Abstract
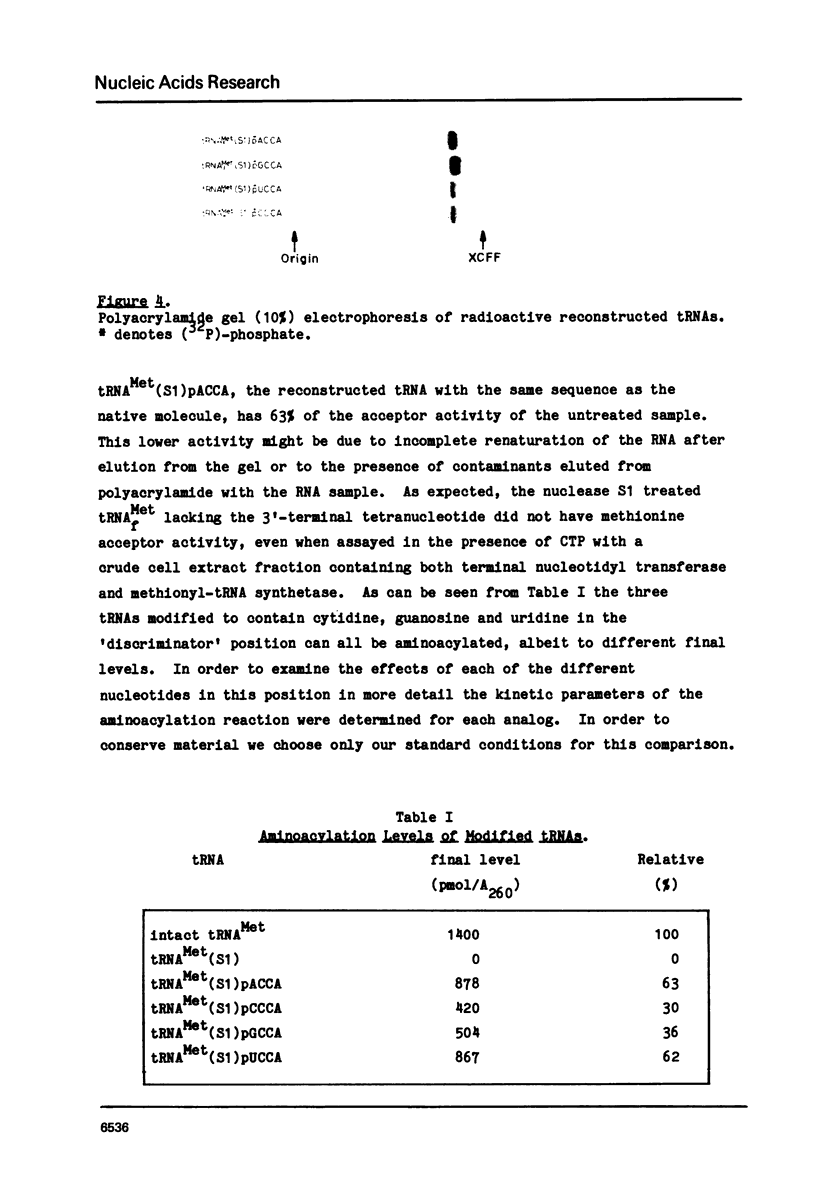
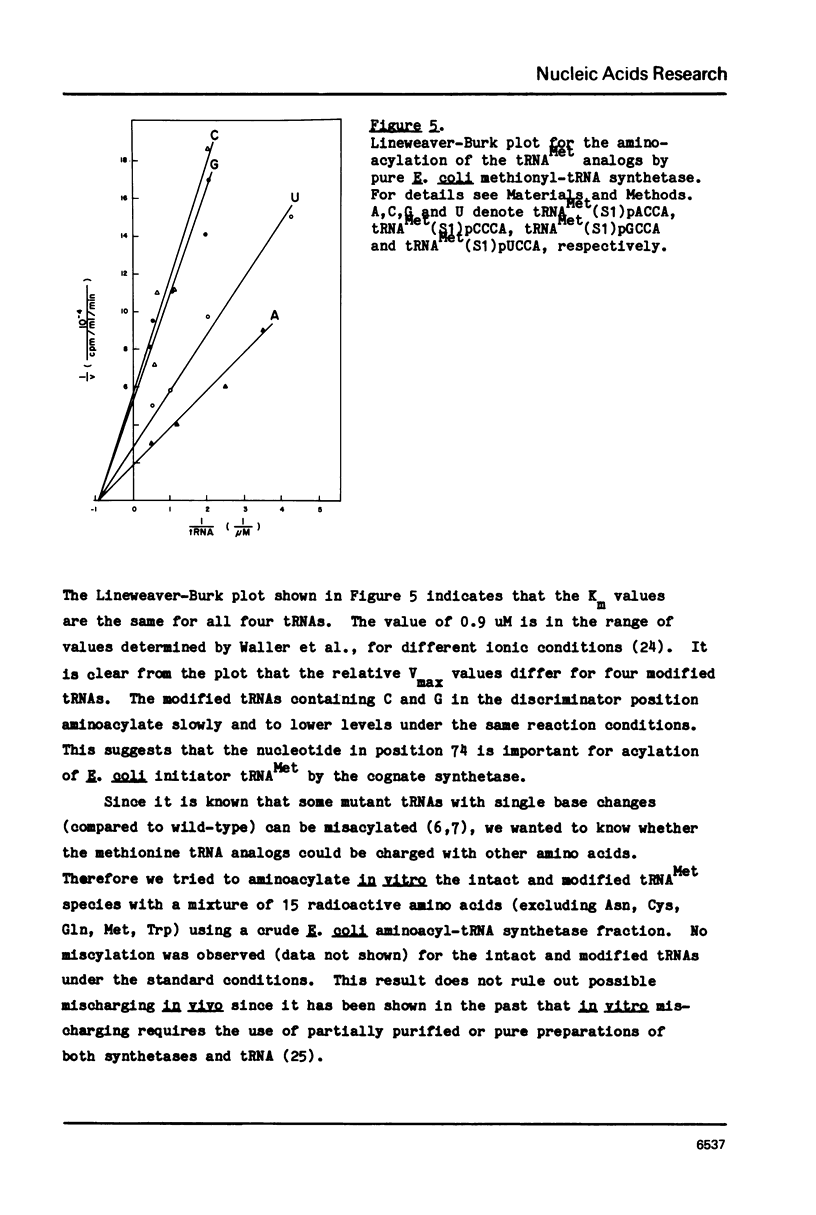
The effect of base changes at the fourth position from the 3'-terminus of Escherichia coli initiator tRNAMet has been studied to test the 'discriminator hypothesis' which proposed that the nucleotide in this position might have a role in the specificity of the aminoacylation reaction. E. coli initiator tRNA lacking the 3'-terminal tetranucleotide was prepared by partial digestion with S1 nuclease. To construct tRNA analogs with different bases in the fourth position this truncated tRNA was joined by RNA ligase to each of four chemically synthesized 2',3'-ethoxy-methylidene tetranucleotides pACCA(em), pCCCA(em), pGCCA(em), and pUCCA(em). In vitro aminoacylation studies showed that all four molecules accepted methionine, albeit with different Vmax values.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanquet S., Iwatsubo M., Waller J. P. The mechanism of action of methionyl-tRNA synthetase from Escherichia coli. 1. Fluorescence studies on tRNAMet binding as a function of ligands, ions and pH. Eur J Biochem. 1973 Jul 2;36(1):213–226. doi: 10.1111/j.1432-1033.1973.tb02903.x. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Enzymatic replacement of the anticodon of yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1982 Mar 2;21(5):855–861. doi: 10.1021/bi00534a007. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Specific interaction of anticodon loop residues with yeast phenylalanyl-tRNA synthetase. Biochemistry. 1982 Aug 17;21(17):3921–3926. doi: 10.1021/bi00260a003. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Seno T., Söll G. Is there a discriminator site in transfer RNA? Proc Natl Acad Sci U S A. 1972 Oct;69(10):3063–3067. doi: 10.1073/pnas.69.10.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSKINSON R. M., KHORANA H. G. STUDIES ON POLYNUCLEOTIDES. XLI. PURIFICATION OF PHENYLALANINE-SPECIFIC TRANSFER RIBONUCLEIC ACID FROM YEAST BY COUNTERCURRENT DISTRIBUTION. J Biol Chem. 1965 May;240:2129–2134. [PubMed] [Google Scholar]
- Harada F., Dahlberg J. E. Specific cleavage of tRNA by nuclease S1. Nucleic Acids Res. 1975 Jun;2(6):865–871. doi: 10.1093/nar/2.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence F., Blanquet S., Poiret M., Robert-Gero M., Waller J. P. The mechanism of action of methionyl-tRNA synthetase. 3. Ion requirements and kinetic parameters of the ATP-PPi exchange and methionine-transfer reactions catalyzed by the native and trypsin-modified enzymes. Eur J Biochem. 1973 Jul 2;36(1):234–243. doi: 10.1111/j.1432-1033.1973.tb02905.x. [DOI] [PubMed] [Google Scholar]
- NIHEI T., CANTONI G. L. STUDIES ON SOLUBLE RIBONUCLEIC ACID: THE ACTION OF SNAKE VENOM PHOSPHODIESTERASE ON SOLUBLE RIBONUCLEIC ACID IN YEAST. J Biol Chem. 1963 Dec;238:3991–3998. [PubMed] [Google Scholar]
- Nishimura S., Harada F., Narushima U., Seno T. Purification of methionine-, valine-, phenylalanine- and tyrosine-specific tRNA from Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):133–148. doi: 10.1016/0005-2787(67)90522-9. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Fujiyama K., Ohashi M., Ikehara M. Studies on transfer ribonucleic acids and related compounds. X. synthesis of the yeast tyrosine tRNA 5'-terminal oligonucleotides. Chem Pharm Bull (Tokyo) 1976 Apr;24(4):570–579. doi: 10.1248/cpb.24.570. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Miyake T., Nagao K., Uemura H., Nishikawa S., Sugiura M., Ikehara M. Elongation of oligonucleotides in the 3'-direction with activated mononucleotides and their analogs using RNA ligase. Nucleic Acids Res. 1980 Feb 11;8(3):601–610. doi: 10.1093/nar/8.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Nishikawa S., Fukumoto R., Uemura H., Tanaka T., Nakagawa E., Miyake T., Ikehara M. Synthesis of 5' fragments of formylmethionine transfer ribonucleic acid and their reconstitution with a natural three-quarter molecule. Eur J Biochem. 1980 Apr;105(3):481–487. doi: 10.1111/j.1432-1033.1980.tb04523.x. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Tanaka S., Tanaka T., Miyake T., Markham A. F., Nakagawa E., Wakabayashi T., Taniyama Y., Nishikawa S., Fukumoto R. Total synthesis of a RNA molecule with sequence identical to that of Escherichia coli formylmethionine tRNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5493–5497. doi: 10.1073/pnas.78.9.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Structural requirements for aminoacylation of Escherichia coli formylmethionine transfer RNA. Biochemistry. 1977 Sep 20;16(19):4256–4265. doi: 10.1021/bi00638a020. [DOI] [PubMed] [Google Scholar]
- Shimura Y., Aono H., Ozeki H., Sarabhai A., Lamfrom H., Abelson J. Mutant tyrosine tRNA of altered amino acid specificity. FEBS Lett. 1972 Apr 15;22(1):144–148. doi: 10.1016/0014-5793(72)80240-0. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Sternbach H., von der Haar F., Cramer F. Enzymatic incorporation of ATP and CTP analogues into the 3' end of tRNA. Eur J Biochem. 1977 Dec;81(3):579–589. doi: 10.1111/j.1432-1033.1977.tb11985.x. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Suzuki M., Ohtsuka E., Nishikawa S., Uemura H., Ikehara M. Purification of T4 RNA ligase by 2', 5'-ADP sepharose chromatography. FEBS Lett. 1979 Jan 1;97(1):73–76. doi: 10.1016/0014-5793(79)80055-1. [DOI] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Wrede P., Woo N. H., Rich A. Initiator tRNAs have a unique anticodon loop conformation. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3289–3293. doi: 10.1073/pnas.76.7.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]