Abstract
Background
Cyclin D1-positive B cells are occasionally found in the mantle zones of reactive lymphoid follicles, a condition that has been called “in situ mantle cell lymphoma”. The clinical significance of this lesion remains uncertain.
Design and Methods
The clinical and pathological characteristics, including SOX11 expression, of 23 cases initially diagnosed as in situ mantle cell lymphoma were studied.
Results
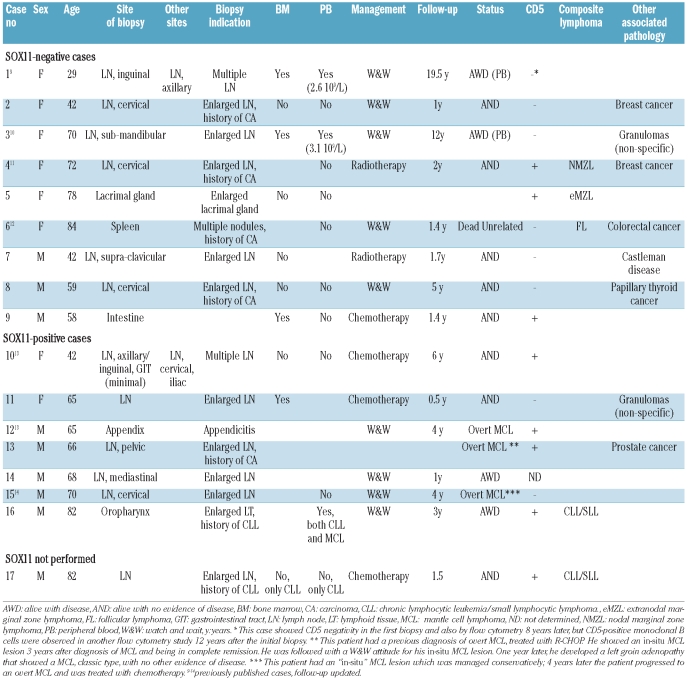
Seventeen of the 23 cases fulfilled the criteria for in situ mantle cell lymphoma. In most cases, the lesions were incidental findings in reactive lymph nodes. The t(11;14) was detected in all eight cases examined. SOX11 was positive in seven of 16 cases (44%). Five cases were associated with other small B-cell lymphomas. In two cases, both SOX11-positive, the in situ mantle cell lymphoma lesions were discovered after the diagnosis of overt lymphoma; one 4 years earlier, and one 3 years later. Twelve of the remaining 15 patients had a follow-up of at least 1 year (median 2 years; range, 1–19.5), of whom 11 showed no evidence of progression, including seven who were not treated. Only one of 12 patients with an in situ mantle cell lymphoma lesion and no diagnosis of mantle cell lymphoma at the time developed an overt lymphoma, 4 years later; this case was also SOX11-positive. The six remaining cases were diagnosed as mantle cell lymphoma with a mantle zone pattern. Five were SOX11-positive and four of them were associated with lymphoma without a mantle zone pattern.
Conclusions
In situ mantle cell lymphoma lesions are usually an incidental finding with a very indolent behavior. These cases must be distinguished from mantle cell lymphoma with a mantle zone pattern and overt mantle cell lymphoma because they may not require therapeutic intervention.
Keywords: in situ mantle cell lymphoma, in situ involvement by mantle cell-lymphoma-like cells, indolent behavior
Introduction
Mantle cell lymphoma (MCL) is a B-cell neoplasm that usually carries the t(11;14)(q13;q32) translocation and constitutively overexpresses cyclin D1.1 Most patients present in an advanced stage, with generalized lymphadenopathy, and frequent involvement of extranodal sites and peripheral blood. The clinical evolution is relatively aggressive with a poor response to conventional therapeutic regimens, frequent relapses and a median overall survival of 3–5 years. This biological behavior has led to the recommendation for early treatment, usually with intensive therapeutic regimens that may include hematopoietic stem cell transplantation.2 However, recent clinical and pathological observations have recognized subsets of patients whose disease has an indolent clinical behavior and who may not need immediate treatment.3–6
It is well-recognized that MCL has a wide spectrum of growth patterns. Most cases have a vaguely nodular and/or diffuse growth pattern, very rare cases have a follicular growth pattern, and a larger minority has a mantle zone growth pattern in which the lymphoma grows as an expanded ring around reactive germinal centers. Although controversial, one study suggested that a mantle zone growth pattern was associated with a better prognosis.7 More recently, isolated reports have described cases with cyclin D1-positive MCL-like cells restricted to the mantle zone of hyperplastic follicles in otherwise reactive lymph nodes.8–14 The restricted distribution of the atypical cells suggests that these lesions may represent an early step in the development of MCL, and the process has been termed “in situ MCL”. A similar phenomenon with a restricted distribution of follicular lymphoma-like cells with BCL2 rearrangement and overexpression within reactive germinal centers has been termed follicular lymphoma in situ (FLIS).15,16 However, it is not known whether either FLIS or “in situ” MCL represent true precursor lesions that will inevitably progress to overt lymphoma, or are simply incidental findings with a low likelihood of progression, analogous to monoclonal gammopathy of unknown significance or monoclonal B-cell lymphocytosis. Thus, the malignant potential of these lesions and their clinical and biological significance are not well known. In fact, some patients have been followed for a long time without antineoplastic therapy9,10 whereas others with the same histological pattern have been treated with conventional therapies including intensive chemotherapy and stem cell transplantation.13
SOX11, a neural transcription factor involved in tissue remodeling during embryogenesis, is a highly sensitive bio-marker of MCL since it is expressed in virtually all MCL, including cyclin D1-negative cases, but is not expressed in any other lymphoid neoplasm with the exception of lymphoblastic lymphoma, some Burkitt lymphomas and T-prolymphocytic leukemia.17–20 SOX11 negativity has been recently associated with a subset of MCL with a very indolent behavior, nonnodal presentation, hypermutated IGHV and simple karyotypes suggesting that this lack of expression may help to identify a particular clinical and biological subtype of MCL.6 The expression of SOX11 in early lesions of MCL and tumors with a mantle zone growth pattern has not been investigated and it is not, therefore, known whether its expression is an early or later event in the development of these tumors.
In this study we investigated a large series of cases previously diagnosed as “in situ MCL”, to define their clinical and pathological significance, their distinction from MCL with a mantle zone growth pattern, and the potential value of SOX11 in the characterization of these lesions.
Design and methods
Case selection
We identified 23 cases initially diagnosed as “in situ MCL” lesions over a period of 8 years (2003–2010); as cited in Table 1,9–14 seven of these cases have been published previously. The following sites participated in the study: National Cancer Institute, Bethesda, MD, USA (6 cases); Hospital Clínic Barcelona, Spain (3 cases); Oslo University Hospital, Oslo, Norway (3 cases); Hammersmith Hospital, London, UK (2 cases); University of Pittsburgh, Pittsburgh, PA, USA (2 cases); Stanford University Medical Center, Stanford, CA, USA (2 cases); Hospital del Mar, Barcelona, Spain (1 case); Hospital of the University of Pennsylvania, Philadelphia, PA, USA (1 case); CHU Purpan, Toulouse, France (1 case); Brigham & Women Hospital, Boston, MA, USA (1 case) and Massachusetts General Hospital, Boston, MA, USA (1 case). This study was approved by the Institutional Review Board of the participating institutions, where required. All 23 cases were detected on the basis of a restricted location of cyclin D1-positive cells within the mantle zones of reactive follicles. Clinical information and follow-up from these patients were obtained from the referring pathologists and/or clinicians to the extent possible. The follow-up of the previously published patients was updated and new histological sections were obtained for additional studies. While the cases were originally diagnosed by the contributing pathologists, all cases were subsequently reviewed by two of the authors (AC-C, EC). In addition, we collected 100 consecutive lymph node biopsies from the Hospital Clinic of Barcelona diagnosed as follicular lymphoid hyperplasia in patients older than 40 years. None of these patients had a previous or simultaneous diagnosis of a lymphoid neoplasm.
Table 1.
Clinical features, follow-up and histopathological findings in in situ MCL lesions.
Histological and immunohistochemical studies
Paraffin-embedded tissue was available from the diagnostic biopsies. Immunohistochemical stains were performed in the 23 cases with a previous diagnosis of “in situ MCL” lesions with a panel of pre-diluted antibodies from DAKO (Glostrup, Denmark) including CD20 (clone L26), IgD (polyclonal), CD23 (SP23), CD5 (clone 4C7), cyclin D1 (clone SP4) and BCL2 (clone 123). We also used CD3 (clone PS1, Novocastra, Newcastle, UK) and SOX11 (Atlas Antibodies, Stockholm, Sweden). The one hundred reactive lymph nodes were stained with cyclin D1 and BCL2. SOX11 staining was performed as previously described20 using a heat-induced retrieval with ER2 BondMax buffer solution for 15 min, a horseradish-peroxidase–linked polymer for 8 min (Define; Vision Biosystems) and 5′-3′ diaminobenzidine for 10 min. Staining with the routine panel of the remaining antibodies was performed using automated immunostainers (Autostainer Link, DAKO or Benchmark, Ventana Medical Systems, Tucson, AZ, USA).
Genetic studies
Classical G-banded cytogenetic studies from peripheral blood or tissue samples were available in four cases. Fluorescence in situ hybridization (FISH) studies were performed in eight cases using a standard procedure previously described21 and dual-fusion DNA probes for IGH@-CCND1 (Abbot Molecular, Abbot Park, IL, USA). One additional case (case 8) was studied with FISH and simultaneous cyclin D1 immunofluorescence using a modification of the immuno-FISH protocol described by Cook et al.22 with a dual color, dual fusion probe for t(11;14)(q13;q32) (Vysis, Downers Grove, IL, USA) and cyclin D1 antibody (clone SP4) on paraffin sections. Two hundred cells specifically identified by nuclear cyclin D1 staining under a 4′-6′-diamidino-2-phenylindole filter were analyzed. Nuclei were scored as positive if at least one fusion signal was present or as negative in all other patterns. Paraffin sections of tonsils were used as negative controls.
Results
Histopathology and immunohistochemical studies
We identified 23 cases initially diagnosed as “in situ MCL” lesions based on the restriction of cyclin D1-positive cells to the mantle zone of reactive follicles. The main clinical and pathological features of these cases are summarized in Tables 1–3. The distribution and expansion of the cyclin D1-positive cells was variable among the cases and upon review we could distinguish two major patterns; one we refer to as an “in situ MCL” pattern and the other was designated as a mantle zone pattern.
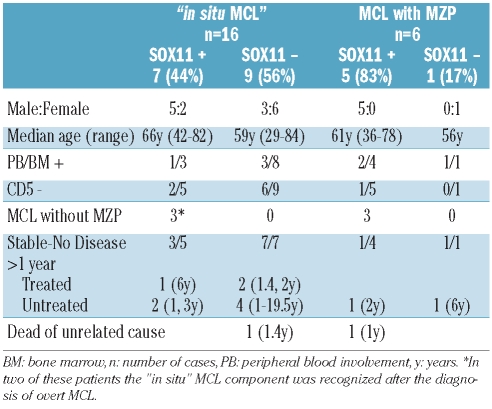
Table 3.
Relationship between SOX11 expression and clinicopathological aspects of “in situ MCL” lesions and MCL with mantle zone growth pattern (MZP).
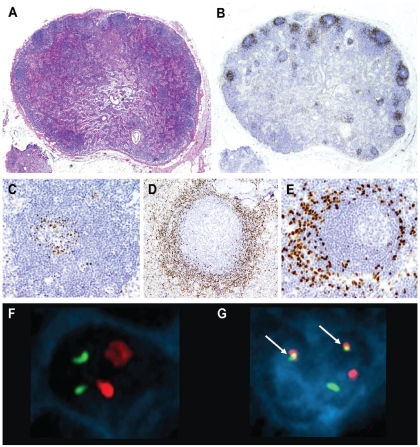
The “in situ MCL” pattern was identified in 17 cases, in which the global architecture of the lymphoid tissue was preserved and the reactive follicles were mainly distributed in the cortical areas with an evident paracortical space among them. The mantle zones of these follicles were usually not expanded and cyclin D1-positive cells were restricted to the mantle zones with only scattered cells in the inter-follicular areas. In some cases the mantle zone was expanded but the cyclin D1-positive cells were restricted only to a few layers and not all follicles contained these cells (Figure 1A-B). In general, cyclin D1-positive cells tended to accumulate in the inner mantle zone but in two cases they were localized at the periphery of the mantle zone and in one case they extended focally into the germinal centers (Figure 1C-E). Similar features were found with involvement localized to otherwise unremarkable mantle zones in lymphoid tissue at extranodal sites as well.
Figure 1.
In situ MCL. (A) The global architecture of the lymph node is preserved (hematoxylin-eosin stain, Olympus BX51, magnification x12.5). (B) Cyclin D1-positive cells are restricted to the mantle zones, which are not expanded (cyclin D1 stain, Olympus BX51, magnification x12.5). (C-E) “In-situ MCL” growth patterns. (C) Few cyclin D1-positive cells accumulate in the inner layer of the mantle zone (cyclin D1 stain, Olympus BX51, magnification x200). (D) Cyclin D1-positive cells that replace almost all the cells in the mantle zone, but the mantle zone is not expanded (cyclin D1 stain, Olympus BX51, magnification x100). (E) Cyclin D1-positive cells at the periphery of the mantle zone (cyclin D1 stain, Olympus BX51, magnification x200). (F, G) FISH and simultaneous cyclin D1 immunofluorescence in case 8. (F) Normal cell with negative nuclear immunofluorescence for cyclin D1; FISH using a dual-fusion probe for t(11;14) shows two red and two green signals corresponding to the presence of CCND1 and IGH genes, respectively, without rearrangement. (G) Cyclin D1 expression is shown by the blue nuclear immunofluorescence and the presence of the t(11;14) by two yellow fusion signals (arrows) in the same cell. (Olympus BX51, magnification x100, acquisition software Applied Imaging Cytovision FISH, size modified after acquisition with Adobe Photoshop).
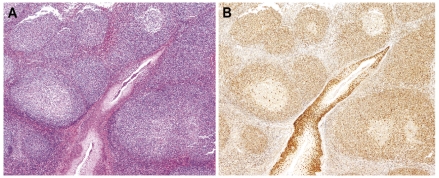
Six of the 23 cases showed a greater expansion of the cyclin D1-positive cells and were designated as MCL with a mantle zone growth pattern. In these cases the architecture of the lymphoid tissue was still largely preserved but with focal effacement, more numerous, crowded, involved follicles and marked reduction of the interfollicular space. The mantle zones were usually expanded and the cyclin D1-positive cells were densely packed replacing virtually all the mantle zone cells. Focal extension of clusters of cyclin D1-positive cells into the interfollicular areas was seen and the mantle zones of different follicles merged focally (Figure 2).
Figure 2.
MCL, mantle zone growth pattern. (A) Mantle zones are expanded and merge (hematoxylineosin stain, Olympus BX51, magnification x40). (B) Cyclin D1-positive cells replace virtually all the mantle zones of reactive follicles (cyclin D1 stain, Olympus BX51, magnification x40).
CD5 was difficult to evaluate in the 17 cases with an “in situ MCL” pattern as they had only few cyclin D1-positive cells. However, in eight cases, comparison between cyclin D1, CD5 and CD3 staining revealed that the cyclin D1-positive cells were CD5-negative. Two of these cases also had clonal tumor cells in the peripheral blood and the flow cytometry study confirmed the CD5 negativity in both. In one of them a flow cytometry study performed during follow-up 12 years after the diagnosis showed that the clonal population had become CD5-positive.
SOX11 was positive in seven of 16 (44%) cases with an “in situ MCL” pattern in which sections were available for staining, and in five of the six (83%) MCL with a mantle zone growth pattern. SOX11 showed a nuclear staining pattern and correlated well with the distribution of the cyclin D1-positive cells. In addition to the cyclin D1-positive cells, endothelial and follicular dendritic cells showed nuclear positivity and were used as internal controls (Figure 3).
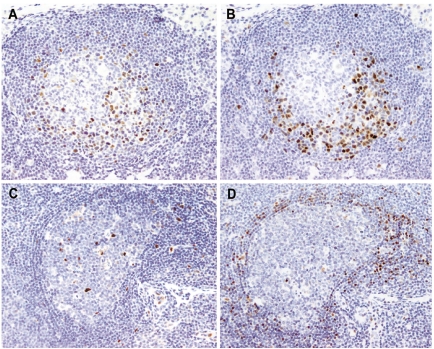
Figure 3.
(A, B) In situ MCL lesion, SOX11-positive case. The distribution of the SOX11-positive cells (A) correlates well with that of the cyclin D1-positive cells (B) (SOX11 stain (A), cyclin D1 stain (B), Olympus BX51, magnification x200). (C, D) “In situ” MCL lesion, SOX11-negative case. SOX11 is negative in the mantle zone cells (C), while cyclin D1-positive cells are seen (D). Note the follicular dendritic cells and the endothelial cells as positive internal controls for SOX11 stain (C) (SOX11 stain (C), cyclin D1 stain (D), Olympus BX51, magnification x200).
A composite lymphoid neoplasm was found in five of the 17 cases with an “in situ MCL” pattern (34%): chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in two cases, marginal zone lymphoma in two cases (one nodal and one extranodal) and follicular lymphoma grade 1–2 with a FLIS component in one case. The CLL diagnosis had been previously established in the two patients with CLL; the “in situ MCL” lesion was only recognized in subsequent biopsies performed based on an enlarged lymph node and oropharyngeal mass, respectively. Two different B-cell clones were observed by a flow cytometry study of peripheral blood in one of these cases (Table 1). Both CLL had a classical phenotype. The composite lymphomas in the other three patients were apparently localized after computed tomography studies (cases 4 and 6), multiple gastrointestinal biopsies (case 5), bone marrow (cases 5 and 17) and peripheral blood (cases 4, 5, 6 and 17) examination. One additional patient had simultaneous Castleman disease of hyaline-vascular type. SOX11 was studied in five of these cases and was negative in four (80%). The SOX11-positive case was associated with CLL/SLL. All the associated lymphoid neoplasms were SOX11-negative.
To explore the frequency of occurrence of incidental “in situ MCL” lesions in reactive lymph nodes and to compare it to the frequency of detection of FLIS, we stained 100 consecutive lymph nodes with follicular hyperplasia for BCL2 and cyclin D1. Two cases showed scattered follicles with strong BCL2 staining of the germinal center cells corresponding to what has been termed FLIS. These cases included a mesenteric lymph node obtained as part of the diagnostic study of a recurrent gastric adenocarcinoma in a 49-year old female and an inguinal lymph node biopsy performed as part of a left inguinal hernioplasty in a 61-year old male. None of the 100 cases had cyclin D1-positive cells in the mantle zones.
Genetics
The t(11;14) was demonstrated in nine of nine cases with an “in situ MCL” pattern of cyclin D1-positive cells by conventional cytogenetics of lymph node or peripheral blood samples (3 cases) and/or by FISH studies (8 cases). In one of these cases we performed a FISH study with simultaneous cyclin D1 immunofluorescence and demonstrated the presence of the t(11;14) in cyclin D1-positive cells (Figure 1F-G). The t(11;14) was identified in 64–95% of the cells analyzed (mean 84%) in the case sections compared to 2–4% (mean 3%) in the tonsil control sections. The t(11;14) was detected in two SOX11-positive and in seven SOX11-negative cases with an “in situ MCL” pattern. The t(11;14) was also confirmed by conventional cytogenetics or FISH in two of two MCL with a mantle zone growth pattern, one SOX11-positive and the other SOX11-negative.
Clinical features associated with “in situ mantle cell lymphoma” lesions
The patients with “in-situ MCL” lesions were nine males and eight females with a median age of 66 years (range, 29–84 years) (Table 1). The diagnosis of “in situ MCL” was most often an incidental finding. The biopsies were performed as part of the staging procedure for a previous diagnosis of carcinoma (5 cases), to evaluate single (5 cases) or multiple (2 cases) enlarged lymph nodes or an enlarged lacrimal gland (1 case), as part of the diagnostic evaluation for CLL (2 cases), and in one case an appendectomy had been performed for acute appendicitis. In one case (intestine sample) the reason is not known. In two cases the “in situ MCL” lesion was discovered after the diagnosis of MCL: in one patient it was detected in a retrospective analysis of a previous appendectomy specimen removed 4 years earlier, and in one patient, it was identified in a lymphadenectomy obtained at surgery for prostatic carcinoma 3 years after the diagnosis and treatment of MCL. Immunostains for cyclin D1 were performed as part of routine immunohistochemical panels in 15 cases, eight of them to complete the study of other lymphoid lesions, clinical suspicion of lymphoma in one case, the finding of atypical cells in the flow cytometry study in one case and after the prospective or retrospective finding of a classic MCL in two cases. The most frequent localization of the “in situ MCL” lesions was nodal; ten cases had a single enlarged lymph node and two had multiple enlarged lymph nodes. Five cases were observed in extranodal locations including small intestine, appendix, lacrimal gland, spleen and oropharynx. Peripheral blood and/or bone marrow involvement was detected in five cases by flow cytometry or bone marrow biopsy (Table 1).
Clinical follow-up of at least 1 year was available for 12 patients with “in situ MCL” lesions and no diagnosis of overt MCL at the time the in situ lesion was discovered. Four were treated with intensive chemotherapy and were alive with no evidence of disease 0.5, 1.4, 4 and 6 years later; two patients received involved-field radiotherapy and had no evidence of disease 1.7 and 2 years later. Eight patients were managed with watchful waiting: two were alive with no evidence of disease, 1 and 5 years later, four were alive with stable disease (atypical circulating cells in the peripheral blood) 1, 3, 12 and 19 years after the diagnosis, one 84 year-old patient died of an unrelated cause 1.4 years after the diagnosis, and one patient developed overt MCL 4 years after the “in situ MCL” diagnosis. This patient who developed overt MCL had not been given any treatment at the time the diagnosis of “in situ MCL” was made.
In one patient (case 12), the in situ component was found in the retrospective examination of an acute appendicitis specimen that had been obtained 4 years before the diagnosis of the overt MCL. In one additional patient (case 13) a diagnosis of stage IV classic MCL had been made 3 years earlier; the patient had been treated with R-CHOP and had achieved a complete remission. The “in situ MCL” lesion was found in a reactive lymph node obtained as part of the surgery for a prostatic adenocarcinoma. No evidence of lymphoma in other sites was observed at the time of the diagnosis of the “in situ MCL” lesion and a watch-and-wait attitude was adopted. However, 1 year later the patient developed left groin adenopathy that showed relapse of classic MCL with no evidence of disease elsewhere.
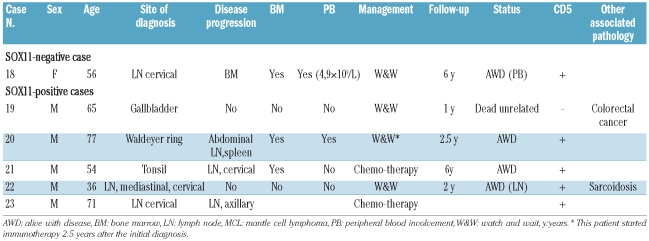
A comparison of the clinical features of the patients with “in situ MCL” lesions according to SOX11 expression is summarized in Table 3. The seven patients with SOX11-positive lesions were five males and two females with a median age of 66 years (range, 42–82 years). One of the three patients examined had peripheral blood involvement and two of six were negative for CD5. Extranodal involvement was seen in three cases. Five patients had a follow-up of more than 1 year; one received chemotherapy and was alive after 6 years. The three “in situ MCL” lesions associated with a metachronous MCL were SOX11-positive in both the in situ lesion and the corresponding overt MCL.
The nine patients with SOX11-negative “in situ MCL” lesions were three men and six women with a median age of 59 years (range, 29–84 years). Differences in the sex prevalence were not statistically significant. Three patients had bone marrow involvement, two of whom also had peripheral blood involvement and six of the nine were negative for CD5. Extranodal involvement was seen in three cases. Eight patients had follow-up of more than 1 year. Three of them were treated, one patient received aggressive chemotherapy and was alive after 1.4 years and two patients received local radiotherapy and were alive after 1.7 and 2 years. Five patients were not treated and had a median survival of 5 years (range, 1–19.5 years); one of these patients died of unrelated causes 1.4 years after the diagnosis. None of the patients developed overt MCL.
Table 2.
Clinical features, follow-up and histopathological findings in MCL with mantle zone growth pattern.
Clinical features of mantle cell lymphoma with a mantle zone growth pattern
Five of the six patients with a MCL with a mantle zone pattern were male with a median age of 60 years (range, 36–78 years). Five of the six cases were SOX11-positive and one was SOX11-negative. CD5 was expressed in five cases and was negative in one. Four patients showed evidence of disseminated disease at diagnosis, three of them had bone marrow involvement, two with peripheral blood involvement, and one had cervical and axillary lymphadenopathy. Two patients were considered to have localized lesions, one in the gallbladder and the other in a cervical lymph node. This latter patient had sarcoidosis and mediastinal lymphadenopathy. Two patients with clinical evidence of dissemination to other lymph nodes at diagnosis were treated with chemotherapy; both cases were SOX11-positive. Follow-up data were available for one of them, who was alive with disease 6 years after diagnosis. Four patients were managed with a watchful waiting approach; three were SOX11-positive and one was negative. The single patient with a SOX11-negative MCL had stable leukemic disease and was alive 6 years after diagnosis. One of the SOX11-positive cases developed disease progression 2 years after the diagnosis and was treated with immunotherapy. The other two SOX11-positive cases were the patient with sarcoidosis who had stable mediastinal lymphadenopathy after 2 years of follow-up, and the other patient died of unrelated causes 1 year after diagnosis.
Discussion
In this study we have recognized two clinically and biologically distinct types of lesions characterized by cyclin D1-positive cells mostly limited to the mantle zones of reactive follicles. The cases we believe belong in the category of “in situ MCL” lesions had relatively few cyclin D1-positive cells restricted to the mantle area and concomitant or subsequent overt MCL was uncommon. The second group, which is best considered as MCL with a mantle zone growth pattern, had more extensive cyclin D1-positive cells with focal extension into the interfollicular areas and a more frequent association with conventional MCL without a mantle zone pattern. This suggests that the “in situ MCL” lesions may correspond to an early step in the lymphomagenesis of MCL, whereas MCL with a mantle zone growth pattern represent partial or early involvement by lymphoma.
Recent studies have found clonal B cells carrying the t(14;18) translocation restricted to the germinal centers in an otherwise reactive lymph node.15,16 These lesions were interpreted as an early step in the lymphomagenesis of follicular lymphoma, and have been termed FLIS. They may correspond to the circulating cells carrying the t(14;18) found in many normal individuals, and appear to have a very low malignant potential. The “in situ MCL” lesions investigated in this and other studies of individual cases14,23,30 may correspond to a similar phenomenon. We detected the t(11;14) translocations in the eight “in situ MCL” lesions studied and in one of them we confirmed that the translocation was present in the cyclin D1-positive cells. These observations support the genetic relationship of these limited lesions to MCL and rule out the possibility that cyclin D1 is overexpressed by other mechanisms as may be occasionally seen in cells of the proliferation centers in CLL.24 Clones carrying the t(11;14) translocation may be found at very low levels in the peripheral blood of healthy individuals using very sensitive methods.25 These clones persist over long periods and in some cases may even expand. Although there is no evidence that these clones progress to overt lymphoma, they may represent a very early event in MCL lymphomagenesis. The “in situ MCL” lesions observed in this study may correspond to the tissue counterpart of these circulating clones.
To evaluate the frequency of “in situ MCL” lesions, we stained 100 consecutive specimens of reactive follicular hyperplasia in adults for cyclin D1 and none of them showed positive cells, indicating that this situation must be uncommon in routine practice. Therefore, the use of cyclin D1 staining in the context of a reactive lymph node does not seem to be necessary. We also stained the same cohort for BCL2 and found two cases with FLIS. These findings parallel in part the observation of clones carrying the t(11;14) and the t(14;18) translocation in the blood of healthy individuals using highly sensitive methods. The t(11;14) was detected only in 7% of individuals whereas the t(14;18) was found in up to 71% of healthy people.25–27 Interestingly, almost all healthy individuals carrying the t(11;14) also had clones with the t(14;18), and clones with this translocation were also found in patients with overt MCL.25 The concomitant identification of clones with both translocations in the same individual may correspond at a molecular level to the frequent association of “in situ MCL” lesions with follicular lymphoma and other indolent small B-cell lymphomas observed in this study. This phenomenon is also seen in FLIS,15,16,28 suggesting that these patients may have a certain susceptibility to develop and expand aberrant B-cell clones.
Most patients with “in situ MCL” lesions presented with a localized enlarged lymph node or extranodal lymphoid tissue. However, six of 17 patients had evidence of more widespread disease. Five patients had bone marrow and/or peripheral blood involvement, and a sixth patient had “in situ MCL” lesions simultaneously in an axillary and inguinal lymph node, as well as minimal infiltration of a gastric biopsy by cyclin D1-positive cells.13 The clinical significance of these disseminated cells is uncertain because their numbers were always limited in all locations and the evolution of disease in three of these patients was very indolent with a stable presence of atypical cells in the peripheral blood for more than 3, 12 and 19 years without any antineoplastic treatment.9,10 The patient with more than one lymph node involved and minimal gastric infiltration was alive with no evidence of disease 6 years later but he had been treated with high-dose immunochemotherapy and stem cell transplantation. The apparent dissemination in these cases may represent the normal circulating nature of lymphoid cells rather than an indication of clinically significant malignant potential. Dissemination of FLIS in the absence of more overt lymphoma is also well-recognized.12,15,16,28 The indolent behavior of the “in situ MCL” lesions is also reinforced by the evolution of four other patients managed with watchful waiting who did not have progression of disease 1, 1, 1.4 and 5 years after diagnosis, although the third died of an unrelated cause at the age of 84.12 Six patients treated with local radiotherapy or chemotherapy were also alive and without evidence of disease 0.5 to 6 years later (median 1.6 years).11,13,14
Only three patients with “in situ MCL” lesions had overt MCL. In two cases the in situ lesion was discovered after the diagnosis of the MCL. In one patient it was detected in a retrospective analysis of a previous appendectomy specimen removed 4 years earlier.13 In the second patient, the “in situ MCL” lesion was identified in a lymphadenectomy obtained at surgery for prostatic carcinoma 3 years after the diagnosis and treatment of MCL. The persistence of cyclin D1-positive cells in the mantle zones of a MCL patient in apparent complete remission highlights the relevance of the mantle zone as a microenvironment favoring the survival of cells with the t(11;14) translocation, not only in early steps of the disease, but also as a potential sanctuary for chemotherapy-resistant cells. This patient relapsed with overt MCL 1 year later. Only one patient without overt MCL at the time of the diagnosis of the in situ lesion developed an overt MCL 4 years later.
The follow-up of the patients in our study suggests that most patients with “in situ MCL” lesions do not develop an overt MCL for long periods of time (1 to 19.5 years), even without any treatment. However, in some cases the lesion may evolve into overt MCL. This evolution to MCL is consistent with recent observations by Racke et al., published in abstract form, in which all seven MCL patients examined had an “in situ MCL” lesion in reactive lymph nodes obtained between 2 and 15 years (median 8 years) prior to the diagnosis of the overt lymphoma.29 A similar case has been recently reported in which an in situ lesion was found in a gastrointestinal tract biopsy 2 years before the diagnosis of overt intestinal MCL.30 This long latency period prior to the development of MCL has also been highlighted by the diagnosis of a MCL 12 years after an allogenic bone marrow transplantation occurring simultaneously in the respective recipient and donor and corresponding to the same neoplastic clone in both patients.31
Although MCL with a mantle zone growth pattern was not always associated with conventional MCL with nodular or diffuse patterns, four of the six patients developed MCL with more extensive infiltration. Three patients, two with bone marrow involvement, showed lymphadenopathy at other sites; two were treated with chemotherapy, whereas one patient with peripheral blood and bone marrow involvement at the time of diagnosis was not treated and developed abdominal lymphadenopathy 1 year later. One patient with bone mar-row dissemination followed with watchful waiting had stable disease in the peripheral blood 6 years after the diagnosis and one untreated patient died of an unrelated cause 1 year after the diagnosis.
These findings highlight the low malignant potential of the “in situ MCL” lesions which is similar to that of FLIS and raises the question of maintaining the term “lymphoma” for these lesions.15,16 In contrast, MCL with a mantle zone pattern appears to correspond to early involvement by conventional MCL similar to partial involvement of a lymph node by follicular lymphoma.16,32 A recent Workshop of the European Association for Hematopathology and the Society for Hematopathology (Uppsala, Sweden, September 2010) proposed the alternative terms of “in situ MCL-like B cells” and “in situ FL-like B cells of uncertain significance” to avoid overdiagnosis and overtreatment of these individuals.33 These recommendations are parallel to the use of monoclonal gammopathy of uncertain significance and monoclonal B-cell lymphocytosis for potentially premalignant phases of plasma cell myeloma and CLL, respectively.
SOX11 is a specific marker of MCL expressed in 78–93% of these tumors.6,18,20 In a recent study, a group of MCL with a very indolent clinical behavior and managed without any treatment for more than 2 years were consistently SOX11-negative. The expression of this bio-marker was less common in the “in situ MCL” lesions (44%) than in the MCL with a mantle zone pattern (5 of 6 cases, 83%), which is a similar proportion to that observed in other overt MCL. Interestingly, the only patient with a SOX11-negative MCL with a mantle zone pattern had very stable disease for 6 years without treatment. Although not statistically significant, it is intriguing that the SOX11-negative “in situ MCL” lesions were mainly found in women (67%) whereas SOX11- positive “in situ MCL” lesions and mantle zone pattern lesions were more common in men (71% and 83%, respectively). Both SOX11-negative and -positive “in situ MCL” lesions had a very indolent behavior. However, the tumors in the three patients who had overt MCL either before or after the diagnosis of “in situ MCL were SOX11-positive in both the “in situ MCL” lesion and the overt MCL. The expression of SOX11 in some “in situ MCL” lesions, the progression of SOX11-positive “in situ MCL” lesion to an overt MCL, and the higher number of cases of SOX11-positive MCL with a mantle zone pattern indicate that SOX11 is already differentially expressed early in the development of “in situ MCL” lesions and suggest that lesions expressing SOX11 may have a greater tendency to progress.
In conclusion, our data suggest that cases diagnosed as “in situ mantle cell lymphoma” include two distinct situations with different clinical impact. Some cases are overt MCL with a mantle zone growth pattern that may have disseminated disease at diagnosis, although some cases may also follow a relatively indolent course. In these cases, a diagnosis of MCL should be made, with a comment regarding the pattern. Other cases, in which the nodal architecture is preserved and cyclin D1-positive cells are restricted to mantle zones of otherwise normal-appearing follicles, appear to have a very low, but still definite risk of progression to overt MCL. In these cases we suggest that a diagnosis of “in situ involvement by mantle cell lymphoma-like cells” should be made, with a note indicating that this does not constitute a diagnosis of lymphoma, and that treatment should not be undertaken based solely on the basis of this finding. In such cases, surveillance of the patients and evaluation for the presence of progressive disease should be considered.
Acknowledgments
We thank Dr J.L. Kutok from Brigham & Women Hospital, Boston, MA, USA, for his kind contribution of one of the cases.
Footnotes
Funding: this work was supported by grants from the European Regional Development Fund; the Comisión Interministerial de Ciencia y Tecnología (CICYT) SAF08-03630; RD06/0020/0039 from Red Temática de Investigación Cooperativa en Cáncer (RTICC); Instituto de Salud Carlos III (ISCIII); Spanish Ministry of Science and Innovation, and the Generalitat de Catalunya 2009SGR992. AC-C has received a fellowship from the University of Costa Rica (UCR) and the Centro de Desarrollo Estratégico e Información en Salud y Seguridad Social (CENDEISSS) of Costa Rica.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Swerdlow SH, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues fourth edition. 2008. [Google Scholar]
- 2.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orchard J, Garand R, Davis Z, Babbage G, Sahota S, Matutes E, et al. A subset of t(11;14) lymphoma with mantle cell features displays mutated IgVH genes and includes patients with good prognosis, nonnodal disease. Blood. 2003;101(12):4975–81. doi: 10.1182/blood-2002-06-1864. [DOI] [PubMed] [Google Scholar]
- 4.Martin P, Chadburn A, Christos P, Weil K, Furman RR, Ruan J, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27(8):1209–13. doi: 10.1200/JCO.2008.19.6121. [DOI] [PubMed] [Google Scholar]
- 5.Eve HE, Furtado MV, Hamon MD, Rule SA. Time to treatment does not influence overall survival in newly diagnosed mantle-cell lymphoma. J Clin Oncol. 2009;27(32):e189–e190. doi: 10.1200/JCO.2009.23.9731. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez V, Salamero O, Espinet B, Sole F, Royo C, Navarro A, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Res. 2010;70(4):1408–18. doi: 10.1158/0008-5472.CAN-09-3419. [DOI] [PubMed] [Google Scholar]
- 7.Majlis A, Pugh WC, Rodriguez MA, Benedict WF, Cabanillas F. Mantle cell lymphoma: correlation of clinical outcome and biologic features with three histologic variants. J Clin Oncol. 1997;15(4):1664–71. doi: 10.1200/JCO.1997.15.4.1664. [DOI] [PubMed] [Google Scholar]
- 8.Richard P, Vassallo J, Valmary S, Missoury R, Delsol G, Brousset P. “In situ-like” mantle cell lymphoma: a report of two cases. J Clin Pathol. 2006;59(9):995–6. doi: 10.1136/jcp.2005.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nodit L, Bahler DW, Jacobs SA, Locker J, Swerdlow SH. Indolent mantle cell lymphoma with nodal involvement and mutated immunoglobulin heavy chain genes. Hum Pathol. 2003;34(10):1030–4. doi: 10.1053/s0046-8177(03)00410-6. [DOI] [PubMed] [Google Scholar]
- 10.Espinet B, Sole F, Pedro C, Garcia M, Bellosillo B, Salido M, et al. Clonal proliferation of cyclin D1-positive mantle lymphocytes in an asymptomatic patient: an early-stage event in the development or an indolent form of a mantle cell lymphoma? Hum Pathol. 2005;36(11):1232–7. doi: 10.1016/j.humpath.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Rodig SJ, Healey BM, Pinkus GS, Kuo FC, Dal CP, Kutok JL. Mantle cell lymphoma arising within primary nodal marginal zone lymphoma: a unique presentation of two uncommon B-cell lymphoproliferative disorders. Cancer Genet Cytogenet. 2006;171(1):44–51. doi: 10.1016/j.cancergencyto.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Roullet MR, Martinez D, Ma L, Fowler MH, McPhail ED, Judkins A, et al. Coexisting follicular and mantle cell lymphoma with each having an in situ component: a novel, curious, and complex consultation case of coincidental, composite, colonizing lymphoma. Am J Clin Pathol. 2010;133(4):584–91. doi: 10.1309/AJCP5RT4MRSDGKSX. [DOI] [PubMed] [Google Scholar]
- 13.Bassarova A, Tierens A, Lauritzsen GF, Fossa A, Delabie J. Mantle cell lymphoma with partial involvement of the mantle zone: an early infiltration pattern of mantle cell lymphoma? Virchows Arch. 2008;453(4):407–11. doi: 10.1007/s00428-008-0621-x. [DOI] [PubMed] [Google Scholar]
- 14.Aqel N, Barker F, Patel K, Naresh KN. In-situ mantle cell lymphoma--a report of two cases. Histopathology. 2008;52(2):256–60. doi: 10.1111/j.1365-2559.2007.02906.x. [DOI] [PubMed] [Google Scholar]
- 15.Cong P, Raffeld M, Teruya-Feldstein J, Sorbara L, Pittaluga S, Jaffe ES. In situ localization of follicular lymphoma: description and analysis by laser capture microdissection. Blood. 2002;99(9):3376–82. doi: 10.1182/blood.v99.9.3376. [DOI] [PubMed] [Google Scholar]
- 16.Jegalian AG, Eberle FC, Pack SD, Mirvis M, Raffeld M, Pittaluga S, et al. Follicular lymphoma in situ: clinical implications and comparisons with partial involvement by follicular lymphoma. Blood. 2011;118(11):2976–84. doi: 10.1182/blood-2011-05-355255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ek S, Dictor M, Jerkeman M, Jirstrom K, Borrebaeck CA. Nuclear expression of the non B-cell lineage Sox11 transcription factor identifies mantle cell lymphoma. Blood. 2008;111(2):800–5. doi: 10.1182/blood-2007-06-093401. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Asplund AC, Porwit A, Flygare J, Smith CI, Christensson B, et al. The subcellular Sox11 distribution pattern identifies subsets of mantle cell lymphoma: correlation to overall survival. Br J Haematol. 2008;143(2):248–52. doi: 10.1111/j.1365-2141.2008.07329.x. [DOI] [PubMed] [Google Scholar]
- 19.Dictor M, Ek S, Sundberg M, Warenholt J, Gyorgy C, Sernbo S, et al. Strong lymphoid nuclear expression of SOX11 transcription factor defines lymphoblastic neoplasms, mantle cell lymphoma and Burkitt’s lymphoma. Haematologica. 2009;94(11):1563–8. doi: 10.3324/haematol.2009.008474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mozos A, Royo C, Hartmann E, De JD, Baro C, Valera A, et al. SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1-negative subtype. Haematologica. 2009;94(11):1555–62. doi: 10.3324/haematol.2009.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuchinka BD, Kalousek DK, Lomax BL, Harrison KJ, Barrett IJ. Interphase cytogenetic analysis of single cell suspensions prepared from previously formalin-fixed and paraffin-embedded tissues. Mod Pathol. 1995;8(2):183–6. [PubMed] [Google Scholar]
- 22.Cook JR, Hartke M, Pettay J, Tubbs RR. Fluorescence in situ hybridization analysis of immunoglobulin heavy chain translocations in plasma cell myeloma using intact paraffin sections and simultaneous CD138 immunofluorescence. J Mol Diagn. 2006;8(4):459–65. doi: 10.2353/jmoldx.2006.050149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edlefsen KL, Greisman HA, Yi HS, Mantei KM, Fromm JR. Early lymph node involvement by mantle cell lymphoma limited to the germinal center: report of a case with a novel “follicular in situ” growth pattern. Am J Clin Pathol. 2011;136(2):276–81. doi: 10.1309/AJCP6KFFGTC8PLVR. [DOI] [PubMed] [Google Scholar]
- 24.O’Malley DP, Vance GH, Orazi A. Chronic lymphocytic leukemia/small lymphocytic lymphoma with trisomy 12 and focal cyclin d1 expression: a potential diagnostic pitfall. Arch Pathol Lab Med. 2005;129(1):92–5. doi: 10.5858/2005-129-92-CLSLLW. [DOI] [PubMed] [Google Scholar]
- 25.Lecluse Y, Lebailly P, Roulland S, Gac AC, Nadel B, Gauduchon P. t(11;14)-positive clones can persist over a long period of time in the peripheral blood of healthy individuals. Leukemia. 2009;23(6):1190–3. doi: 10.1038/leu.2009.31. [DOI] [PubMed] [Google Scholar]
- 26.Roulland S, Navarro JM, Grenot P, Milili M, Agopian J, Montpellier B, et al. Follicular lymphoma-like B cells in healthy individuals: a novel intermediate step in early lymphomagenesis. J Exp Med. 2006;203(11):2425–31. doi: 10.1084/jem.20061292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roulland S, Lebailly P, Lecluse Y, Briand M, Pottier D, Gauduchon P. Characterization of the t(14;18) BCL2-IGH translocation in farmers occupationally exposed to pesticides. Cancer Res. 2004;64(6):2264–9. doi: 10.1158/0008-5472.can-03-3604. [DOI] [PubMed] [Google Scholar]
- 28.Montes-Moreno S, Castro Y, Rodriguez-Pinilla SM, Garcia JF, Mollejo M, Castillo ME, et al. Intrafollicular neoplasia/in situ follicular lymphoma: review of a series of 13 cases. Histopathology. 2010;56(5):658–62. doi: 10.1111/j.1365-2559.2010.03529.x. [DOI] [PubMed] [Google Scholar]
- 29.Racke F, Simpson S, Christian B, Blum K, Hasserjian R, Zhao W. American Society of Hematology. San Diego, CA: 2010. Dec 6, 2010. Evidence of long latency periods prior to development of mantle cell lymphoma. [Abstract] [Google Scholar]
- 30.Neto AG, Oroszi G, Protiva P, Rose M, Shafi N, Torres R. Colonic in situ mantle cell lymphoma. Ann Diagn Pathol. 2011 Aug 11; doi: 10.1016/j.anndiagpath.2011.05.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Christian B, Zhao W, Hamadani M, Sotomayor EM, Navarro W, Devine SM, et al. Mantle cell lymphoma 12 years after allogeneic bone marrow transplantation occurring simultaneously in recipient and donor. J Clin Oncol. 2010;28(31):e629–e632. doi: 10.1200/JCO.2010.29.8992. [DOI] [PubMed] [Google Scholar]
- 32.Adam P, Katzenberger T, Eifert M, Ott MM, Rosenwald A, Muller-Hermelink HK, et al. Presence of preserved reactive germinal centers in follicular lymphoma is a strong histopathologic indicator of limited disease stage. Am J Surg Pathol. 2005;29(12):1661–4. doi: 10.1097/01.pas.0000173233.29741.38. [DOI] [PubMed] [Google Scholar]
- 33.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–32. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]