Abstract
Associating sensory cues with aversive outcomes is a relatively basic process shared across species. Yet higher-order cognitive processes likely contribute to associative fear learning in many circumstances, especially in humans. Here we ask whether fears can be acquired based on conceptual knowledge of object categories, and whether such concept-based fear conditioning leads to enhanced memory representations for conditioned objects. Participants were presented with a heterogeneous collection of images of animals and tools. Objects from one category were reinforced by an electrical shock, whereas the other category was never reinforced. Results confirmed concept-based fear learning through subjective report of shock expectancy, heightened skin conductance responses, and enhanced 24 hour recognition memory for items from the conditioned category. These results provide novel evidence that conditioned fear can generalize through knowledge of object concepts, and sheds light on the persistent nature of fear memories and category-based fear responses symptomatic of some anxiety disorders.
Keywords: emotional arousal, generalization, fear conditioning, associative learning, phobia, posttraumatic stress disorder
As a survival mechanism, many species are equipped with the ability to learn and remember which stimuli in the environment present a threat. Given that a known threat can take many forms, it is also important to generalize learning beyond a specific instance and extend defensive behaviors towards other exemplars that might portend the same negative outcome. For example, escape from a predator dictates future avoidance of that animal if it is encountered under different conditions, as well as avoidance of other animals that strongly resemble a known threat. Accordingly, an organism with an advanced capacity to detect similarities between unique but related stimuli may be at an advantage to avoid harm in a dynamic environment. As the ability to abstract from a learning episode on the basis of conceptual knowledge is a hallmark of human cognition, we examined whether categorical knowledge for object concepts influences associative fear learning processes and retention of fear memories.
An understanding of how species learn to fear and remember potentially threatening stimuli or situations has been advanced from laboratory studies of fear conditioning. In these studies, an innocuous conditioned stimulus (CS; e.g. a tone) will produce an array of defensive conditioned responses (CR; e.g., freezing) if the CS reliably predicts a biologically aversive unconditioned stimulus (US; e.g. an electrical shock). Previous studies on the generalization of conditioned learning have shown that CRs often extend to unreinforced stimuli that resemble the CS along some basic perceptual feature dimension, such as tone pitch, size, or color (Honig and Urcuioli, 1981; Pavlov, 1927). Of course, in real world situations a stimulus of potential relevance can approximate a threatening stimulus along multiple dimensions (Shepard, 1987). For example, in posttraumatic stress disorder, conditioned fear memories for a traumatic event may be triggered by a range of stimuli or situations that are only indirectly related to the episode and cannot be explained merely by perceptual feature similarity (e.g., anniversary dates, media coverage, or mementos of a war). In other anxiety disorders, such as specific phobia, fears can generalize categorically across stimuli that diverge greatly in perceptual features (e.g., in blood-injection phobia, the sight of a needle, nurse’s uniform, or hospital corridor). Fear may also enhance long-term declarative memory for a host of information associated with an aversive experience, thereby leading to persistent and intrusive memories for items that evoke an emotional reaction but are not necessarily intrinsically threatening. As stimuli related to a learned threat can take on multiple forms, it remains a great challenge to predict which stimuli might attain fear value and enter into long-term memory as affectively significant.
Here we sought to determine whether conceptual knowledge that links heterogeneous exemplars of an object category can form the basis for fear acquisition and retention using a Pavlovian conditioning procedure. We employed a differential fear conditioning paradigm using basic-level exemplars from two distinct superordinate object categories (animals and tools) as CSs. Objects from one category were intermittently paired with an aversive electrical shock US, whereas objects from the other category were never reinforced. Participants learned through experience which category presented a threat and which category was safe. This approach differs considerably from the standard conditioning procedure in which a single CS is repeatedly reinforced during acquisition training, and subsequent generalization tests then present unreinforced stimuli that parametrically vary from the CS along a basic sensory dimension (e.g. Guttman and Kalish, 1956). In the present study, exemplars are never repeated. Instead, participants are required to generalize beyond each instance in order to successfully predict the US. The key information that links exemplars is based on conceptual knowledge of relationships among the category members (which may include some perceptual information) and abstraction to the superordinate category level. We predicted that psychophysiological indices of sympathetic arousal (i.e., skin conductance responses, SCRs) and declarative ratings of US expectancy would be greater to the basic-level CS exemplars from within the reinforced superordinate category than from the unreinforced superordinate category.
We also sought to determine whether long-term declarative memory was selectively enhanced for exemplars from the superordinate category that acquired fear value through the conditioning procedure. Numerous studies have shown that long-term item memory is enhanced by emotional arousal (Cahill and McGaugh, 1998; LaBar and Cabeza, 2006; Mather and Sutherland, 2011). For example, individuals preferentially remember memoranda that are intrinsically arousing (e.g., violent scene) relative to those that are affectively neutral (e.g., an office scene). Despite a wealth of research on the mechanisms of fear conditioning (LeDoux, 2000) and declarative memory for emotionally-arousing items (Murty et al., 2010), these two areas of research have rarely overlapped. Although declarative memory for the CS-US association has been examined as a consequence of conditioned learning (LaBar and Disterhoft, 1998; Shanks, 2010), it has been challenging to determine how the conditioning process has altered the strength of the memory representation for the CS, given that only a single exemplar is typically presented during training. Thus, in the present study we asked participants to return 24 hours after the initial fear acquisition session for a surprise recognition memory test. We predicted that memory would be enhanced for those CS exemplars from the superordinate object category that had been reinforced by the US relative to the exemplars from the unreinforced category. Moreover, we predicted that the memory advantage would generalize to those CS exemplars that came from the reinforced superordinate category but were not directly followed by electric shock.
Method
Participants
Twenty-six healthy volunteers (12 females, median age = 19 years) provided written informed consent in accordance with the Duke University Institutional Review Board guidelines. Two subjects were excluded from the final analysis due to an overall lack of measurable electrodermal activity, which precludes an examination of differential conditioning.
Stimulus materials
Stimuli consisted of 80 unique basic level exemplars of tools (N = 40) and animals (N = 40) presented on a white background. Exemplars were chosen on the basis of published category norms (e.g. Van Overschelde et al., 2004) to ensure a range of highly typical (e.g. dog and hammer) and atypical (e.g., auger and leaf insect) items. Highly threat-relevant items (e.g., knives and snakes) were not included so as to mitigate potential arousal bias towards these objects (Öhman and Mineka, 2001). Stimulus presentation was controlled with Presentation Software (Neurobehavioral Systems, Albany, CA). The aversive US consisted of a 6-ms electrical shock delivered to the right wrist, calibrated for each participant prior to the start of the experiment using an ascending staircase procedure so the subjective experience of the shock was rated as “annoying but not painful” to the participants [see Dunsmoor et al. (2009) for similar procedures].
US expectancy
On each trial, participants were instructed to rate their level of expectancy for receiving the US using a rating bar controlled with a mouse. The rating bar appeared below the CS and ranged from 0 (“sure the US will not occur on this trial”) to 5 (“uncertainty whether the US will occur on this trial”) to 10 (“sure the US will occur on this trial”). Participants were accustomed to the use of the rating bar during a practice session that included trials with random objects (unrelated to the task stimulus set) and no US presentations. Expectancy was calculated as the final location of the rating bar at stimulus offset. Participants were not instructed of the CS-US contingencies and were not told that each animal and tool image would only be presented once during the conditioning session. In addition, participants were not informed that Day 2 would include a recognition memory test (incidental encoding).
Skin conductance responses (SCRs)
SCRs were collected throughout the experiment on Day 1 from the hypothenar eminence of the palmar surface of the left hand as the dependent measures of sympathetic arousal in response to the CS and US. SCRs were scored according to our previous criteria (Dunsmoor et al., 2011). In brief, an SCR was considered related to stimulus presentation if the trough-to-peak response began between 1–4 s after stimulus onset, lasted between 0.5 and 5.0 s, and was > 0.02 microsiemens (μS). Responses that did not fit these criteria were scored as zero. SCRs were square root transformed for normalization prior to statistical analysis. The psychophysiological recordings and shock administration were controlled with the MP-150 BIOPAC system (BIOPAC systems, Goleta, CA).
Fear conditioning procedures
Fear conditioning occurred over 4 training runs that each included 20 CSs (10 tools and 10 animals) presented in a pseudorandomized order such that no more than 2 images of tools (or animals) occurred in a row. We used 4 different stimulus presentation orders to counterbalance the presentation of animal and tool exemplars across subjects. Stimulus presentation lasted for 6 s, during which time participants rated their shock expectancy. A white fixation cross on a black background followed the offset of each trial for 10–12 s. For each participant, one object category (e.g., animals) was designated the CS+, and 50% of exemplars from this category were reinforced with delivery of the shock US. The other object category (e.g., tools) served as the CS−, and none of its exemplars were reinforced with a shock US. Category assignment was counterbalanced across subjects.
Recognition memory procedures
Participants returned 24 hours later for a surprise recognition memory test. This test included the 80 previously seen images and 40 new images (20 tools and 20 animals). Participants rated whether each image was new or old and their level of memory confidence on a 4-point scale (“definitely new,” “maybe new,” “maybe old,” “definitely old”). Prior research has shown that emotion has a larger impact on memory for items recalled with high confidence or a sense of recollection rather than those items recalled with low confidence or accompanied by a sense of mere familiarity (Dolcos et al., 2005; Ochsner, 2000; Talarico et al., 2004). Therefore we focused the present analyses on high confidence trials only and used corrected recognition procedures by subtracting high confidence false alarms. Following each memory judgment, the item was rated for its categorical typicality, but these ratings were not used in the present analyses.
Results
Behavioral ratings
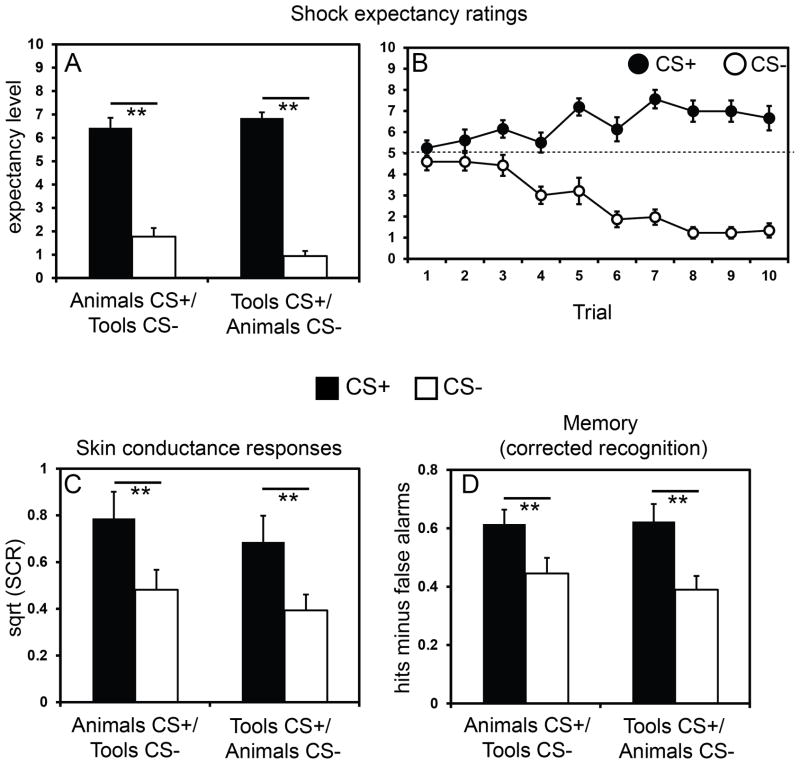
Repeated-measures ANOVA of the US expectancy data, using CS type (CS+, CS−) as a within-subjects factor and group (animal CS+/tool CS−, tool CS+/animal CS−) as a between-subjects factor, showed a main effect of CS type, F(1, 22) = 216.40, p < .001, ηp2 = .908. There was no effect of group (p = .481) and no interaction with group (p = .096), indicating that declarative CS-US contingency learning was similar regardless of whether participants were fear conditioned to animals or tools (Fig 1A). Figure 1B illustrates that CS-US contingency learning occurred rapidly, within the first run of 10 CS+ and 10 CS− presentations. Note that US expectancy levels for the CS+ did not reach ceiling. This is likely due to the use of partial CS-US pairing, such that participants could not be absolutely certain the US would occur on any given CS+ trial.
Figure 1.
Behavioral results. (A) Participants rated expectancy for the US higher on CS+ trials versus CS− trials, and there was no effect of category (tool or animal) on US expectancy ratings. (B) Differential ratings of US expectancy emerged early during training. Dashed line indicates chance level of certainty as to whether or not the US would be delivered. (C) Differential SCRs were observed between CS+ and CS− items, and there was no effect of category (tool or animal) on differential SCRs. (D) 24-hour delayed recognition memory for CS+ items was significantly greater than that for CS- items, and these effects were not driven by which category served as the CS+ or CS−. Error bars reflect ± standard error (SEM). ** = p < .001.
Skin conductance responses
Repeated-measures ANOVA of SCRs revealed a main effect of CS type, F (1, 22) = 34.71, p < .001, ηp2 = .612, indicating that subjects acquired differential autonomic reactions to the category of objects that predicted the US versus the control category (Fig 1C). Notably, there was no effect of group (p = .463) and no interaction with group (p = 0.907), demonstrating that participants conditioned equally well to animals and tools.
24-hour delayed recognition memory
Figure 1D shows that high confidence recognition for CS+ items was superior to that for CS− items, F (1, 22) = 20.31, p < .001, ηp2 = .480. There was no effect of group (p = .697) and no interaction with group (p = .484). High confidence false alarm rates were low for both the CS+ (mean ± SEM: .06 ± .01) and the CS− (.05 ± .02) category, and there was no difference in false alarm rates between the two conditions (p = .819). Finally, we examined whether the direct presentation of the US disproportionately affected memory for CS+ items that were paired with the shock (half of the CS+ trials) relative to unreinforced CS+ trials (the other half of CS+ trials). There was no difference in the memory performance for CS+ items paired with shock versus CS+ items presented alone (p = .532), suggesting that the emotional enhancement of memory generalized to items from within the same category, regardless of whether or not the item was directly reinforced by the shock US.
Discussion
Fear conditioning
In summary, our results show that conceptual knowledge is utilized during associative fear learning in humans, and that concept-based fear learning has unique effects on memory retention for items that have attained fear value. The ability to extract conceptual information and abstract from a learning experience is an essential characteristic of human cognition; but how this ability interacts with evolutionarily conserved systems like fear conditioning is unclear. A long standing challenge in understanding the role of higher-level cognitive systems (like conceptual representations) in emotional learning has been reliance on animal models of fear conditioning. While these models are increasingly important to understand the mechanics of fear, they may not be sufficient to fully interpret the way in which humans acquire, express, and generalize learned fears. These findings confirmed that participants quickly learn the relationship between a superordinate category and an aversive stimulus, and acquire differential fear responses to exemplars within the superordinate category that have become feared.
These fear conditioning results are particularly noteworthy given that each trial contained a unique exemplar and participants were responsible for learning the superordinate relationship to the US in order to effectively predict when the US would occur. If participants had attended solely to each instance (e.g., cow= US, dog ≠ US, hammer ≠ US etc.) without extrapolating to the superordinate category (e.g., animal ≈ US, tool ≠ US), then learning rates and SCRs would have been highly irregular. That US expectancy rates rapidly dissociated between the CS+ and CS− categories shows that participants quickly used conceptual knowledge to generalize beyond the basic level exemplar associations with the US. Thus category-specific knowledge was swiftly and effectively implemented to judge the likelihood of receiving the US. These findings are consistent with the contemporary view that higher-order cognitive systems interact with basic conditioning mechanisms (Davey, 1992; Pessoa, 2008), and demonstrate how cognitive representations can mediate conditioned learning (Holland, 1990; Rescorla, 1988). These findings are also in line with human behavioral studies demonstrating the role of rule-based knowledge (Shanks and Darby, 1998), propositional knowledge (Mitchell et al., 2009), and verbal processes (Vervliet et al., 2010) during associative learning and generalization. The present study complements and extends prior research on human fear generalization, which has predominantly employed perceptual dimensions (Dunsmoor et al., 2009; Lissek et al., 2008; Vervliet et al., 2004). It is well established that similarity promotes generalization of conditioned learning, such that stimuli more closely resembling the CS+ evoke considerably stronger CRs than perceptually dissimilar stimuli (Honig and Urcuioli, 1981; Pavlov, 1927). The role of conceptual similarity on generalization of conditioned learning has received far less attention (Dunsmoor et al., 2011) [see also Maltzman (1977) and Razran (1939) for early examples of generalization of semantic conditioning using verbal stimuli]. Although the ability to extract higher-order regularities during learning is not unique to humans (Honey and Hall, 1989; Wasserman et al., 1992), humans often utilize inductive reasoning and linguistics during learning in order to extract large amounts of information from a given instance (Landauer and Dumais, 1997). Conceptually-based forms of fear generalization may utilize similar mechanisms involved in other non-similarity based forms of conditioned learning, such as mediated generalization or sensory preconditioning (Gewirtz and Davis, 2000; Honey and Hall, 1989; Wasserman et al., 1992). In this way, prior experience with categorically related stimuli (or knowledge about interrelated concepts) facilitates the transfer of new learning from one stimulus to the next despite differences in physical form. Thus, one could speculate that an individual unaware of the connection between a known threat and a conceptually related stimulus would fail to generalize fear accordingly. Conversely, the ability to detect numerous connections to a known threat may prove maladaptive, if information acquired during a highly negative event is generalized to a wide network of interrelated knowledge -- as exemplified in posttraumatic stress disorder (Ehlers et al., 2004; Foa et al., 1989).
Of course, as metric features help determine category membership it is not possible to completely rule out the influence of low-level perceptual features on these fear conditioning results. For example, pictures of tools often contain more straight lines than pictures of animals. Thus, it is possible that fear expression is initiated purely on the basis of perceptual features that help differentiate animals from tools. To minimize the reliance on perceptual strategies, we incorporated a range of exemplars that varied in shape and appearance (e.g., four-legged mammals, fish, birds, insects, etc.). We also ensured that random samplings of CS+ images were paired with shock as a safeguard to prevent participants from relying on a particular perceptual feature to predict the US. Studies of patients with category-specific deficits in semantic knowledge due to focal brain damage (Capitani et al., 2003) and neuroimaging studies on the processing and storage of object properties (Martin, 2007) demonstrate that certain categories (e.g., tools and animals) are partially organized in the brain according to domain-specific properties. These areas along the ventral visual stream and prefrontal cortex respond somewhat broadly to a variety of objects from within the same categorical boundaries (Binder et al., 2009), whereas regions in early visual cortex are more sensitive to changes in perceptual form (Tootell et al., 1998). We predict, based on these behavioral results, that areas important for visual object recognition and conceptual knowledge interact with areas important for associating neutral and aversive stimuli (i.e., amygdala) to modify the emotional interpretation of categorical information. Such neuroscience evidence would complement the behavioral evidence presented here that fear learning can generalize beyond simple perceptual features (e.g., straight edges) to include conceptual knowledge of object categories.
Long-term memory enhancement for fear conditioned stimuli
Results from the 24 hour recognition memory test revealed that conceptually related items from a category that had attained fear value were preferentially remembered relative to items from another “safe” category. These results have implications for understanding the memory enhancing effects of arousal and memory biases in anxiety disorders. For instance, a wealth of research has implicated the role of stress hormone release on memory consolidation in rodents and humans (Cahill and McGaugh, 1998). Endogenous stress hormone activation induced after item encoding enhances long term memory for emotionally-significant items (Cahill et al., 2003). In addition, studies have shown selective memory enhancement for neutral information encoded in an arousing context (Anderson et al., 2006; Mather and Sutherland, 2011). Interestingly, in this study, feared CS+ trials without shock were remembered just as well as CS+ trials that included shock, which suggests that the shock itself did not necessarily enhance memory for the preceding CS+ (Anderson et al., 2006). The present findings are instead consistent with the hypothesis that neutral information that attains a high priority through top-down factors (in this case, stimuli predicting an electric shock) is preferentially remembered versus neutral information of low priority (i.e., stimuli not predictive of shock) (Mather and Sutherland, 2011). In this way, the state of fear induced by the anticipation for an impending US may be sufficient to provide deep memory encoding for the signaling item, irrespective of the actual outcome (i.e., whether or not the shock is delivered). A “levels of processing” account (Craik and Lockhart, 1972) may describe how internal affective states during a negative experience play a key role in mediating individual differences in memory for the details of an event. For example, an individual with emotional expectations may remember details from a wholly unremarkable event, provided that a level of physiological arousal has been generated by this expectation.
Understanding the linkages between fear learning processes and memory enhancement may have important implications for characterizing memory biases in anxiety disorders. In this regard, conceptual processes may be particularly relevant for patients whose behavioral sequelae are manifested categorically (e.g., Specific Phobias).
It is important to note that arousal-mediated memory enhancement can be driven by multiple processes, including increased attention during encoding, rehearsal, and post-encoding consolidation (McGaugh, 2006). Dissociating these effects in fear conditioning is complicated by the fact that both explicit and implicit systems are involved in mediating fear expression (LaBar and Disterhoft, 1998). For instance, conditioned fear can be acquired in the absence of contingency awareness (Knight et al., 2009), and patients with bilateral damage to the hippocampus acquire conditioned fear but do not retain declarative memory for the conditioned stimuli (Bechara et al., 1995; LaBar and Phelps, 2005). These previous findings suggest that fear conditioning is not always tied to the ability to form long-term declarative memories. Attentional factors may also account for why CS+ items were preferentially remembered, as a level of uncertainty for receiving the US enhances attention to a CS+ whereas attention to the “safe” CS− is reduced (Pearce and Hall, 1980). Further research is needed to explore how conditioning and declarative memory processes interact, particularly in the case of trauma in which intrusive vivid memories can be triggered by a range of innocuous information tangential to the traumatic experience (Ehlers et al., 2004).
Conclusion
This study yielded three key findings regarding the relationship between conceptual knowledge for object categories and associative fear learning. First, participants quickly learned the contingencies between a superordinate object category and delivery of an aversive shock, suggesting that conceptual knowledge can be rapidly recruited to learn about threats in the environment. Second, differential autonomic responses emerged between stimuli from the reinforced category and those from the safe category. Finally, 24-hour recognition memory performance was enhanced for exemplars from the reinforced category relative to the safe category, even if the individual item was not itself followed by shock the previous day. These findings have particular implications for better understanding anxiety disorders marked by overgeneralization of fear to harmless stimuli and the persistent nature of fearful memories. Although several models of human anxiety disorders have invoked fear conditioning processes as an explanatory mechanism (e.g. Brewin, 2001; Ehlers et al., 2004), probing declarative memory in human fear conditioning often involves questions on contingency awareness for only one or two CSs. As a result, the typical human fear conditioning experiment is ill-suited to address how humans acquire and retain long-term declarative memories for a range of threat-related stimuli. The approach developed in this experiment provides a novel way test the hypothesis that emotional arousal enhances long-term memory within the domain of conditioned learning, wherein neutral information acquires meaning through experience.
Highlights.
Humans rapidly form an association between object concepts and an electric shock
Concept-based fear learning was expressed through increases in expectancy and arousal
24 hour declarative memory is enhanced for fear conditioned stimuli vs. control stimuli
Acknowledgments
We thank Vishnu Murty for helpful comments and Philip Kragel for assistance with coding the presentation graphics program. This work was supported by NSF grant 0745919 and NIH grants R01 DA027802 and F31 MH090682.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AK, Wais PE, Gabrieli JDE. Emotion enhances remembrance of neutral events past. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1599–1604. doi: 10.1073/pnas.0506308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR. A cognitive neuroscience account of posttraumatic stress disorder and its treatment. Behav Res Ther. 2001;39:373–393. doi: 10.1016/s0005-7967(00)00087-5. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning & Memory. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosciences. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Capitani E, Laiacona M, Mahon B, Caramazza A. What are the facts of semantic category-specific deficits? A critical review of the clinical evidence. Cogn Neuropsychol. 2003;20:213–261. doi: 10.1080/02643290244000266. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing - framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Davey GCL. Classical-conditioning and the acquisition of human fears and phobias - A review and synthesis of the literatures. Advances in Behaviour Research and Therapy. 1992;14:29–66. [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Mitroff SR, LaBar KS. Generalization of conditioned fear along a dimension of increasing fear intensity. Learning & Memory. 2009;16:460–469. doi: 10.1101/lm.1431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, White AJ, LaBar KS. Conceptual similarity promotes generalization of higher order fear learning. Learn Mem. 2011;18:156–160. doi: 10.1101/lm.2016411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Hackmann A, Michael T. Intrusive re-experiencing in post-traumatic stress disorder: phenomenology, theory, and therapy. Memory. 2004;12:403–415. doi: 10.1080/09658210444000025. [DOI] [PubMed] [Google Scholar]
- Foa EB, Steketee G, Rothbaum BO. Behavioral cognitive conceptualizations of post-traumatic stress disorder. Behavior Therapy. 1989;20:155–176. [Google Scholar]
- Gewirtz JC, Davis M. Using Pavlovian higher-order conditioning paradigms to investigate the neural substrates of emotional learning and memory. Learning & Memory. 2000;7:257–266. doi: 10.1101/lm.35200. [DOI] [PubMed] [Google Scholar]
- Guttman N, Kalish HI. Discriminability and stimulus-generalization. Journal of Experimental Psychology. 1956;51:79–88. doi: 10.1037/h0046219. [DOI] [PubMed] [Google Scholar]
- Holland PC. Event representation in Pavlovian conditioning: image and action. Cognition. 1990;37:105–131. doi: 10.1016/0010-0277(90)90020-k. [DOI] [PubMed] [Google Scholar]
- Honey RC, Hall G. Acquired equivalence and distinctiveness of cues. J Exp Psychol-Anim Behav Process. 1989;15:338–346. [PubMed] [Google Scholar]
- Honig WK, Urcuioli PJ. The legacy of Guttman and Kalish (1956) - 25 years of research on stimulus-generalization. Journal of the Experimental Analysis of Behavior. 1981;36:405–445. doi: 10.1901/jeab.1981.36-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Waters NS, Bandettini PA. Neural substrates of explicit and implicit fear memory. Neuroimage. 2009;45:208–214. doi: 10.1016/j.neuroimage.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Disterhoft JF. Conditioning, awareness, and the hippocampus. Hippocampus. 1998;8:620–626. doi: 10.1002/(SICI)1098-1063(1998)8:6<620::AID-HIPO4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behav Neurosci. 2005;119:677–686. doi: 10.1037/0735-7044.119.3.677. [DOI] [PubMed] [Google Scholar]
- Landauer TK, Dumais ST. A solution to Plato’s problem: The latent semantic analysis theory of acquisition, induction, and representation of knowledge. Psychol Rev. 1997;104:211–240. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, Grillon C. Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behav Res Ther. 2008;46:678–687. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltzman I. Orienting in classical conditioning and generalization of the galvanic skin-response to words : an overview. Journal of Experimental Psychology-General. 1977;106:111–119. [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspect Psychol Sci. 2011;6:114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Make mild moments memorable: add a little arousal. Trends Cogn Sci. 2006;10:345–347. doi: 10.1016/j.tics.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ, De Houwer J, Lovibond PF. The propositional nature of human associative learning. Behav Brain Sci. 2009;32:183. doi: 10.1017/S0140525X09000855. [DOI] [PubMed] [Google Scholar]
- Murty VP, Ritchey M, Adcock RA, LaBar KS. fMRI studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia. 2010;48:3459–3469. doi: 10.1016/j.neuropsychologia.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN. Are affective events richly recollected or simply familiar? The experience and process of recognizing feelings past. J Exp Psychol Gen. 2000;129:242–261. doi: 10.1037//0096-3445.129.2.242. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. Oxford University Press; London: 1927. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87:532–552. [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Razran GHS. A quantitative study of meaning by a conditioned salivary technique (Semantic conditioning) Science. 1939;90:89–90. doi: 10.1126/science.90.2326.89-a. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioning - Its not what you think it is. American Psychologist. 1988;43:151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Shanks DR. Learning: From Association to Cognition. Annual Review of Psychology. 2010;61:273–301. doi: 10.1146/annurev.psych.093008.100519. [DOI] [PubMed] [Google Scholar]
- Shanks DR, Darby RJ. Feature- and rule-based generalization in human associative learning. J Exp Psychol-Anim Behav Process. 1998;24:405–415. doi: 10.1037//0097-7403.24.2.136. [DOI] [PubMed] [Google Scholar]
- Shepard RN. Toward a universal law of generalization for psychological scienc. Science. 1987;237:1317–1323. doi: 10.1126/science.3629243. [DOI] [PubMed] [Google Scholar]
- Talarico JM, LaBar KS, Rubin DC. Emotional intensity predicts autobiographical memory experience. Mem Cognit. 2004;32:1118–1132. doi: 10.3758/bf03196886. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani NK, Vanduffel W, Liu AK, Mendola JD, Sereno MI, Dale AM. Functional analysis of primary visual cortex (V1) in humans. Proc Natl Acad Sci U S A. 1998;95:811–817. doi: 10.1073/pnas.95.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overschelde JP, Rawson KA, Dunlosky J. Category norms: An updated and expanded version of the Battig and Montague (1969) norms. Journal of Memory and Language. 2004;50:289–335. [Google Scholar]
- Vervliet B, Kindt M, Vansteenwegen D, Hermans D. Fear generalization in humans: Impact of verbal instructions. Behav Res Ther. 2010;48:38–43. doi: 10.1016/j.brat.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Vansteenwegen D, Eelen P. Generalization of extinguished skin conductance responding in human fear conditioning. Learning & Memory. 2004;11:555–558. doi: 10.1101/lm.77404. [DOI] [PubMed] [Google Scholar]
- Wasserman EA, Devolder CL, Coppage DJ. Nonsimiliarity-based conceptualization in pigeons via secondary or mediated generaliation. Psychological Science. 1992;3:374–379. [Google Scholar]