, and efficiency of vector to human transmission are too limited to adequately characterize these components (Table 1). Rather than analyzing the sensitivity of the model outcome to each of these parameters individually, we combined their lowest estimates to create a lower-limit low transmissibility scenario, their highest estimates to create a worst-case high scenario, and central estimates to create a moderate scenario. For the ease of discussion, we classify these scenarios in terms of R0, the basic reproductive number. In the case of a vector-borne virus such as YFV, R0 can be defined as the average number of human infections resulting from a single human infection (calculation described in Supplemental Information).
Table 1.
Parameters for the low, moderate, and high transmissibility scenarios
| Parameter | Low | Moderate | High | Source |
|---|---|---|---|---|
| Female mosquitoes per person* | 1 | 2 | 4 | 52 |
| Vector longevity (days)* | 10.6† | 10.6† | 10.6† | 19 |
| Human blood meals per mosquito per day | 0.5 | 0.7 | 1 | 29, 35, 36, 53, 54 |
| Efficiency of human to vector transmission | 0.2 | 0.5 | 0.5 | 29, 31–36 |
| Efficiency of vector to human transmission | 0.5 | 0.5 | 1 | 32 |
| Extrinsic incubation period (days)* | 6.9† | 6.9† | 6.9† | 18 |
| Human infectious period (days) | 3 | 3 | 4 | 55 |
| Peak R0 (at 36°C and 100% relative humidity) | 0.42 | 4.1 | 90 |
These factors are dependent on local climate and thus, exhibit significant spatiotemporal variation. Their values here correspond to 36°C and 100% relative humidity.
These factors are considered well-estimated and thus, do not vary between models.
Each simulated epidemic is seeded by introducing infected humans to a single city at a specified day of the year. The model is discrete with daily time steps, and all interactions are stochastic. A number of epidemics are simulated to generate a range of possible outcomes starting from a given scenario.
Probabilistic models
A full description of these models can be found in Supplemental Information. The models are parameterized the same as the stochastic metapopulation model. With pi,j as the probability of travel from city i to city j and NIi,t as the number of infected individuals in city i at time t, the probability of infected individuals traveling from a particular city, i′, to any other city by time T can be written as:
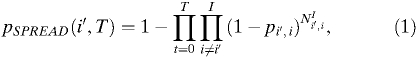 |
where i is the city index for cities i = 1, 2, …, I and I is the total number of cities. The probability of introduction from any other city to city i′ by time T can be written as:
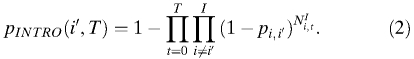 |
If the time series NIi,t is unknown, the equation may be reformulated to assume that all we know is an estimate of the number of people who have been infected and the rates of travel. Equation 1, describing the probability of an infected traveler leaving city i′, can be simplified to be a function of cumulative infected person-days in city i′, Xi′:
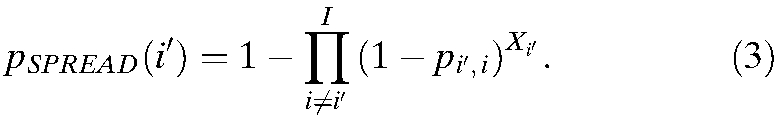 |
To assess the probability of novel autochthonous transmission events, we used branching process analysis.20 In the case of vector-borne infections, an infectious human generates a random number of infectious vectors from a distribution determined by the vector density, feeding rate, transmission efficiency, and probability of a vector surviving the extrinsic incubation period. An infectious vector, likewise, may give rise to any number of infectious humans dependent on the feeding rate, transmission efficiency, and vector longevity. To analyze the probability of extinction in a single step, we analyzed the value g(0) for the respective probability generating function g(s).20 In this case, we use a composite probability-generating function to analyze the probability that three processes—infectious individuals traveling from city i to j, infection of vectors in city j, and infection of humans in city j—result in zero new human cases at time t:
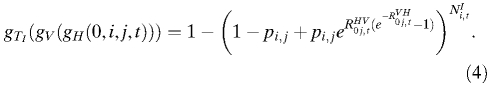 |
This equation requires 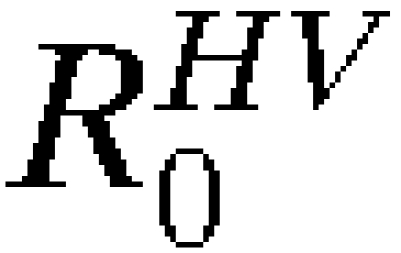 and
and 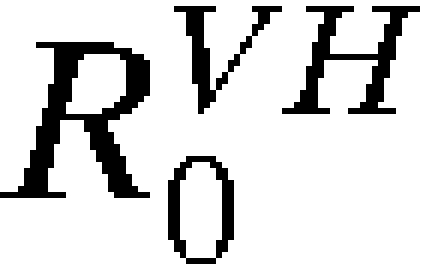 , the average number of infectious vectors produced per infectious human and the average number of infectious humans produced per infectious vector, respectively, both of which exhibit spatiotemporal variation and thus, are subscripted (i,t). As above, introduction may occur from different cities and at different time points, and therefore, the probability of novel autochthonous transmission in city i′ by time T is:
, the average number of infectious vectors produced per infectious human and the average number of infectious humans produced per infectious vector, respectively, both of which exhibit spatiotemporal variation and thus, are subscripted (i,t). As above, introduction may occur from different cities and at different time points, and therefore, the probability of novel autochthonous transmission in city i′ by time T is:
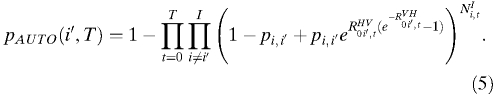 |
This probability can also be estimated in the absence of complete information by modifying Equation 5 to use infected person-days, X, for each potential source city rather than a daily number of infected individuals:
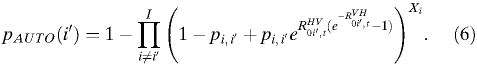 |
The probability of spread from a given city, i′, resulting in autochthonous transmission in any other city is:
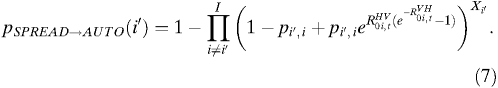 |
In the presence of vaccination, 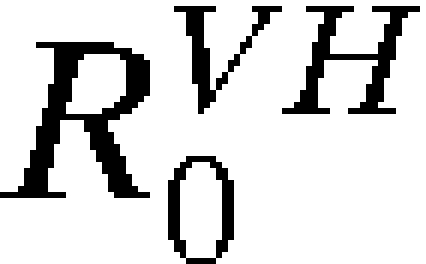 is replaced by the effective reproductive number,
is replaced by the effective reproductive number, 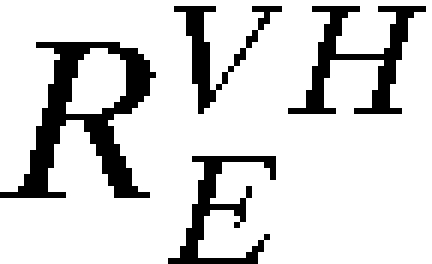 , indicating transmissibility in a partially vaccinated population:
, indicating transmissibility in a partially vaccinated population:
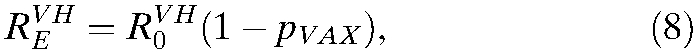 |
where pVAX is the proportion of the population that has been effectively vaccinated.
Results
R0
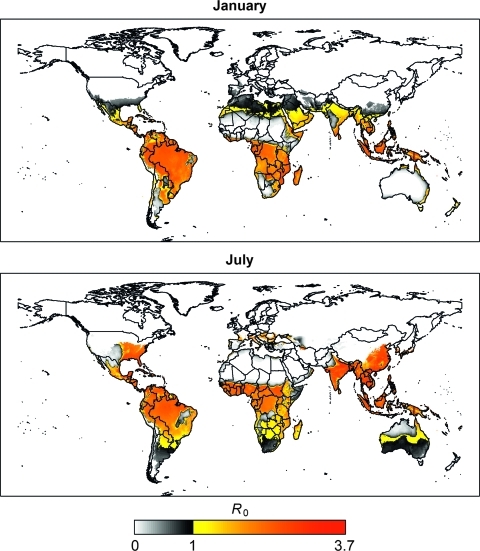
We first established three different model parameter sets and characterized them in terms of R0, which indicates the average number of infected humans produced by a single infected human in a completely naïve population. Using low, moderate, and high literature estimates of the parameters for the human infectious period, vector density, vector biting rate, efficiency of human to vector transmission, and efficiency of vector to human transmission, we estimated R0 values to be 0.42, 4.1, and 90 under the respective scenarios at peak transmissibility conditions (Table 1). Although these R0 estimates classify transmissibility at 36°C and 100% relative humidity, transmissibility in the model is adapted to reflect temporal and geographic variation of local climate. Figure 2 shows global climate-adjusted estimates of R0 based on the moderate transmissibility scenario for January and July.
Figure 2.
Estimated R0 in January and July for an average year. R0 is a measure of transmission potential under idealized contact conditions and does not account for important extant determinants of transmission, such as the prevalence of vaccination or personal protective practices.
Spread without vaccination
Before the outbreak-associated vaccination campaign in 2008, reported vaccine coverage in Paraguay was 34%,17 but vaccination efforts had been concentrated on children throughout the country and people in rural border areas rather than in Asunción.2 We, therefore, assumed that the population of Asunción was 100% susceptible. Furthermore, to simulate a worst-case scenario, we assumed that all other populations were also 100% susceptible. The first cases in Paraguay were reported in January of 2008, and therefore, we ran 1,000 simulations with the introduction of a single incubating individual to Asunción on January 1. Given the local climate at that time of year, the initial R0 values in Asuncion were 0.047, 0.46, and 10 for the low, moderate, and high scenarios, respectively.
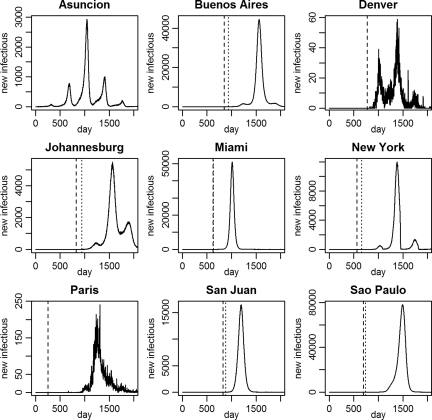
In the low transmissibility scenario, only 2.3% of the simulations resulted in local transmission, with a maximum of seven additional cases occurring (Table 2). Because transmission under this scenario was so limited and spread to other cities did not occur, it was not considered in later experiments. Under the moderate transmission scenario, a single introduction led to additional human transmission in 128 (12.8%) of the simulations. In 108 (84.4%) of these outbreaks, the outbreak involved only local transmission, affecting a median of 2 persons with a range of 1–981 persons. In two outbreaks, infectious individuals arrived in other cities but did not initiate any additional transmission. In the other 20 (15.6%) simulations, however, large epidemics occurred, affecting 450,000–550,000 people in Asunción, and international spread occurred, resulting in YFV pandemics. Figure 3 shows epidemic curves for a selection of cities under the moderate R0 model. Under the high R0 scenario, nine (0.9%) simulations resulted in only small-scale local transmission (one to three additional cases), one of which resulted in a single infected traveler going to New York (Table 2). In 689 (68.9%) other simulations, there were large local outbreaks leading to pandemics.
Table 2.
Occurrence of local YFV transmission in Asunción, infected travelers, and autochthonous transmission in other cities in simulations under different transmissibility scenarios
| R0 parameterization | Transmission in Asuncion (%)* | Infected travelers (%)* | Transmission in other cities (%)* |
|---|---|---|---|
| Low | 2.3 | 0.0 | 0.0 |
| Moderate | 12.8 | 2.2 | 2.0 |
| High | 69.8 | 69.0 | 68.9 |
| Moderate with vaccination | 6.9 | 0.1 | 0.0 |
| High with vaccination | 64.0 | 57.4 | 57.4 |
Percentage of 1,000 simulations.
Figure 3.
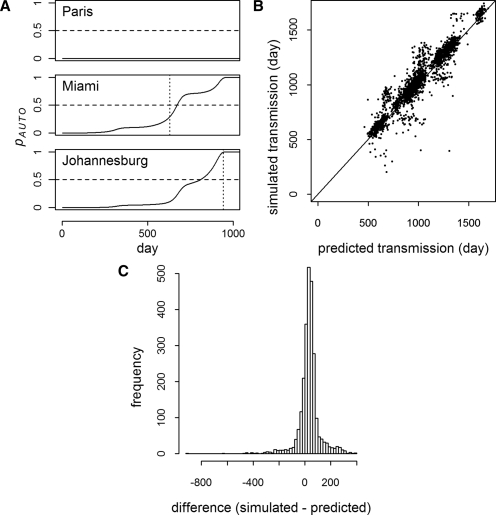
Pandemic simulation. The lines indicate the number of individuals becoming infectious each day for nine representative cities in a single moderate R0 pandemic simulation.YFV transmission is initiated in Asunción, where transmission follows a seasonal pattern. The first introductions to other cities (dashed lines) began with Paris on day 254. The first autochthonous transmission outside of Asunción (dotted lines) occurred in Miami on day 629 (after introduction on day 622). Repeated introductions and seasonal variation in transmissibility may lead to recurring epidemics. In Denver and Paris, the climate does not support YFV transmission, and therefore, all of the infectious individuals in those locations are returning or visiting travelers from areas where transmission is ongoing.
Dynamics were monitored locally for both potential introduction (i.e., the presence of infectious individuals) and autochthonous transmission, which was evidenced by a locally acquired human infection (Figure 3). In the moderate R0 model, the first international spread of YFV by an infected or infectious traveler from Asunción occurred at a median of 259 days (range = 14–561 days) after introduction into Asunción. At the time of introduction, a median of 1,013.5 infections (range = 3–6,363 infections) had occurred in Asunción. The first international autochthonous transmission occurred after 596.5 days (range = 203–1,310 days) when there had been 10,654 infections (range = 1,045–61,240 infections) in Asunción. In the high R0 model, both introduction and autochthonous transmissions occurred earlier at a median of 53 (range = 3–80 days) and 68 days (range = 27–94 days), respectively. This finding corresponded to a median of 756 infections (range = 5–8,735 infections) occurring before the earliest foreign introduction event and 9,468 infections (range = 28–140,681 infections) before the first foreign autochthonous transmission event.
The first three cities to which YFV was introduced by infected travelers in both the moderate and high transmissibility scenarios were Paris, London, and New York. Autochthonous transmission occurred earliest, on average, in New York, Miami, and Singapore in the moderate R0 model and Miami, Sao Paulo, and Singapore in the high R0 model. This order of initiation of autochthonous transmission varied greatly between simulations. For example, although Miami was, on average, the first city to experience autochthonous transmission in the high R0 model, in 25.7% of the simulations, 10 or more cities experienced transmission before Miami.
Spread with vaccination
We then repeated simulations for both the moderate and high transmissibility scenarios under the assumption that the population of each city had been vaccinated at the last reported coverage rate for its respective country. In Asunción, for example, 67% of the population was assumed to be immune, because the estimated YFV vaccine coverage for Paraguay was 67% after the 2008 vaccination campaign. Under these conditions, local transmission occurred in 69 and 640 of 1,000 simulations in the moderate and high transmission scenarios, respectively (Table 2). In the 6.9% of moderate scenario simulations in which transmission occurred, transmission was limited to 1–12 new local infections with a single infected traveler but no subsequent transmission. In the high R0 model, 57.4% of the simulations resulted in pandemics. When pandemics occurred, the total number of infections globally was reduced by ∼26% (307.2–307.8 million with vaccination versus 416.4–416.8 without vaccination).
Probabilistic models
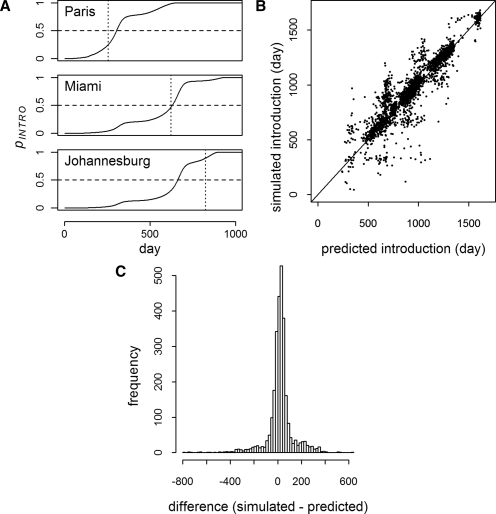
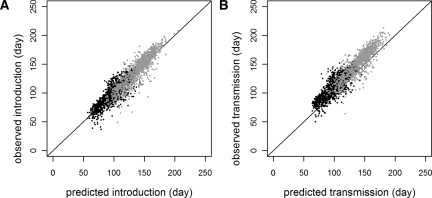
For each simulation, the probabilities of introduction, pINTRO, and introduction leading to autochthonous transmission, pAUTO, were calculated using the theoretical models. Figure 4A shows the increase in pINTRO over the course of a single simulation for three cities. Introduction is predicted when pINTRO = 0.5. In the moderate R0 model, there were 2,802 simulated introductions of a possible 140,000 (1,000 simulations for 140 cities). On average, predicted introduction was 22 days (middle 95% = −253–277 days) (Figure 4B and C) and 2 days (middle 95% = −23–23 days) before introduction in the moderate and high R0 scenario simulations, respectively. Of the 2,802 simulated introduction events, 2,800 were predicted for a sensitivity of greater than 99% and a negative predictive value (NPV) of greater than 99%. With 20 false positives, both the specificity and positive predictive value (PPV) were also greater than 99%. In the high R0 model, all but 1 of 96,460 introductions were predicted for a sensitivity and NPV of greater than 99%. Specificity was greater than 98% with 689 false positives, and the PPV was greater than 99%.
Figure 4.
Probability of introduction events (moderate R0). A shows the probability of introduction (solid line) for three cities as a function of time in a single simulation. For each city, the threshold, pINTRO = 0.5, is indicated by the horizontal dashed line, and the time of actual first introduction in the simulation is indicated by the vertical dotted line. B shows simulated versus predicted introduction times for all cities where introduction was predicted and occurred (N = 2,800). C is a histogram of the time difference between the predicted and simulated introductions in B (mean difference = 22 days, middle 95% = -253–277 days).
The onset of autochthonous transmission is predicted at pAUTO = 0.5. In the simulations, autochthonous transmission was predicted on average 33 days (middle 95% = −171–242 days) (Figure 5) and 11 days (middle 95% = −14–33 days) before occurrence in the moderate and high R0 simulations, respectively. In the moderate R0 model, autochthonous transmission was predicted on 2,580 occasions, of which 2,560 had simulated transmission events (PPV > 99%). Specificity was also greater than 99% with no false negatives, and sensitivity and NPV were 100%. The high R0 model also had no false negatives (sensitivity = 100%, NPV = 100%). There were 689 false positives, however, with specificity and PPV approximately 99%.
Figure 5.
Probability of autochthonous transmission (moderate R0). A shows the probability of autochthonous transmission (solid line) for three cities as a function of time in a single simulation (the same simulation as Figure 4). For each city, the threshold, pAUTO = 0.5, is indicated by the horizontal dashed line, and the time of the first locally acquired human infection in the simulation is indicated by the vertical dotted line (no transmission was predicted in Paris, and none occurred in the simulation). B shows the timing of simulated versus predicted autochthonous transmission events for all cities where simulated autochthonous transmission occurred (N = 2,560). C is a histogram of the difference between the simulations and predictions in B (mean difference = 33 days, middle 95% = −171–242 days).
With preexisting vaccination, only the high R0 model led to autochthonous transmission in other areas (Table 2). For introduction and autochthonous transmission, sensitivity, specificity, PPV, and NPV were all greater than 99%. Both the prediction and occurrence of introduction and autochthonous transmission were delayed when vaccination was incorporated (Figure 6).
Figure 6.
The effect of vaccination on simulated and predicted events (high R0). A shows simulated and predicted introduction times (N = 2,000, sampled randomly from the complete set) for the high R0 model with (grey) and without (black) prior vaccination. B shows the simulated and predicted times of the onset of autochthonous transmission under both conditions.
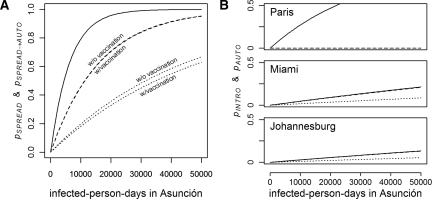
We also assessed the probabilistic models in the case where complete data on an epidemic is unknown. Figure 7A shows the probability of spread, pSPREAD, from Asunción using the travel parameters presented here under increasing cumulative infected person-days and the probability of spread resulting in autochthonous transmission, pSPREAD→AUTO, in at least one other city based on the number of infected person-days and 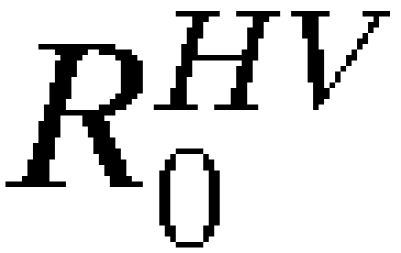 and
and 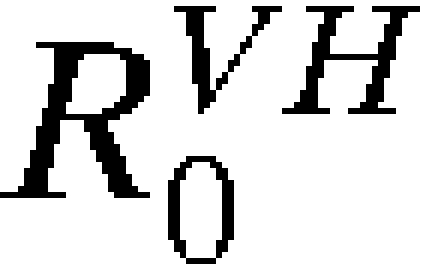 on January 1. The probability of spread leading to autochthonous transmission is delayed compared with the probability of spread, and it is further delayed with decreased R0 or the presence of preexisting vaccination in other cities. With 10,000 infected person-days, for example, the probability of spread having already occurred is approximately 0.8, and the probability of autochthonous transmission having occurred in another city is approximately 0.5 under the high R0 model and 0.2 under the moderate R0 model. Note that an average human infection results in 7.6 infected person-days (4.6 days incubating and 3 days infectious), and therefore, 10,000 infected person-days is roughly equivalent to a cumulative total of 1,300 people infected.
on January 1. The probability of spread leading to autochthonous transmission is delayed compared with the probability of spread, and it is further delayed with decreased R0 or the presence of preexisting vaccination in other cities. With 10,000 infected person-days, for example, the probability of spread having already occurred is approximately 0.8, and the probability of autochthonous transmission having occurred in another city is approximately 0.5 under the high R0 model and 0.2 under the moderate R0 model. Note that an average human infection results in 7.6 infected person-days (4.6 days incubating and 3 days infectious), and therefore, 10,000 infected person-days is roughly equivalent to a cumulative total of 1,300 people infected.
Figure 7.
Probability of spread. A shows the relationship between accumulating infected person-days in Asunción and the risk of spread to at least one other city. The solid line is the probability of an infected traveler departing Asunción, pSPREAD, and the dashed and dotted lines are the probabilities of the spread of autochthonous transmission to any other city, pSPREAD→AUTO, occurring under the high and moderate R0 models, respectively. The influence of vaccination is shown as indicated, with hardly any difference in the high R0 model. pSPREAD→AUTO is calculated based on R0 on January 1. B shows the probability of introduction (solid) and autochthonous transmission (dashed, high R0; dotted, moderate R0) to three cities from Asunción. In Paris, pAUTO is almost zero under both the moderate and high R0 scenarios. In Miami and Johannesburg, pAUTO under the high R0 scenario is approximately equivalent to pINTRO.
The modified infected person-day equations can also be used to calculate the probability of introduction to a particular city. Figure 7B shows how the cumulative probabilities of introduction and autochthonous transmission in three cities follow the number of infected person-days in Asunción. In the case of Paris, introduction is highly probable, but R0 is so low on January 1 that the probability of autochthonous transmission is virtually zero. Meanwhile, when R0 is high, such as in Miami and Johannesburg in the high R0 model, the probability of introduction leading to autochthonous transmission is nearly equivalent to the probability of introduction.
Discussion
The model described here is the first model to mechanistically address the potential for a vector-borne pathogen, such as YFV, to spread around the world through infected airline travelers. It was built using our best understanding of the dynamics of Ae. aegypti mosquitoes, YFV infection, and global travel and was designed to assist in assessing the probabilities of spread of YFV in the event of an urban epidemic. To put this model into a real life context, we applied it to an actual outbreak that occurred in Asunción, Paraguay, in 2008. Below, we discuss our estimation of YFV transmission dynamics, what the models suggest about the outbreak in Asunción, our findings regarding the probability of introduction and autochthonous transmission of YFV, the effect of existing vaccine coverage, and the limitations of the data and models.
YFV transmission dynamics
We assessed three transmission scenarios representing drastically different estimations of YFV virus transmissibility and pandemic potential (Table 1). Although all three scenarios incorporate plausible estimates for individual parameters, it is likely that the moderate parameter set represents the most realistic scenario. The lowest estimate that we evaluated for R0 was 0.42, too low to reliably cause epidemics even under the most favorable environmental conditions. The highest estimate for R0 was 90, extremely high compared with related dengue viruses for which estimates range from 0 to 103 but with median estimates in the range of 1 to 6.21–27 Moreover, given that most YFV epidemics are small or progress slowly,1 it is more likely that R0 for YFV is generally much lower, closer to 1 than 90.
Between the low and high R0 estimates, there is much parameter flexibility. Although our moderate model likely overestimates some parameters, it likely underestimates others, leading to a middle ground. Although this likelihood cannot be explicitly tested, each parameter that we used falls within a reasonable range (more details in Supplemental Information), and the estimated geographic areas where transmission is favored (Figure 2) correspond to the known, historical, and estimated spatial distributions of YFV and dengue virus transmission.28 The YFV R0 estimates from the moderate model are also similar to those estimates in previous studies.25,29,30 Note that R0 is not an absolute determinant of potential transmission; many other factors such as vaccination rates, vector control programs, and personal protective measures may also determine whether transmission occurs.
Further refining estimates of R0 would be difficult because of the complexity of the underlying components. For example, various studies estimate that the average human to vector efficiency of YFV transmission is much less than 0.5, the estimate under the moderate scenario.31–34 At lower efficiency estimates, however, R0 quickly drops below one, even under ideal environmental conditions. For YFV to cause even occasional epidemics, as it does, either this efficiency has been routinely underestimated or there are other components that have been underestimated.
Asunción
In the actual Asunción outbreak, a total of nine locally acquired infections were confirmed. Using the moderate R0 parameter set to simulate the introduction of a single infected individual into Asunción, we found that small local outbreaks occurred in 10.8% of the simulations. An outbreak like the one that was reported is, thus, a distinct possibility, although no further transmission was a more common result in simulations (87.2%).
It is possible that we underestimated the probability of local outbreaks by underestimating YFV transmissibility in Asunción. In our high R0 model, the frequency of local outbreaks was higher, with local transmission occurring in 70.7% of the simulations. However, in 98% of those outbreaks, a pandemic occurred, an eventuality that did not occur in the real outbreak.
We also lack a complete description of the actual outbreak. An infected individual with a travel history to rural areas with ongoing transmission was never identified, and the true number of people infected is likely underestimated, because many infected individuals may be asymptomatic. However, if more than one infected person had arrived, the probability of a local epidemic would have been substantially higher. For example, given that 10.8% of introductions in the moderate R0 model resulted in local transmission, if six infected people arrived, the probability of local transmission would be almost 50% (1 − [1 − 0.108]6).
The most probable explanation for the short-lived outbreak in Asunción is that it was self-limited because of a relatively inhospitable environment (low local R0) and that spread beyond Asunción did not occur, because with so few individuals infected, spread is unlikely to occur. Using Equation 3, with a total of nine infected individuals and average duration of infection of 8 days, the probability of at least one infected individual leaving Asunción is approximately 0.01.
Probability of introduction by travelers
The first event of interest relative to the potential spread of YFV by travelers is the appearance of an incubating or infectious individual in a population where YFV is absent. The simulations presented here can be used to directly estimate the probability of spread under the assumptions that we have presented. In the moderate R0 model, international introduction from Asunción was rare, occurring in 2.2% of simulations. However, in 90.9% of those simulations, YFV-infected travelers eventually reached every city in the model, leading to a pandemic. Thus, although the probability of spread is low, the consequences may be drastic. In the high R0 model, both of these events were more common, with 69.0% of simulations resulting in international spread and 99.9% of spread resulting in pandemics.
Focusing on the simulations in which pandemics did occur in the moderate R0 model, the median time to spread was 259 days, but spread occurred as soon as 14 days after the initial case was introduced to Asunción. At the time of the earliest spreading events, the median outbreak size in Asunción was just over 1,000 people and spread occurred with as little as 3 people infected. This timing, in terms of both actual time and the number of people infected, shows that outbreaks could quickly spread to other locations before being recognized.
The probabilistic models were highly sensitive and specific for the prediction of introduction in the simulations and tended to predict introduction before actual introduction (Figure 4). As Equation 3 makes clear, the cities with the highest rates of travel are the ones where the first introductions are expected. In our model, Asunción had the highest rates of travel to Paris, London, and New York, the cities where introduction occurred earliest in the simulations. After the initial spread, the situation becomes more complicated, because there are multiple sources of infected individuals.
In the midst of an ongoing outbreak, precise data on the number of people infected and the timing of their infectious periods is generally not available. Therefore, it may be of more use to estimate the risk of spread using an estimate of cumulative infected person-days. As presented in Equation 3, this estimate and an estimate of travel rates are sufficient to estimate both the probability of infected travelers leaving a given city and the probability of infected travelers arriving in a given city.
Probability of introduced autochthonous transmission
Assessing the risk of introduction is only the first step. Often more critical is assessing whether introduction will lead to autochthonous transmission. The only additional information needed to estimate the probability of autochthonous transmission after introduction is the transmission components 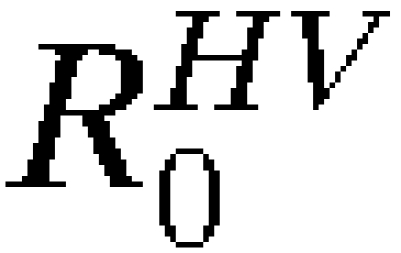 and
and 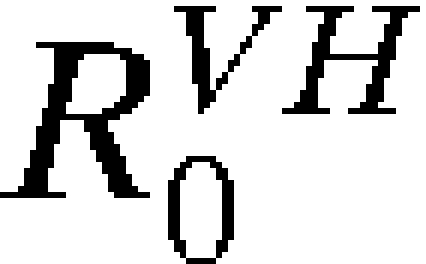 for the time and location of interest (Equation 5). As discussed above, we have estimated R0 and its subcomponents mechanistically, with reassuring concordance with historical observations and environmental suitability models.
for the time and location of interest (Equation 5). As discussed above, we have estimated R0 and its subcomponents mechanistically, with reassuring concordance with historical observations and environmental suitability models.
Using probability generating functions to estimate the probability of one or more autochthonous infections, we reliably predicted our simulations of these events (Figure 5). We also estimated the probability of autochthonous transmission occurring in other cities based solely on the cumulative number of infectious person-days in a source city, showing that the probability of autochthonous transmission depends on both the probability of introduction and the efficiency of local transmission (Figure 7B). The stochasticity of these processes contributes to the high degree of variability in the city where the earliest autochthonous infections occurred in the simulations.
Prior vaccination
Prior vaccination in Asunción reduced the probability of outbreaks (Table 1). This finding is because of both reduced individual susceptibility (direct effect) and reduced rate of vector to human transmission, because some infectious vectors feed on immune humans (indirect effect). Because the number of local infections is a key determinant of the probability of international spread, vaccination in Asunción reduces the frequency of spread (Table 1), and the slower growth of those epidemics that do occur leads to a delay in spread (Figure 6).
Despite the decreased probability of a seed epidemic and slower spread when these epidemics did occur, pandemics still occurred. Overall, the probability of autochthonous transmission in other cities is slightly decreased, reflecting the decreased transmissibility in the cities with high vaccine coverage (Figure 7A). Previous vaccination also contributed to a global reduction in the number of persons affected by approximately 26% or 100 million persons. Thus, although prior vaccination decreases the probability of spread occurring and slows its pace, the potential for a major global health problem persists.
Although pandemics may occur in the presence of prior vaccination, in our simulations, they only occurred in the high R0 model. Under the more realistic assumptions of the moderate R0 model, they did not occur, suggesting that previous vaccination in the population where the first infections occur may be sufficient to prevent international spread. We did not assess the critical threshold for vaccination coverage, but optimal coverage rates can be derived based on R0 values.29,35,36 Preventive vaccination may seem a logical control measure, but there are also problems with vaccine supply, cost, and safety.7–12 In future work, we will evaluate the potential impact of both preventive vaccination and reactive interventions, such as local vaccination and vector control, vaccination of travelers, and restriction of travel.
Limitations
Two important sources of uncertainty are the parameterizations of the travel network and YFV transmission dynamics (Table 1). The former requires more data,14 and the latter is partly captured in the different R0 scenarios. However, even within a given scenario, there is likely more variability than we could reasonably incorporate. Different vector densities and contact rates, for instance, may vary greatly between cities based on housing characteristics and other factors that cannot be reliably assessed on a global scale. It is also not necessarily true that Ae. aegypti are present in all of the areas where YFV transmission may occur in our model.28 In some areas, Ae. aegypti has been replaced by Ae. albopictus,37 another competent vector.32,34,38,39 Because the geographical distributions of the two species are dynamic and imprecisely known and because the relative importance of each species to YFV transmission is not well-understood, we did not attempt to model any differences between them.
Beyond the parameterization assumptions above, one of the most important assumptions that we make is that local transmission is a mass action-based process. There is ample evidence to suggest that virus transmission by Ae. aegypti is highly focal,40–43 thus treating each city as a single pool of individuals all experiencing equal exposure risk masks significant underlying heterogeneity. However, our primary interest is the probability of spread between populations, and the local heterogeneity is likely of little importance. Perhaps most critical to the subject of interest here is the simple fact that not all travelers are equivalent. It is well-documented that travelers visiting friends and family are more likely to stay longer, stay in homes rather than hotels, and be infected by pathogens while traveling.44–49 Unfortunately, adding more local heterogeneity for human and vector interaction would require parameterization beyond the reach of available data, especially when applied globally.
Lastly, we made significant simplifications regarding the immune status of the populations. We assumed either complete susceptibility or partial immunity on the population scale because of vaccination at a level consistent with the reported country-wide rates, which do not necessarily reflect immunity in the cities. Furthermore, vaccines are not the only source of immunity. Some populations have experienced natural exposure, and others may have acquired some degree of cross-immunity because of exposure to other flaviviruses. For example, cross-protection afforded by prior dengue virus exposure is a principal hypothesis for why YFV has not emerged in Asia, where competent vectors and dengue viruses are ubiquitous.50,51 Because of these complications and a lack of data to address them on a global scale, more accurate estimation of YFV susceptibility is a formidable challenge.
General conclusions
The models presented here provide general approaches to assessing the risk of vector-borne disease spread by infected travelers. Despite their limitations, these models may serve as useful tools and starting points for future models of vector-borne disease spread and interventions designed to reduce the risk of spread. The models also represent formal hypotheses about the YFV transmission system and travel network, which is detailed in Materials and Methods and Supplemental Information. We found that the most critical predictors of disease spread are the rates of travel, number of infected individuals, general transmission parameters (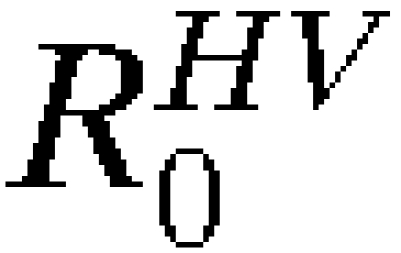 and
and 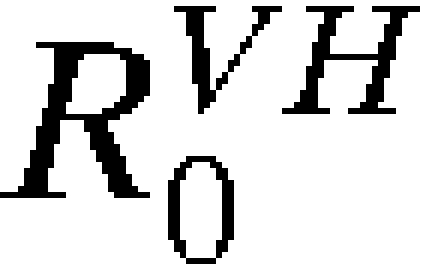 ), and vaccination rates when vaccines are concerned. With estimates of these components, calculation of the probability of introduction and autochthonous transmission can easily be estimated for any ongoing outbreak. Meanwhile, as improved estimates of transmission components and travel rates become available, they can be incorporated into complete mechanistic models, enabling more detailed analyses of a wider variety of potential outcomes.
), and vaccination rates when vaccines are concerned. With estimates of these components, calculation of the probability of introduction and autochthonous transmission can easily be estimated for any ongoing outbreak. Meanwhile, as improved estimates of transmission components and travel rates become available, they can be incorporated into complete mechanistic models, enabling more detailed analyses of a wider variety of potential outcomes.
Supplementary Material
ACKNOWLEDGMENTS
This work was partly supported by the Centers for Disease Control and Prevention Preparedness Modeling Initiative.
Note: Supplemental information appears online at www.ajtmh.org.
Footnotes
Authors’ addresses: Michael A. Johansson and Neysarí Arana-Vizcarrondo, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, San Juan, Puerto Rico, E-mails: mjohansson@cdc.gov and nnarana@gmail.com. Brad J. Biggerstaff and J. Erin Staples, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Fort Collins, CO, E-mails: bbiggerstaff@cdc.gov and estaples@cdc.gov. Nancy Gallagher and Nina Marano, Division of Global Migration and Quarantine, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: ngallagher@cdc.gov and nmarano@cdc.gov.
References
- 1.Vainio J, Cutts F. Yellow Fever. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 2.Pan American Health Organization Outbreak of yellow fever in Paraguay. Epidemiol Bull. 2008;27:2. [Google Scholar]
- 3.Soper FL. The elimination of urban yellow fever in the Americas through the eradication of Aedes aegypti. Am J Public Health Nations Health. 1963;53:7–16. doi: 10.2105/ajph.53.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson SE, Hull BP, Tomori O, Bele O, LeDuc JW, Esteves K. Yellow fever: a decade of reemergence. JAMA. 1996;276:1157–1162. [PubMed] [Google Scholar]
- 5.Gubler DJ. Aedes aegypti and Aedes aegypti-borne disease control in the 1990s: top down or bottom up. Charles Franklin Craig Lecture. Am J Trop Med Hyg. 1989;40:571–578. doi: 10.4269/ajtmh.1989.40.571. [DOI] [PubMed] [Google Scholar]
- 6.Heintze C, Velasco Garrido M, Kroeger A. What do community-based dengue control programmes achieve? A systematic review of published evaluations. Trans R Soc Trop Med Hyg. 2007;101:317–325. doi: 10.1016/j.trstmh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Nathan N, Barry M, Van Herp M, Zeller H. Shortage of vaccines during a yellow fever outbreak in Guinea. Lancet. 2001;358:2129–2130. doi: 10.1016/S0140-6736(01)07185-9. [DOI] [PubMed] [Google Scholar]
- 8.Yellow fever preparedness. Lancet. 2008;371:786. doi: 10.1016/S0140-6736(08)60352-9. [DOI] [PubMed] [Google Scholar]
- 9.Roukens AH, Visser LG. Yellow fever vaccine: past, present and future. Expert Opin Biol Ther. 2008;8:1787–1795. doi: 10.1517/14712598.8.11.1787. [DOI] [PubMed] [Google Scholar]
- 10.Lindsey NP, Schroeder BA, Miller ER, Braun MM, Hinckley AF, Marano N, Slade BA, Barnett ED, Brunette GW, Horan K, Staples JE, Kozarsky PE, Hayes EB. Adverse event reports following yellow fever vaccination. Vaccine. 2008;26:6077–6082. doi: 10.1016/j.vaccine.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Whittembury A, Ramirez G, Hernandez H, Ropero AM, Waterman S, Ticona M, Brinton M, Uchuya J, Gershman M, Toledo W, Staples E, Campos C, Martinez M, Chang GJ, Cabezas C, Lanciotti R, Zaki S, Montgomery JM, Monath T, Hayes E. Viscerotropic disease following yellow fever vaccination in Peru. Vaccine. 2009;27:5974–5981. doi: 10.1016/j.vaccine.2009.07.082. [DOI] [PubMed] [Google Scholar]
- 12.Staples JE, Gershman M, Fischer M. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:1–27. [PubMed] [Google Scholar]
- 13.Codeco CT, Luz PM, Struchiner CJ. Risk assessment of yellow fever urbanization in Rio de Janeiro, Brazil. Trans R Soc Trop Med Hyg. 2004;98:702–710. doi: 10.1016/j.trstmh.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Johansson MA, Arana-Vizcarrondo N, Biggerstaff BJ, Staples JE, Gallagher N, Marano N. On the treatment of airline travelers in mathematical models. PLoS One. 2011;6:e22151. doi: 10.1371/journal.pone.0022151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.New M, Lister D, Hulme M, Makin I. A high-resolution data set of surface climate over global land areas. Clim Res. 2002;21:1–25. [Google Scholar]
- 16.Christophers SR. Aedes aegypti (L.): The Yellow Fever Mosquito. Cambridge, United Kingdom: The University Press; 1960. [Google Scholar]
- 17.World Health Organization Department of Immunization Vaccines and Biologicals Vaccine Preventable Diseases: Monitoring System. 2009. http://www.who.int/immunization_monitoring/en/globalsummary/timeseries/tsincidenceyfe.htm Available at. Accessed November 4, 2009.
- 18.Johansson MA, Arana-Vizcarrondo N, Biggerstaff BJ, Staples JE. Incubation periods of yellow fever virus. Am J Trop Med Hyg. 2010;83:183–188. doi: 10.4269/ajtmh.2010.09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Focks DA, Haile DG, Daniels E, Mount GA. Dynamic life table model for Aedes aegypti (Diptera: Culicidae): analysis of the literature and model development. J Med Entomol. 1993;30:1003–1017. doi: 10.1093/jmedent/30.6.1003. [DOI] [PubMed] [Google Scholar]
- 20.Getz WM, Lloyd-Smith JO. In: Disease Evolution: Models, Concepts, and Data Analysis. Feng Z, Dieckmann U, Levin SA, editors. Providence, RI: American Mathematical Society; 2006. pp. 87–109. (Basic methods for modeling the invasion and spread of contagious disease). [Google Scholar]
- 21.Koopman JS, Prevots DR, Vaca Marin MA, Gomez Dantes H, Zarate Aquino ML, Longini IM, Jr, Sepulveda Amor J. Determinants and predictors of dengue infection in Mexico. Am J Epidemiol. 1991;133:1168–1178. doi: 10.1093/oxfordjournals.aje.a115829. [DOI] [PubMed] [Google Scholar]
- 22.Marques CA, Forattini OP, Massad E. The basic reproduction number for dengue fever in Sao Paulo State, Brazil—1990–1991 epidemic. Trans R Soc Trop Med Hyg. 1994;88:58–59. doi: 10.1016/0035-9203(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson NM, Donnelly CA, Anderson RM. Transmission dynamics and epidemiology of dengue: insights from age-stratified sero-prevalence surveys. Philos Trans R Soc Lond B Biol Sci. 1999;354:757–768. doi: 10.1098/rstb.1999.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massad E, Burattini MN, Coutinho FA, Lopez LF. Dengue and the risk of urban yellow fever reintroduction in Sao Paulo State, Brazil. Rev Saude Publica. 2003;37:477–484. doi: 10.1590/s0034-89102003000400013. [DOI] [PubMed] [Google Scholar]
- 25.Favier C, Degallier N, Rosa-Freitas MG, Boulanger JP, Costa Lima JR, Luitgards-Moura JF, Menkes CE, Mondet B, Oliveira C, Weimann ET, Tsouris P. Early determination of the reproductive number for vector-borne diseases: the case of dengue in Brazil. Trop Med Int Health. 2006;11:332–340. doi: 10.1111/j.1365-3156.2006.01560.x. [DOI] [PubMed] [Google Scholar]
- 26.Chowell G, Diaz-Duenas P, Miller JC, Alcazar-Velazco A, Hyman JM, Fenimore PW, Castillo-Chavez C. Estimation of the reproduction number of dengue fever from spatial epidemic data. Math Biosci. 2007;208:571–589. doi: 10.1016/j.mbs.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Nagao Y, Koelle K. Decreases in dengue transmission may act to increase the incidence of dengue hemorrhagic fever. Proc Natl Acad Sci USA. 2008;105:2238–2243. doi: 10.1073/pnas.0709029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers DJ, Wilson AJ, Hay SI, Graham AJ. The global distribution of yellow fever and dengue. Adv Parasitol. 2006;62:181–220. doi: 10.1016/S0065-308X(05)62006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monath TP, Nasidi A. Should yellow fever vaccine be included in the expanded program of immunization in Africa? A cost-effectiveness analysis for Nigeria. Am J Trop Med Hyg. 1993;48:274–299. doi: 10.4269/ajtmh.1993.48.274. [DOI] [PubMed] [Google Scholar]
- 30.Massad E, Coutinho FA, Burattini MN, Lopez LF. The risk of yellow fever in a dengue-infested area. Trans R Soc Trop Med Hyg. 2001;95:370–374. doi: 10.1016/s0035-9203(01)90184-1. [DOI] [PubMed] [Google Scholar]
- 31.Tabachnick WJ, Wallis GP, Aitken TH, Miller BR, Amato GD, Lorenz L, Powell JR, Beaty BJ. Oral infection of Aedes aegypti with yellow fever virus: geographic variation and genetic considerations. Am J Trop Med Hyg. 1985;34:1219–1224. doi: 10.4269/ajtmh.1985.34.1219. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell CJ, Miller BR, Gubler DJ. Vector competence of Aedes albopictus from Houston, Texas, for dengue serotypes 1 to 4, yellow fever and Ross River viruses. J Am Mosq Control Assoc. 1987;3:460–465. [PubMed] [Google Scholar]
- 33.Lourenco de Oliveira R, Vazeille M, de Filippis AMB, Failloux AB. Oral susceptibility to yellow fever virus of Aedes aegypti from Brazil. Mem Inst Oswaldo Cruz. 2002;97:437–439. doi: 10.1590/s0074-02762002000300031. [DOI] [PubMed] [Google Scholar]
- 34.Johnson BW, Chambers TV, Crabtree MB, Filippis AM, Vilarinhos PT, Resende MC, Macoris Mde L, Miller BR. Vector competence of Brazilian Aedes aegypti and Ae. albopictus for a Brazilian yellow fever virus isolate. Trans R Soc Trop Med Hyg. 2002;96:611–613. doi: 10.1016/s0035-9203(02)90326-3. [DOI] [PubMed] [Google Scholar]
- 35.Massad E, Coutinho FA, Burattini MN, Lopez LF, Struchiner CJ. Yellow fever vaccination: how much is enough? Vaccine. 2005;23:3908–3914. doi: 10.1016/j.vaccine.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Codeco CT, Luz PM, Coelho F, Galvani AP, Struchiner C. Vaccinating in disease-free regions: a vaccine model with application to yellow fever. J R Soc Interface. 2007;4:1119–1125. doi: 10.1098/rsif.2007.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller BR, Monath TP, Tabachnick WJ, Ezike VI. Epidemic yellow fever caused by an incompetent mosquito vector. Trop Med Parasitol. 1989;40:396–399. [PubMed] [Google Scholar]
- 39.Lourenco de Oliveira R, Vazeille M, de Filippis AM, Failloux AB. Large genetic differentiation and low variation in vector competence for dengue and yellow fever viruses of Aedes albopictus from Brazil, the United States, and the Cayman Islands. Am J Trop Med Hyg. 2003;69:105–114. [PubMed] [Google Scholar]
- 40.Morrison AC, Gray K, Getis A, Astete H, Sihuincha M, Focks D, Watts D, Stancil JD, Olson JG, Blair P, Scott TW. Temporal and geographic patterns of Aedes aegypti (Diptera: Culicidae) production in Iquitos, Peru. J Med Entomol. 2004;41:1123–1142. doi: 10.1603/0022-2585-41.6.1123. [DOI] [PubMed] [Google Scholar]
- 41.Mammen MP, Pimgate C, Koenraadt CJ, Rothman AL, Aldstadt J, Nisalak A, Jarman RG, Jones JW, Srikiatkhachorn A, Ypil-Butac CA, Getis A, Thammapalo S, Morrison AC, Libraty DH, Green S, Scott TW. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med. 2008;5:e205. doi: 10.1371/journal.pmed.0050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honorio NA, Nogueira RMR, Codeco CT, Carvalho MS, Cruz OG, Magalhaes MDFM, de Araujo JMG, de Araujo ESM, Gomes MQ, Pinheiro LS, Pinel CD, Lourenco-de-Oliveira R. Spatial evaluation and modeling of dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2009;3:e545. doi: 10.1371/journal.pntd.0000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazquez-Prokopec GM, Kitron U, Montgomery B, Horne P, Ritchie SA. Quantifying the spatial dimension of dengue virus epidemic spread within a tropical urban environment. PLoS Negl Trop Dis. 2010;4:e920. doi: 10.1371/journal.pntd.0000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ackers ML, Puhr ND, Tauxe RV, Mintz ED. Laboratory-based surveillance of Salmonella serotype Typhi infections in the United States: antimicrobial resistance on the rise. JAMA. 2000;283:2668–2673. doi: 10.1001/jama.283.20.2668. [DOI] [PubMed] [Google Scholar]
- 45.Steinberg EB, Bishop R, Haber P, Dempsey AF, Hoekstra RM, Nelson JM, Ackers M, Calugar A, Mintz ED. Typhoid fever in travelers: who should be targeted for prevention? Clin Infect Dis. 2004;39:186–191. doi: 10.1086/421945. [DOI] [PubMed] [Google Scholar]
- 46.Leder K, Tong S, Weld L, Kain KC, Wilder-Smith A, von Sonnenburg F, Black J, Brown GV, Torresi J. Illness in travelers visiting friends and relatives: a review of the GeoSentinel Surveillance Network. Clin Infect Dis. 2006;43:1185–1193. doi: 10.1086/507893. [DOI] [PubMed] [Google Scholar]
- 47.Skarbinski J, James EM, Causer LM, Barber AM, Mali S, Nguyen-Dinh P, Roberts JM, Parise ME, Slutsker L, Newman RD. Malaria surveillance—United States, 2004. MMWR Morb Mortal Wkly Rep. 2006;55:23–37. [PubMed] [Google Scholar]
- 48.Fenner L, Weber R, Steffen R, Schlagenhauf P. Imported infectious disease and purpose of travel, Switzerland. Emerg Infect Dis. 2007;13:217–222. doi: 10.3201/eid1302.060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.US Department of Commerce International Trade Administration . Profile of US Resident Travelers Visiting Overseas Destinations: 2008 Outbound. Washington, DC: Manufacturing and Services, Office of Travel and Tourism Industries; 2009. [Google Scholar]
- 50.Theiler M, Anderson CR. The relative resistance of dengue-immune monkeys to yellow fever virus. Am J Trop Med Hyg. 1975;24:115–117. doi: 10.4269/ajtmh.1975.24.115. [DOI] [PubMed] [Google Scholar]
- 51.Monath TP. The absence of yellow fever in Asia: hypotheses. A cause for concern? Virus Inf Exch Newsl South East Asia West Pac. 1989;6:106–107. [Google Scholar]
- 52.Focks DA, Brenner RJ, Hayes J, Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am J Trop Med Hyg. 2000;62:11–18. [PubMed] [Google Scholar]
- 53.Pant CP, Yasuno M. Field studies on the gonotrophic cycle of Aedes aegypti in Bangkok, Thailand. J Med Entomol. 1973;10:219–223. doi: 10.1093/jmedent/10.2.219. [DOI] [PubMed] [Google Scholar]
- 54.Scott TW, Amerasinghe PH, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. J Med Entomol. 2000;37:89–101. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- 55.Hindle E. The transmission of yellow fever. Lancet. 1930;219:835–842. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.