Abstract
Objective
Age-related cognitive impairments have been attributed to deficits in inhibitory processes that mediate both motor restraint and sensory filtering. However, behavioral studies have failed to show an association between tasks that measure these distinct types of inhibition. In the present study, we hypothesized neural markers reflecting each type of inhibition may reveal a relationship across inhibitory domains in older adults.
Methods
Electroencephalography (EEG) and behavioral measures were used to explore whether there was an across-participant correlation between sensory suppression and motor inhibition. Sixteen healthy older adult participants (65-80 years) engaged in two separate experimental paradigms: a selective attention, delayed-recognition task and a stop-signal task.
Results
Findings revealed no significant relationship existed between neural markers of sensory suppression (P1 amplitude; N170 latency) and markers of motor inhibition (N2 and P3 amplitude and latency) in older adults.
Conclusions
These distinct inhibitory domains are differentially impacted in normal aging, as evidenced by previous behavioral work and the current neural findings. Thus a generalized inhibitory deficit may not be a common impairment in cognitive aging.
Significance
Given that some theories of cognitive aging suggest age-related failure of inhibitory mechanisms may span different modalities, the present findings contribute to an alternative view where age-related declines within each inhibitory modality are unrelated.
Keywords: inhibition, EEG, sensory suppression, motor inhibition, stop signal
1. Introduction
Normal aging has been associated with increased difficulty in managing conflicting situations in both the sensory and motor domains (Hasher et al., 1999; Lustig et al., 2007). For example, studies have revealed age-related deficits in restraining a response (Kok, 1999; May and Hasher, 1998) and ignoring/suppressing visually distracting information (Alain and Woods, 1999; Chao and Knight, 1997; Clapp and Gazzaley, 2010; Gazzaley et al., 2008; Gazzaley et al., 2005; West and Alain, 2000; Zanto et al., 2010a; Zanto et al., 2010b). More specifically, older adults show diminished response inhibition when required to modify an executed plan of action (Andres et al., 2008; Bedard et al., 2002; Kramer et al., 1994; Potter and Grealy, 2008), as well as visual suppression deficits in the presence of distracting information (Clapp and Gazzaley, 2010; Gazzaley et al., 2008; Hasher et al., 1999).
Both motor inhibition and sensory suppression are believed to be mediated by top-down control processes originating from the prefrontal cortex (Andres et al., 2008; Aron et al., 2003; Gazzaley and D'Esposito, 2007). Given that the prefrontal cortex has been shown to undergo significant age-related structural and functional alterations (Dumitriu et al., 2010; Rajah and D'Esposito, 2005; Raz et al., 1997; Tisserand and Jolles, 2003; Tisserand et al., 2004; van Boxtel et al., 2001), it is possible that aging is associated with a general inhibitory deficiency that may be a universal feature of cognitive aging. For example, Hasher and Zacks' (Hasher et al., 1999) inhibitory deficit theory suggests that an age-related decline in inhibitory processing efficiency is reflected by deficits in both the sensory domain (Andres and Van der Linden, 2000; Clapp and Gazzaley, 2010; Gazzaley et al., 2008; Gazzaley et al., 2005; Vallesi and Stuss, 2010; Zanto et al., 2010a; Zanto et al., 2010b), as well as in motor inhibition processes (Butler et al., 1999; May and Hasher, 1998; Nielson et al., 2002). The inhibitory deficit hypothesis proposed by Hasher and Zacks (Hasher et al., 1999) and supported by others (Alain and Woods, 1999; Butler and Zacks, 2006; Ossmann and Mulligan, 2003; West and Alain, 2000) suggests that an underlying failure of inhibitory mechanism(s) may account for an age-related decline in cognitive performance across different modalities.
Alternatively, age-related inhibitory deficits could reflect deterioration of independent neural networks supporting different functions (Greenwood, 2000), rather than a decline involving common inhibitory mechanisms. Indeed, evidence of inhibition devolving as a unitary construct or degrading equivalently across individuals has not been supported by behavioral studies. Several age-related studies have reported non-significant correlations among different inhibitory measures including response compatibility, negative priming, stop signal inhibition, spatial precuing, the Wisconsin Card Sorting Test (WCST), a Stroop task, and garden path sentences (Connelly and Hasher, 1993; Davidson and Glisky, 2002; Kramer et al., 1994; Rush et al., 2006; Shilling et al., 2002). These findings suggest that while the operational characteristic of interference suppression and motor inhibition may follow similar mechanisms, these inhibitory processes are independent, even in the presence of age-related cognitive declines. However, Collette et al. (2009) have argued that there are inherent difficulties in isolating inhibitory processes with behavioral measures due to the integrative nature of inhibitory functioning, i.e., the act of inhibition engages a series of mechanisms that act in concert with other cognitive processes. Thus, previous reports of nonsignificant correlates of inhibitory behavioral measures may reflect an insensitivity of these measures in probing this relationship. Here we hypothesize that the use of measures that probe the underlying neural processes associated with these distinct types of inhibition may provide a unique vantage point to better characterize these age-related inhibitory declines.
Gazzaley et al. have repeatedly examined sensory suppression utilizing both functional MRI and electroencephalography (EEG) during the engagement of a selective attention, delayed-recognition task in order to study both top-down enhancement and suppression of visual representations during a memory encoding period (Clapp and Gazzaley, 2010; Clapp et al., 2010; Gazzaley et al., 2008; Gazzaley et al., 2005; Gazzaley et al., 2007; Zanto et al., 2010a; Zanto et al., 2010b). These studies in younger adults have repeatedly shown an increase in neural activity for relevant stimuli versus a passive condition, as well as a decrease in activity for irrelevant stimuli versus the same passive condition. These changes in selective attention lead to a modulation of activity at the early stages of visual processing (Khoe et al., 2005; Lopez et al., 2004; Schoenfeld et al., 2007), as reflected by greater P1 peak amplitudes (∼100 ms) and earlier N170 peak latencies (∼170 ms), with these event-related potentials (ERPs) localized to the lateral extrastriate corticies (Gomez Gonzalez et al., 1994). With respect to aging, while older adults show intact neural markers of increased cortical activity for task relevant stimuli relative to younger adults (i.e., enhancement), they also display a deficit in the suppression of cortical activity associated with task-irrelevant stimuli (Clapp and Gazzaley, 2010; Gazzaley et al., 2008; Zanto et al., 2010a). Specifically related to the study at hand, the characterization of the age-related effects on sensory suppression on these ERPs has been reproduced on several occasions (Clapp and Gazzaley, 2010; Gazzaley et al., 2008; Zanto et al., 2010a), supporting the reliability of these neural measures.
Studies of response inhibition have regularly utilized the go/nogo paradigm (Butter, 1969; Passingham, 1972), where participants are instructed to respond (go) or not to respond (nogo) to predefined stimuli. ERPs associated with nogo stimuli have shown two consistent components: a fronto-central negative deflection developing at 200–400ms post-stimulus (nogo N2), and a subsequent medial central positivity (nogo P3) (Eimer, 1993; Kok, 1986; Pfefferbaum et al., 1985). While the nogo N2 has been associated with a participant's recognition of their need for inhibition (Kok, 1986; Smith et al., 2008), the nogo P3 component has been considered a more precise indicator of the effectiveness of motor inhibition engaged (Falkenstein et al., 1995; Pfefferbaum et al., 1985; Smith et al., 2008; Vallesi et al., 2009). Age-related ERP studies of the go/nogo task have consistently demonstrated delayed peak latencies for both the N2 and P3 components in older adults (Fallgatter et al., 1999; Horvath et al., 2009; Pfefferbaum and Ford, 1988; Tachibana et al., 1996); however, it has been suggested that this slowing may reflect a general slowing with age versus a selective age-related slowing of inhibition processes (Falkenstein et al., 2002; Vallesi et al., 2009). Vallesi et al. (2009) have recently suggested these effects may reflect older adults strategically using more time to process conflicting stimuli; thus, a purer measure of motor inhibition may be revealed by controlling for strategies at an individual level.
Better control of strategies can be achieved using a stop signal task (Logan et al., 1984) with a dynamically changing “stop” stimuli based on a participant's inhibition accuracy (cf. Rubia et al., 2003). This task has a greater inherent inhibitory load than a go/nogo task in that participants attempt to inhibit an already triggered movement rather than withholding a prepotent response. In addition, the use of a staircase algorithm to ensure participants inhibit on 50% of trials (thus failing on the other 50%) ensures that each individual performs at the limit of their respective inhibitory capacity, without being able to resort to a ‘slowing’ strategy. Similar to the nogo P3, the stop signal P3 has also consistently been interpreted as the more precise indicator of the effectiveness of inhibition compared to the N2 (Beste et al., 2010; Kok et al., 2004; Ramautar et al., 2004). Each component in this N2/P3 complex has been associated with inhibition; however, the inhibitory role of the P3 has been strengthened by studies showing that its source is in or near the primary motor or premotor cortices (Kok et al., 2004; Ramautar et al., 2004). However, to date there has not been an EEG study of the stop signal task in older adults that characterizes how these components change with age.
In the present study, we assessed established neural markers of motor inhibition (N2 and P3 amplitude and latency) and sensory suppression (P1, N170) using a stop signal task and a selective delayed-recognition task, respectively, in the same cohort of older individuals. The goal was to evaluate these measures with an across-participant regression analysis to determine if there was a relationship between the impact of aging on motor inhibition and sensory suppression at the neural level. We hypothesized that those older individuals who showed greater markers of inhibition of motor responses would also show greater sensory suppression abilities. This would suggest a common system of inhibitory control equivalently impacted by aging. Alternatively, age-related declines in the underlying networks mediating each type of inhibition type may not degrade in an equivalent fashion, leading to a non-significant relationship between these factors. This distinction would inform previous behavioral reports that described the construct of inhibition as a distinct set of processes depending on modality (Andres et al., 2008; Collette et al., 2009; Kramer et al., 1994).
2. Methods
2.1. Participants
Twenty healthy older individuals (70.6 ± 6.7 years; range 65–80 years; 11 males) were recruited from the greater San Francisco community through fliers and senior centers, gave consent to participate in the study, and were paid $15/hour for their participation in the study which lasted 3 hours on average. In order to participate, individuals consented to be screened through a UCSF approved online battery of 89 questions that ensured they were right-handed, have normal to corrected vision, and had no medical or neurological history that would likely impact experimental behavioral or neural measures (see http://gazzlabrecruitment.com/limesurveygazzlab/). More specifically, within the 89 question battery are questions probing for potential neurological condition (e.g. schizophrenia, previous head traumas, stroke), previous and current use of psychotropic, hormonal, cardiovascular and cold medications (participants are excluded for current use of psychotropic and thyroid medications) and if there are any physical or mental conditions that may interfere with daily activities (e.g. migraine headaches, substance abuse, neuropathy). Three participants were excluded from the analysis as their performance on the stop signal task suggested that they did not follow the instructions, given that their rate of failed inhibition on the stop signal task was greater than 85%. Another participant whose mean response time (RT) was greater than 2.5 SD from the group mean was excluded. Thus 16 (9 male) participants were included in the analysis.
2.2. Neurocognitive assessments and functioning
Participants were evaluated on 2 separate measures probing for potential cognitive impairments and depression (Mini-Mental State Evaluation (MMSE): Folstein et al., 1975; Geriatric Depression Scale (GDS): Yesavage et al., 1982), and 11 neuropsychological tests prior to experimental testing. This was done to thoroughly characterize the cognitive status of our participants in multiple domains and ensure that their cognitive faculties were comparable to that of their age-matched peers. All participants tested within two standard deviations of the normative values established for each of the following measures, supporting their inclusion in the present study (see Supplementary Table S1). The neuropsychological evaluations consisted of tests designed to assess verbal learning (CVLT-II: Delis et al., 2000; Kramer et al., 1994), visual-spatial function (copy of a modified Rey-Osterrieth figure), visual-episodic memory (memory for details of a modified Rey-Osterrieth figure), visual-motor sequencing (trail making test A and B: Tombaugh, 2004), phonemic fluency (words beginning with the letter ‘D’), semantic fluency (animals), calculation ability (arithmetic), executive functioning (Stroop interference test: Stroop, 1935), working memory (backward digit span, WAIS-R: Wechsler, 1981), and speed of processing (digit symbol, WAIS-R: Wechsler, 1981). All neuropsychological test scores are summarized in Supplementary Table S1.
2.3.1 Experimental Procedures and Stimuli: Selective attention, delayed-recognition task
The paradigm was composed of three different conditions (see Figure 1). Each condition consisted of the same basic temporal design, such that they all required viewing four images: two faces and two scenes presented in randomized order, each being displayed for 800 ms (200 ms between images), followed by a 9000 ms delay period in which the images were to be remembered and mentally rehearsed. After the delay, a third image appeared for 1000 ms (Probe). The participant was asked to respond with a button press (as quickly as possible without sacrificing accuracy) indicating whether the third image (Probe) matched one of the previous images. Following this period, a 3500 ms presentation of the fixation cross was used to fill the intertrial interval (ITI). The tasks differed in the instructions given at the beginning of each run.
Figure 1.
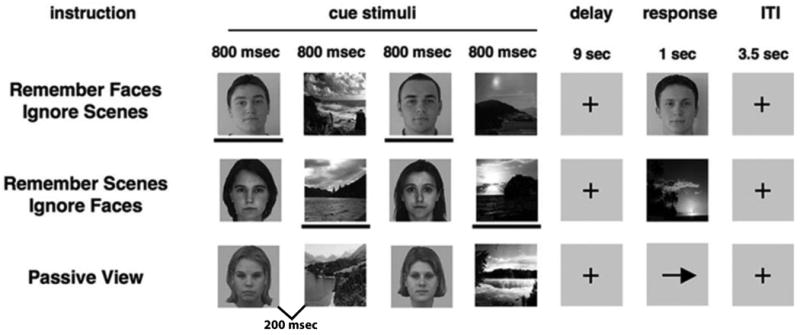
Schematic illustration of the selective attention, delayed-recognition task. In this task, participants were required to report with a button press whether the probe stimulus matched one of the previously presented stimuli (a face or scene stimulus). In the passive view response period, an arrow was presented, and participants were required to make a button press indicating the direction of the arrow. The lines below the stimuli are used to highlight task-relevance in this illustration and were not present in the actual task.
For the Face Memory condition, the participants were asked to remember only the face stimuli and to ignore the scene stimuli. Correspondingly for the Scene Memory condition, participants were asked to remember only the scene stimuli and ignore the faces. When the probe image appeared, it was composed of a face in the face memory conditions, or a scene in the scene memory conditions. In the Passive View, participants were instructed to relax and view the stimuli without trying to remember them. Instead of a probe image, an arrow was presented where participants were required to make a button press indicating the direction of the arrow. The task was presented in 3 separate runs (20 trials each) with each of the three conditions in random order, resulting in 60 trials per condition with 120 EEG segments (2 stimuli/trial). Conditions and stimuli were counterbalanced across participants. The stimuli consisted of grayscale images of faces and natural scenes. All face and scene images were novel across all conditions and across all runs of the experiment. Images were 225 pixels wide and 300 pixels tall (14-18 cm). The face stimuli consisted of a variety of neutral-expression male and female faces across a large age range. The sex of the face stimuli was held constant within each trial, and each stimulus was used in only one trial.
2.3.2 Experimental Procedures and Stimuli: Stop signal task
Participants saw arrows (1000 ms duration) pointing either to the left or to the right, with a jittered inter-stimulus interval (ISI) between 1.6 and 2.0 seconds to optimize statistical efficiency (see Figure 2). Participants were instructed to make a button response with their right index (arrow pointing left) or middle (arrow pointing right) finger corresponding to the arrow direction as fast as possible. However, arrows pointing left or right were followed by a single arrow pointing upward on 25% of the trials. This vertical arrow signaled the participants to inhibit their motor response (‘STOP’). The time interval of 250 ms between go-signal and stop signal onsets changed idiosyncratically, according to each participant's performance. It became 50 ms longer after a successful inhibition (making it harder to inhibit) and 50 ms shorter after an unsuccessful inhibition (making it easier to inhibit). The tracking algorithm ensured that the task was equally challenging and difficult for each individual, providing approximately 50% successful inhibition (SI) and 50% failed inhibition (FI) trials.
Figure 2.
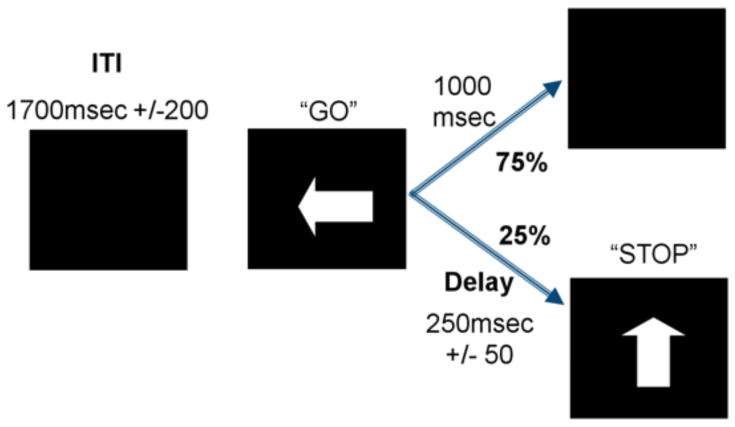
Schematic illustration of the stop signal task. The interval between horizontal (“GO” signal; 75% of trials) and vertical arrows (“STOP” signal; 25% of trials) in the stop trials becomes shorter/longer in steps of 50 ms depending on each participant's performance, ensuring 50% of correctly inhibited and 50% failed stop trials for each participant.
Participants were told to react as quickly as possible while maintaining a high level of accuracy. The primacy of the reactions to the “GO” stimuli was emphasized at the end of each block of trials, reminding participants not to delay their response in anticipation of the stop signal, as it would not always be possible to withhold their response after detection of the stop signal. To encourage participants to respond as quickly as possible, participants' mean RT to the “GO” trials was displayed following each block of 100 trials, along with the message, “The fastest average response time for your age group is currently 552 ms, so try to reach or beat it!” This time was taken from the mean of 6 piloted older adult participants who performed only the behavioral version of the task (data not presented here). Participants also performed 1 run of 80 trials (without any stop trials) to assess basic reaction time (RT) function on ‘GO’ trials, as well as 1 block of 80 trials of the stop signal task (with stop trials) as practice.
2.4 EEG Recording
Participants were seated in an armchair in a dark room with the screen 85cm from the participants' eyes. Neural data were recorded using an Active Two head cap (Cortech-Solutions) with a BioSemiActiveTwo 64-channel EEG acquisition system in conjunction with BioSemiActiView software (Cortech-Solutions), which utilizes a fixed gain that allows for an input range of +262 to -262mV (for hardware specifications, see http://www.biosemi.com/activetwo_full_specs.htm). EEG recordings were also taken at five external electrodes: bilateral mastoid (M1, M2), right EOG (REOG), left EOG (LEOG), and inferior EOG under the right eye (IEOG). In addition, The BioSemi Active Two system utilizes a feedback loop between two separate electrodes (the common mode sense active electrode (CMS) located between the POz & PO3, and the driven right leg passive electrode (DRL) located between POz & PO4) to drive the reference voltage. Thus, any one electrode could act as the reference; in our case, the average reference was composed of the mean voltage of all 64 channels (calculated offline). Signals were amplified and digitized at 1,024 Hz with a 16-bit resolution. All electrode offsets were <25 kΩ. Antialiasing filters were used and data were band-pass filtered between 0.01-100 Hz during data acquisition.
2.5 EEG Data Analysis
Preprocessing was conducted using Analyzer software (Brain Vision, LLC) then exported to EEGLAB (http://sccn.ucsd.edu/eeglab) for subsequent analyses. Blinks and eye-movement artifacts were removed through an independent components analysis (ICA), with the raw EEG-data then referenced to an average reference composed of the mean voltage of all 64 channels off-line. Epochs then cleaned of excessive peak-to-peak deflections using a voltage threshold of 75 mV. For the delayed-recognition task, epochs were created beginning 200 ms before stimulus onset (i.e. each face or scene image) and ending 800 ms afterwards (-200 to 0 ms baseline corrected). Face and scene trials were separately segmented and averaged with only the encoding-period segments from correct trials considered. Our focus was directed at ERPs associated with face stimuli, given that the ERP analysis of scene stimuli had previously not shown significant enhancement or suppression in young adults (Gazzaley et al., 2008) and matched the approach taken by previous studies (Clapp and Gazzaley, 2010; Gazzaley et al., 2008). ERP statistics were calculated using amplitudes and latencies obtained from each participant, with these values obtained via an 8 ms window centered around each participant's peak amplitude deflection for each the component of interest (± 4 ms), replicating the electrode of interest procedure (see 2.6.1 for more details) used by Gazzaley et al. for this paradigm (Clapp and Gazzaley, 2010; Gazzaley et al., 2008).
For the stop signal task, SI and FI trials were time-locked to the onset of the stop signal. To account for SI and FI waveforms being contaminated with activity related to the preceding “GO” stimulus, the regression method outlined by Knyazev et al. (2008) was utilized. This method, unlike the ADJAR method (cf. Woldorff, 1993), allows for corrections on a single trial basis. Epochs beginning 800 ms before “STOP” stimulus onset and ending 1200 ms after stimulus onset were utilized to ensure capturing any contamination from the preceding “GO” stimulus. Responses time-locked to the “GO” stimulus were averaged across the trials for each participant at every electrode site. Next, for each single trial, the respective EEG waveform was regressed on the averaged response ERP and residuals were used for the subsequent analysis of ERPs time-locked to the stop signal. These regressed waveforms were then segmented into 1000 ms epochs (-200 to 800 ms) and utilized in all subsequent analyses. “GO” ERPs were excluded from subsequent analyses to focus solely on trials with an inhibitory component.
2.6.1 Electrode Selection: Selective attention, delayed-recognition task
To select electrodes for statistical analyses, we utilized an electrode of interest (EOI) approach to identify the electrode most sensitive to the neural responses associated with the task stimuli (Bach and Hoffmann, 2000; Berry et al., 2009; Gazzaley et al., 2008; Hoffmann et al., 1999; Hoffmann et al., 2001; Maurer and Bach, 2003; Mercier et al., 2009; Rutman et al., 2010). For consistency with the approach used by Gazzaley et al. (Gazzaley et al., 2008), we combined responses to all stimuli of one class (i.e., faces) that were viewed throughout the experiment (i.e., collapsed across all tasks), and chose the posterior electrode (among the following posterior electrodes: P10, PO8, P8, O2, P9, PO7, P7, and O1) with the largest response at the group level. The P1 component was identified as the first positive deflection appearing between 50 and 150 ms after stimulus onset at electrode P10. The N170 component was identified as the maximal negative peak between 120 and 220 ms after stimulus onset, also at electrode P101. Planned paired t-tests were used to analyze each modulation index (enhancement: relevant - passive; suppression: passive - irrelevant) for each ERP component at the EOI for both latency and amplitude, as previously utilized for this paradigm (Berry et al., 2009; Clapp and Gazzaley, 2010; Clapp et al., 2010; Gazzaley et al., 2008; Zanto et al., 2010a).
2.6.2 Electrode Selection: Stop signal task
Based on previous ERP studies using the stop signal task (Kok et al., 2004; Liotti et al., 2005; Overtoom et al., 2002; Pliszka et al., 2000; Ramautar et al., 2004; Schmajuk et al., 2006), we selected time windows around the stop-N2 (180–260 ms) and the stop-P3 components (260–480 ms) at midline electrodes (Fz, FCz, Cz, CPz, Pz) for analysis. Using the same approach described above, we selected an EOI from these electrodes for each ERP by collapsing across SI and FI conditions, which led to the use of the FCz electrode in each case. Given that the P3 component has consistently been interpreted as the more precise indicator of the effectiveness of motor inhibition as compared to the N2 (Falkenstein et al., 1995; Pfefferbaum et al., 1985; Roberts et al., 1994), our subsequent analyses focused on this component during SI trials. However, we report the findings of the same analyses using the N2 component to provide full disclosure given that other studies have also associated this component with inhibition processes. FI trials were not included in this analysis due to the potential that error-related activation may contaminate the underlying inhibition processes and also to mirror the delayed-recognition task analysis, which only examined correct trials.
2.7.1 Analytical approach: Selective delayed-recognition task
For the selective delayed-recognition task, we recorded each participant's accuracy and response time to the probe stimuli for attended faces, passively viewed faces, and attended scenes (where participants ignored faces). For the neural markers (P1 peak amplitude, N1 peak latency), two separate Repeated Measures ANOVAs were run to test for a main effect among the three conditions (attended faces, passively viewed faces, ignored faces). When a main effect was observed, ERP modulation indices of top-down enhancement (attend – passive) and suppression (ignore – passive) were further evaluated using paired t-test. Given that we previously utilized this task in older adults, we also quantitatively compared behavioral and neural measures from the current dataset to those of our previous study (Gazzaley et al., 2008) to assess consistency of these measures.
2.7.2 Analytical approach: Stop signal task
For the stop signal task, we recorded each participant's response time to ‘GO’ stimuli, their mean stop signal delay, and then calculated SSRT from these variables to assess each individual's inhibition response time. We also qualitatively compared performance of our older adult cohort on the stop signal task with previous studies from others utilizing this task in older adults to reveal that performance of our cohort was similar to previous findings. To ensure that SSRT effects were not driven by executive speed of processing declines, we correlated this measure with RT to ‘GO’ trials during the task, RT from the Response time task, and performance on the digit symbol task. For the neural markers (ERP type: N2 peak amplitude, P300 peak amplitude, N2 peak latency, P300 peak latency), four separate Repeated Measures ANOVAs were run to test for a main effect among successful vs. failed inhibition trials for each ERP measure.
2.7.3 Analytical approach: Neural inter-task relationships
To test for relationships between these sensory and motor inhibition tasks, each of the neural ERP measures established for each task (Sensory: P1 amplitude, N1 latency; Motor: N2 & P3 amplitude, N2 & P3 latency) were entered into a Pearson's bivariate correlation matrix with the two-tailed level of significance set at p=.05. The use of all measures in the correlation matrix ensured that any potential relationship(s) between these measures of inhibition could emerge. In addition, to ensure the data followed assumptions of a normal distribution, we performed the Shapiro-Wilk test of normality on each of the measures entered into the correlational analysis. This approach was adopted given: i) the relatively modest sample size in the present study, ii) the cognitive aging literature would theoretically support either a significant or non-significant relationship amongst these neural measures, and iii) the most informative relationship between these different ERPs has not been clearly established. We also computed 95% confidence intervals for our sample size at a significance level of p= .05 (thus, an r-value of .497 would reach significance), as well as for each individual inter-task correlation to probe the range of correlations observed. Given that behavioral inter-task relationships testing between sensory & motor inhibition have already shown no inter-task relationship, we focused our correlational analyses on neural markers (although behavioral correlations are evaluated and reported).
3. Results
3.1.1 Behavioral results: Selective attention, delayed-recognition task
Recognition accuracy (hits + correct rejections/total responses) for faces and scenes (faces: 86.3% ± 10.9; scenes: 81.1% ± 11.9) were comparable to previous results using this paradigm in older adults (faces: 88.2% ± 10.3, p> .75; scenes: 83.0% ± 9.1, p> .75; (Gazzaley et al., 2008)). Similarly, the RTs observed in each condition (faces: 1187 ms ± 268 ms; scenes: 1269 ms ± 259 ms) were also comparable to the RTs previously reported (faces: 1280 ms ± 294 ms; p> .15; scenes: older 1362 ms ± 306 ms; p> .24; (Gazzaley et al., 2008)). See Table 1.
Table 1. Behavioral results: mean (standard dev.).
| Stop signal task | |
|
| |
| “GO” trial accuracy | 95.5% (5.3) |
| “GO” trial RT | 574 ms (102) |
| % of Successfully inhibited stop trials | 50.9% (7.5) |
| Stop signal delay | 272 ms (102) |
| SSRT | 302 ms (50) |
| RT task | |
|
| |
| RT task accuracy | 98.9% (2.5%) |
| RT task RT | 491 ms (109) |
| Selective, delayed-recognition task | |
|
| |
| Accuracy for faces | 86.3% (10.9) |
| RT for faces | 1187 ms (268) |
| Accuracy for scenes | 81.1% (11.9) |
| RT for scenes | 1269 ms (259) |
| Accuracy for passive view | 98.3% (3.7) |
| RT for passive view | 745 ms (224) |
3.1.2 Behavioral results: Stop signal task
Participant accuracy and RTs on “GO” trials for the stop signal task were 95.5% ± 5.3% and 574 ms ± 102. The mean percentage of successfully inhibiting on “STOP” trials was 50.9% ± 7.5%, demonstrating the effectiveness of the adaptive staircase algorithm that was designed to achieve 50% successful inhibition. The mean stop signal onset following the “GO” stimuli was 272 ms ± 102 ms, with a mean stop signal reaction time (SSRT) of 302 ms ± 50 ms; i.e., the time required by a participant to successfully inhibit a movement (mean “GO” RT - stop signal onset; see Table 1). While the mean stop signal onset correlated with participant's age (r= .52, p< .05), SSRT did not (r= -.01, p> .90). Importantly, older adults' SSRT was comparable to values from previously reported age-related stop signal studies (329 ms: Bedard et al., 2002; Kramer et al., 1994). SSRT did not correlate with participant's RT to “GO” trials during the stop signal task (r= .13, p> .60), the RT task (r= .03, p> .80), or digit symbol performance (r= .12, p> .60), suggesting that this measure of motor inhibition was not driven by executive speed of processing declines.
3.2.1 Neural results: Selective attention, delayed-recognition task
Following previous work from this laboratory (Clapp and Gazzaley, 2010; Gazzaley et al., 2008; Zanto et al., 2010a), ERP modulation analyses for face stimuli focused on P1 amplitude and N170 latency as indices of top-down enhancement (attend – passive) and suppression (ignore – passive). Figure 3 displays the EOI grand averaged waveforms time-locked to the onset of face stimuli for attended, ignored and passively viewed stimuli (see Supplementary Figure S1 for full 64 electrode montage). There was an effect of condition for the N170 latency (F(2,30) = 5.65, p< .05) unlike P1 amplitude (F(2,30) = 1.20, p> .30), with the values for each condition shown in Table 2. Neural enhancement of relevant stimuli was significant, as the N170 latency was earlier when attended versus passively viewed (t(15) = -3.35, p< .005; Fig. 3), with these findings being comparable to previous results using this task in older adults (p> .35 for each component, Gazzaley et al., 2008); however, significant P1 enhancement to the relevant stimuli was not evident (t(15) = .59, p> .45; Fig. 3). Importantly, suppression of irrelevant stimuli was not present for either the P1 amplitude or the N170 latency (i.e., N170 latency for ignored faces was not different than passively viewed faces (t(15) = -.72, p> .45); P1 amplitude for ignored faces was not different than passively viewed faces (t(15) = -1.35, p> .20), with these findings being the same as previous results using this identical paradigm in older adults (p> .30 for each component, Gazzaley et al., 2008, Fig. 3). Thus, these results replicate previous findings that older adults do not display significant suppression indices for P1 amplitude and N170 latency (Clapp and Gazzaley, 2010; Gazzaley et al., 2008; Zanto et al., 2010a).
Figure 3.
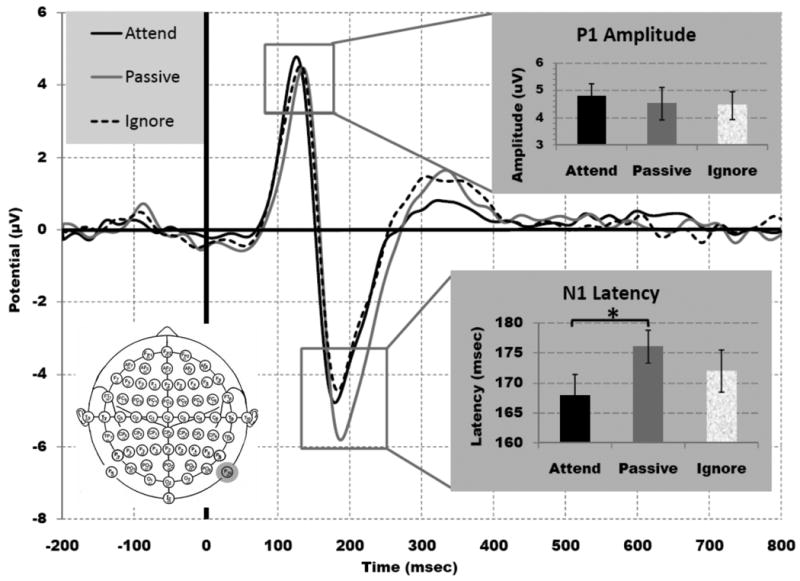
ERPs from the selective attention, delayed-recognition task at the electrode of interest where the effect was greatest across all conditions, P10 (grey circle). The solid black line indicates attended faces, dashed black line indicates ignored faces and the solid gray line represents the passive view condition. Both P1 amplitude and the N170 latency displayed a suppression deficit, while only N170 latency also showed an enhancement effect.
Table 2. Mean peak amplitudes and latencies (standard error).
| Stop signal task (successful inhibition) | |
|
| |
| N2 amplitude | -1.3 (.3) |
| N2 latency | 217 (8) |
| P3 amplitude | 1.5 (.4) |
| P3 latency | 341 (12) |
| Selective, delayed-recognition task | |
|
| |
| P1 amplitude | |
| Attend | 4.8 (.5) |
| Passive | 4.5 (.6) |
| Ignore | 4.3 (.6) |
| P1 latency | |
| Attend | 118 (2) |
| Passive | 123 (4) |
| Ignore | 121 (3) |
| N170 amplitude | |
| Attend | -5.1 (.7) |
| Passive | -6.0 (.7) |
| Ignore | -5.2 (.6) |
| N170 latency | |
| Attend | 168 (4) |
| Passive | 177 (3) |
| Ignore | 173 (4) |
3.2.2 Neural results: Stop signal task
A N2/P3 complex was observed over the electrode of interest for the SI and FI trials, with the values for each condition shown in Table 2. Figure 4 displays grand average of ERPs time-locked to the onset of the stop signal stimuli for both the SI and FI trials at the FCz electrode (see Supplementary Figure S2 for full 64 electrode montage). No significant difference was observed between SI and FI trials for N2 amplitude (F(1,15) = 3.17, p> .10) or P3 amplitude (F(1,15) = .009, p> .90). However, a trial type difference was observed with respect to N2 peak latency, such that SI trials had an earlier peak onset than FI trials (F(1,15) = 8.57, p< .01), with the same pattern observed for P3 peak latency (F(1,15) = 10.13, p< .01). Given that young adults typically display increased N2 & P3 amplitudes on FI versus SI trials (Knyazev et al., 2008; Kok et al., 2004; Ramautar et al., 2004; van Boxtel et al., 2001), these findings suggest age-related changes in response inhibition may be better reflected through peak latency versus peak amplitude. This interpretation is supported by P3 latency for SI trials showing a positive correlation with SSRT (r> .60, p< .05), unlike N2 latency (r< .10, p> .80), P3 amplitude (r< .30, p> .20), and N2 amplitude (r< .30, p> .20). Finally, participants mean RT to “GO” stimuli did not correlate with any of the ERP measures (r> .19, p> .45 for all comparisons), suggesting individual RT was not the underlying factor driving the observed ERP effects.
Figure 4.
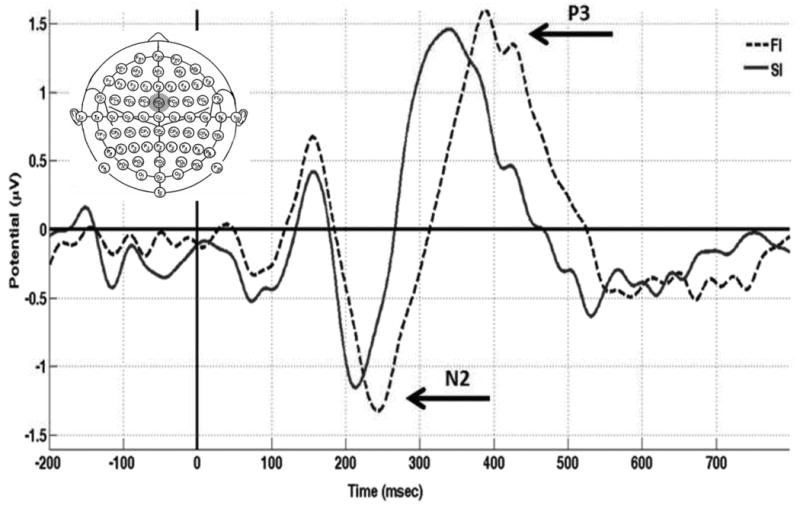
ERPs time-locked to STOP signal for successful inhibition (SI; bold line) and failed inhibition (FI; dotted line) stop trials averaged across all participants at the electrode of interest where the effect was greatest across all conditions, electrode FCz (grey circle). There was a significant difference between conditions for the latency, but not for amplitude, for each component (N2/P3).
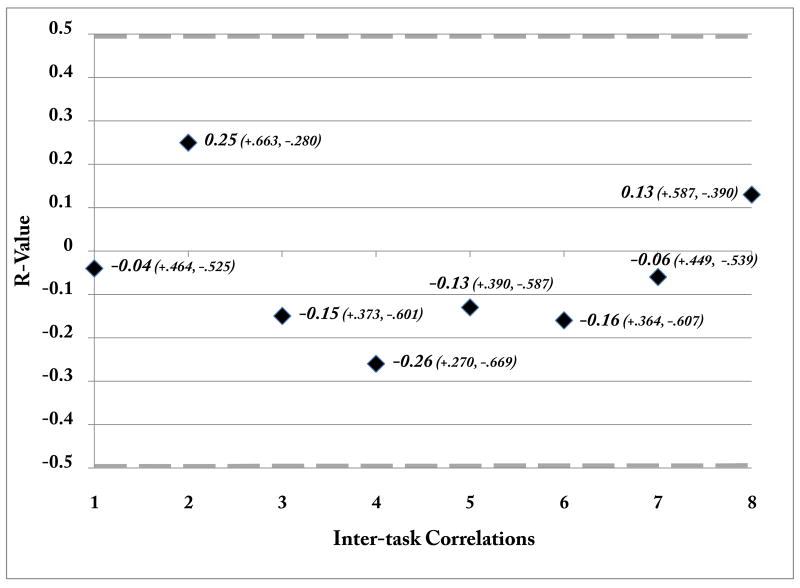
3.3 Inter-task inhibition correlations
While we did observe a correlation in RT between tasks (r = .722, p< .0001), there was no correlation between SSRT (measure of motor inhibition) and either face stimuli RT (r< .13, p>.60) or accuracy (r< .03, p>.90) on the delayed-recognition task, which is line with previous behavioral work that showed no significant correlation between sensory and motor inhibition measures (Kramer, Humphrey, 1994). To evaluate the potential relationship between neural indices of motor and sensory suppression, across-participant regression analyses were performed using neural modulation indices of sensory suppression (N170 peak latency and P1 peak amplitude indices) and neural measures of motor inhibition (P3 peak latency & amplitude; N2 peak latency & amplitude). Note that positive and negative values reflecting the direction of each ERP component's amplitude were utilized for this analysis, as opposed to absolute values. Furthermore, the Shapiro-Wilk test of normality revealed that there was a normal distribution for each of the measures entered into the correlational analysis (p> .120 for each measure). This analysis revealed no significant correlations (Table 3) among any of the inter-task measures (p> .45 for all comparisons), although there was a significant correlation between N2 and P3 peak amplitudes within the stop-signal task (r= -.83, p< .0001). Furthermore, the inter-task correlations observed show a consistent small range of r-values, with 6 out of the 8 falling between .2 and -.2 (see Figure 5). These results are consistent with previous behavioral findings that revealed no relationship between motor inhibition and sensory suppression in older adults (Kramer et al., 1994).
Table 3. Inter-task neural correlations.
| Neural Measure | N170 Lat | P1 Amp | P3 Lat | P3 Amp | N2 Lat | N2 Amp |
|---|---|---|---|---|---|---|
| 1. N170 Peak Latency (Sensory Suppression; N170 Lat) | - | - | - | - | - | - |
| 2. P1 Peak Amplitude (Sensory Suppression; P1 Amp) | -0.17 | - | - | - | - | - |
| 3. P3 Peak Latency (Motor Inhibition; P3 Lat) | -0.04 | -0.13 | - | - | - | - |
| 4. P3 Peak Amplitude (Motor Inhibition; P3 Amp) | 0.25 | -0.16 | -0.03 | - | - | - |
| 5. N2 Peak Latency (Motor Inhibition; N2 Lat) | -0.15 | -0.06 | 0.37 | 0.32 | - | - |
| 6. N2 Peak Amplitude (Motor Inhibition; N2 Amp) | -0.26 | 0.13 | -0.09 | -.83** | -0.29 | - |
Correlation is significant at the 0.001 level (2-tailed).
Figure 5.
Inter-task correlations. R-values for each inter-task correlation are presented, with the 95% confidence interval for each correlation presented in parentheses. The grey dashed line at -.497 and +.497 represent p< .05 level of significance.
4. Discussion
In the current study, EEG was used to elucidate the relationship between response inhibition and the suppression of irrelevant visual information (i.e. sensory suppression). Non-significant correlations for each neural measure suggest that aging is not associated with a generalized inhibitory deficit. Rather, these results support previous behavioral findings regarding independence of inhibitory abilities in the setting of age-related cognitive changes. This study also marks the first evaluation of ERPs in older adults performing the stop signal task. These findings are discussed further with respect to theoretical implications posed in the cognitive aging literature.
Aging and sensory suppression
These results replicate previous findings that older adults do not exhibit the signatures of early neural suppression when viewing irrelevant visual stimuli. More specifically, older adults showed comparable behavioral performance on the working memory task (Gazzaley et al., 2008) and cortical activity associated with task-irrelevant stimuli (P1 amplitude, N170 latency: Clapp and Gazzaley, 2010; Gazzaley et al., 2008; Zanto et al., 2010a) as in previous studies. It is unclear why the present cohort of older adults did not show a significant P1 enhancement effect as previous work; however, there was enhancement as well as the previously characterized (lack of) suppression observed for the N170 component, supporting its use as the primary measure of sensory suppression. These findings provide further support for the inhibitory deficit hypothesis of cognitive aging (Hasher and Zacks, 1988; Hasher et al., 1999) that attributes multiple facets of cognitive decline (notably WM) in older adults to an inability to suppress irrelevant information.
Aging and motor inhibition
As previously mentioned, older adults' performance during the stop signal task was comparable to those previously reported in age-related stop signal studies (Bedard et al., 2002; Kramer et al., 1994), suggesting that the paradigm was sensitive to age-related effects. In each of those studies, older adults' SSRT was greater than that observed in young adults, suggesting that the present data would also be reflective of an age-related deficit in motor inhibition in this population. Importantly, this age-related slowing of SSRT has been shown to not be simply a reflection of declining speed of processing: Andres et al. (2008) demonstrated that when speed of processing was taken into account, the speed of inhibiting an executed action remained slower in older adults. These findings are further supported by Shilling et al. (Shilling et al., 2002), whose inclusion of age as a continuous variable facilitated parsing out how inhibitory deficits can be affected by age as opposed to other factors like slowed response time.
Older participants showed a qualitatively comparable N2/P3 waveform complex to previous ERP studies using a stop signal task in young adults (Dimoska et al., 2006; Kok et al., 2004; Ramautar et al., 2004; van Boxtel et al., 2001). However, older adults displayed equivalent N2 and P3 peak amplitudes in FI vs. SI trials, while N2 and P3 peak latencies exhibited a consistently later peak in FI vs. SI trials, a pattern similarly observed in younger adults. Given that the N2 is thought to reflect aspects of conflict/error processing (Gajewski et al., 2010; Kok et al., 2004; Ramautar et al., 2004), the equivalent N2 amplitudes may reflect older adults interpreting SI and FI trials as having an equivalent degree of conflict. Interestingly, there was a strong negative correlation between N2 and P3 peak amplitudes during successfully inhibited trials, suggesting individuals with a greater N2 showed a correspondingly larger P3. This relationship suggests that these pre-motor inhibitory processes may influence the ability to successfully inhibit an executed action (cf. Beste et al., 2010). However, these interpretations should be considered speculative given that the present study was not designed to dissect age-related interactions between conflict and inhibition.
Young adults with the fastest RTs to “GO” stimuli have shown increased P3 peak amplitudes during a stop signal task (Dimoska et al., 2006). These authors surmised that these fast-acting individuals require equally fast acting inhibition processes to inhibit their “GO” process. In the present study, there was no amplitude difference observed between SI and FI trial types for the P3 component, nor did P3 amplitude correlate with either RT to “GO” stimuli or SSRT. This finding suggests that P3 amplitude in older adults may not be the most accurate metric of evaluating effective inhibition processes. Rather, the correlation between SSRT and P3 latency suggests P3 latency may be a more indicative measure for evaluating effective motor inhibition processes in older adults. However, given that this relationship has not been observed in stop signal studies of young adults, further investigation is warranted to support the interpretation posed here.
Theoretical implications
The present results inform the conclusions of three prominent age-related hypotheses: the dedifferentiation hypothesis, the inhibitory deficit hypothesis, and the frontal theory of aging. Li and Lindenberger's (1999) dedifferentiation theory suggests older adults' asymmetry reductions reflect an inability to engage specific neural mechanisms, with support for this theory evidenced by correlations between distinct cognitive measures increasing with age (Baltes and Lindenberger, 1997; Mitrushina and Satz, 1991). However, the prediction of a melding of inhibition-related processes was not supported by the results here, suggesting that the distinctness of inhibition mechanisms is preserved with aging.
Hasher and Zacks' (1988; 1999) inhibitory deficit theory proposes decreased inhibitory processing efficiency accounts for deficits in both cognitive and motor inhibitory domains. Previous work has extended the principles of this theory to span different inhibitory domains in older adults using behavioral metrics (Butler and Zacks, 2006; Ossmann and Mulligan, 2003). However, the pressing question for this theory is whether these declines are equivalent across domains for given individuals. Again, the present findings suggest no relationship between inhibitory measures across individuals, suggesting the possibility of an over-generalization of the inhibitory deficit hypothesis in this regard. These results also corroborate previous findings that failed to observe a disproportionate preservation of motor or perceptual inhibitory performance (Germain and Collette, 2008; Kramer et al., 1994), arguing against a generalized failure of inhibitory mechanism with aging.
Finally, the frontal lobe hypothesis (Arbuckle and Gold, 1993; Greenwood, 2000; Hartley, 1993; West, 1996) proposes that age-related inhibitory declines are a direct reflection of changes in the integrity of the frontal cortex. This hypothesis associates degradation of frontal cortices with the increased intrusion of irrelevant information (Andres et al., 2006; Gazzaley and D'Esposito, 2007; Kok, 1999) and with increased difficulty inhibiting irrelevant responses (Andres et al., 2008; Mani et al., 2005; Wild-Wall et al., 2008). Kramer et al.'s (1994) examination of whether age-related decreases in inhibitory processing is generalized across distinct behavioral measures of inhibition led to the conclusion that these failures are specific rather than general in nature. This proposal was spurred by i) the presence of age-related deficits on the stop signal task and the WCST, given each tasks' association with frontal lobe dysfunction (Arbuckle and Gold, 1993; Drewe, 1974; Heaton, 1981; Kramer et al., 1994; Milner, 1963; Raz et al., 2000; Ridderinkhof et al., 2002) and ii) limited evidence for associations among inhibitory measures acquired from different tasks.
While Kramer et al. (1994) suggested that the pattern of data obtained fit well with the frontal lobe model of aging, they also acknowledge the possibility that multiple inhibitory networks decrease at different rates during the course of aging. The present study cannot directly address this hypothesis as the low spatial resolution associated with EEG prevents the confirmation of the distinct deterioration of these frontal regions. However, the ERPs used here do provide further evidence for what Kramer et al. (1994) suspected: age-related declines within each inhibitory mechanism do not lead to a correlated decline or a generalized inhibitory function. While frontal lobe hypothesis appears able to account for inhibitory failures in each domain, it remains unclear how it would account for differential age-related prefrontal declines and their impacts upon distinct types of inhibition.
Indeed, one persistent critique of the frontal lobe hypothesis involves conflicting evidence regarding whether frontal regions are truly more sensitive than other regions in their age-related declines (Greenwood, 2000). As non-prefrontal regions engaged in these tasks may be differentially impacted by aging, how this nondescript degradation may have affected the observed findings is unclear. In spite of these effects, the primary source of top-down modulation for these tasks has been shown to engage distinct prefrontal regions. Using fMRI, the delayed-recognition task used here has localized its source of top-down modulation to the left middle frontal gyrus (Clapp et al., 2010; Gazzaley et al., 2007), while fMRI of the stop signal task has suggested that inhibition is being mediated by the right inferior frontal gyrus (Aron et al., 2003; Li et al., 2008; Rubia et al., 2003). Thus, the present findings point to a differential decline for each type of inhibition in aging supporting anatomically distinct control regions/networks for each type of inhibition rather than an equivalent age-related impact of aging.
5. Conclusions
The present findings reveal that inhibitory functions are differentially impacted across older individuals and suggest that inhibition is mediated by distinct processes underlying specific cognitive demands. This interpretation is consistent with behavioral findings by Kramer et al. (1994), concluding that age-related inhibitory decline is specific, rather than general in nature. Thus, age-related brain changes do not result in a generalized state of inhibitory impairment. However, more stringent characterization of these findings would still be appropriate, given that one could argue that the non-significant correlations observed could have resulted from having a relatively small sample size or elevated within-task variability. While the confidence intervals calculated for each individual correlation are relatively large and prevent us from making definitive statements about no effect being present, the actual values for each correlation are consistent. Thus, while our interpretations of a null effect in the present study show a consistent pattern, they should not be interpreted as a definitive non-effect. In addition, while the present ERP findings for the delayed working memory task showed remarkable consistency with respect to previous work from this laboratory, the replication of older adult ERPs using the stop signal task would provide another example of the age-related variability for this task. Nevertheless, the present findings provide new evidence regarding the association of age-related changes in inhibitory control in addressing an important question in the aging literature that has only received relatively faint consideration in the past.
Supplementary Material
Supplementary Figure S1. 64 electrode montage of the ERPs from the selective attention, delayed-recognition task averaged across all participants. The black line indicates the ERP to attended faces stimuli, the red line reflects ERPs to passively viewed faces, and the blue line reflects the ERPs to ignored face stimuli.
Supplementary Figure S2. 64 electrode montage of the ERPs from the stop-signal task averaged across all participants. The blue line indicates ERPs time-locked to STOP signal for successful inhibition (SI) stop trials, while the red line reflects ERPs time-locked to the STOP signal for failed inhibition (FI) stop trials.
Highlights.
Older individuals showed neural markers of sensory enhancement and suppression in line with previous work (Gazzaley et al., 2008).
ERP markers of motor inhibition (N2 and P3 amplitude and latency) are presented for the first time in a cohort of older individuals performing a stop-signal task.
Distinct inhibitory functions (motor, sensory) are differentially impacted in normal aging, as evidenced by behavioral and neural findings using EEG.
Acknowledgments
We thank N Barbhaiya, K Lyman, and B Yang, for their help with data collection and analysis, B Benson for support with the stop signal task, G Knyazev for assisting with the scripting of the component overlap removal procedure, J Boccanfuso for assistance with all of the neuropsychological evaluation measures, and J Bollinger, J Kalkstein, E Morsella, J Pa, A Thangavel, P Wais, and T Zanto for edits on earlier versions of this manuscript. This work was supported by NIH R01-AG030395 (AG).
Footnotes
P10 was also the electrode of interest identified using the same paradigm in older adults for both the P1 and N170 components (Gazzaley et al., 2008).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alain C, Woods DL. Age-related changes in processing auditory stimuli during visual attention: evidence for deficits in inhibitory control and sensory memory. Psychol Aging. 1999;14:507–19. doi: 10.1037//0882-7974.14.3.507. [DOI] [PubMed] [Google Scholar]
- Andres P, Guerrini C, Phillips LH, Perfect TJ. Differential effects of aging on executive and automatic inhibition. Dev Neuropsychol. 2008;33:101–23. doi: 10.1080/87565640701884212. [DOI] [PubMed] [Google Scholar]
- Andres P, Parmentier FB, Escera C. The effect of age on involuntary capture of attention by irrelevant sounds: a test of the frontal hypothesis of aging. Neuropsychologia. 2006;44:2564–8. doi: 10.1016/j.neuropsychologia.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Andres P, Van der Linden M. Age-related differences in supervisory attentional system functions. J Gerontol B Psychol Sci Soc Sci. 2000;55:P373–80. doi: 10.1093/geronb/55.6.p373. [DOI] [PubMed] [Google Scholar]
- Arbuckle TY, Gold DP. Aging, inhibition, and verbosity. J Gerontol. 1993;48:P225–32. doi: 10.1093/geronj/48.5.p225. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–6. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Bach M, Hoffmann MB. Visual motion detection in man is governed by non-retinal mechanisms. Vision research. 2000;40:2379–85. doi: 10.1016/s0042-6989(00)00106-1. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Dev Neuropsychol. 2002;21:93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Rutman AM, Clapp WC, Gazzaley A. Practice-related improvement in working memory is modulated by changes in processing external interference. J Neurophysiol. 2009;102:1779–89. doi: 10.1152/jn.00179.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Willemssen R, Saft C, Falkenstein M. Response inhibition subprocesses and dopaminergic pathways: basal ganglia disease effects. Neuropsychologia. 2010;48:366–73. doi: 10.1016/j.neuropsychologia.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Butler KM, Zacks RT. Age deficits in the control of prepotent responses: evidence for an inhibitory decline. Psychol Aging. 2006;21:638–43. doi: 10.1037/0882-7974.21.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler KM, Zacks RT, Henderson JM. Suppression of reflexive saccades in younger and older adults: age comparisons on an antisaccade task. Mem Cognit. 1999;27:584–91. doi: 10.3758/bf03211552. [DOI] [PubMed] [Google Scholar]
- Butter CM. Perseveration in extinction and in discriminative reversal tasks following selective frontal ablations in Macaca mulatta. Physiol Behav. 1969;4:163–71. [Google Scholar]
- Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cereb Cortex. 1997;7:63–9. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- Clapp WC, Gazzaley A. Distinct mechanisms for the impact of distraction and interruption on working memory in aging. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Gazzaley A. Mechanisms of working memory disruption by external interference. Cereb Cortex. 2010;20:859–72. doi: 10.1093/cercor/bhp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Germain S, Hogge M, Van der Linden M. Inhibitory control of memory in normal ageing: dissociation between impaired intentional and preserved unintentional processes. Memory. 2009;17:104–22. doi: 10.1080/09658210802574146. [DOI] [PubMed] [Google Scholar]
- Connelly SL, Hasher L. Aging and the inhibition of spatial location. J Exp Psychol Hum Percept Perform. 1993;19:1238–50. doi: 10.1037//0096-1523.19.6.1238. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cogn Affect Behav Neurosci. 2002;2:174–86. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Dimoska A, Johnstone SJ, Barry RJ. The auditory-evoked N2 and P3 components in the stop-signal task: indices of inhibition, response-conflict or error-detection? Brain Cogn. 2006;62:98–112. doi: 10.1016/j.bandc.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Drewe EA. The effect of type and area of brain lesion on Wisconsin card sorting test performance. Cortex. 1974;10:159–70. doi: 10.1016/s0010-9452(74)80006-7. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–15. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol Psychol. 1993;35:123–38. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Inhibition-related erp components: Variations with modality, age, and time-on-task. J Psychophysiol. 2002;16:167–75. [Google Scholar]
- Falkenstein M, Koshlykova NA, Kiroj VN, Hoormann J, Hohnsbein J. Late ERP components in visual and auditory Go/Nogo tasks. Electroencephalogr Clin Neurophysiol. 1995;96:36–43. doi: 10.1016/0013-4694(94)00182-k. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Mueller TJ, Strik WK. Age-related changes in the brain electrical correlates of response control. Clin Neurophysiol. 1999;110:833–8. doi: 10.1016/s1388-2457(99)00022-x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Kleinsorge T, Falkenstein M. Electrophysiological correlates of residual switch costs. Cortex. 2010;46:1138–48. doi: 10.1016/j.cortex.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D'Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci U S A. 2008;105:13122–6. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, D'Esposito M. Top-down modulation and normal aging. Ann N Y Acad Sci. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, et al. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17 1:i125–35. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain S, Collette F. Dissociation of perceptual and motor inhibitory processes in young and elderly participants using the Simon task. J Int Neuropsychol Soc. 2008;14:1014–21. doi: 10.1017/S135561770808123X. [DOI] [PubMed] [Google Scholar]
- Gomez Gonzalez CM, Clark VP, Fan S, Luck SJ, Hillyard SA. Sources of attention-sensitive visual event-related potentials. Brain Topogr. 1994;7:41–51. doi: 10.1007/BF01184836. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. The frontal aging hypothesis evaluated. J Int Neuropsychol Soc. 2000;6:705–26. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- Hartley JT. Aging and prose memory: tests of the resource-deficit hypothesis. Psychol Aging. 1993;8:538–51. doi: 10.1037//0882-7974.8.4.538. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks R. The psychology of learning. Academic Press; 1988. Working memory, comprehension, and aging: a review and a new view. [Google Scholar]
- Hasher L, Zacks RT, Rahhal TA. Timing, instructions, and inhibitory control: some missing factors in the age and memory debate. Gerontology. 1999;45:355–7. doi: 10.1159/000022121. [DOI] [PubMed] [Google Scholar]
- Heaton R. A manual for the Wisconsin Card Sorting Test. Odessa, FL: Psychological Assessment Resources; 1981. [Google Scholar]
- Hoffmann M, Dorn TJ, Bach M. Time course of motion adaptation: motion-onset visual evoked potentials and subjective estimates. Vision research. 1999;39:437–44. doi: 10.1016/s0042-6989(98)00186-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann MB, Unsold AS, Bach M. Directional tuning of human motion adaptation as reflected by the motion VEP. Vision research. 2001;41:2187–94. doi: 10.1016/s0042-6989(01)00112-2. [DOI] [PubMed] [Google Scholar]
- Horvath J, Czigler I, Birkas E, Winkler I, Gervai J. Age-related differences in distraction and reorientation in an auditory task. Neurobiol Aging. 2009;30:1157–72. doi: 10.1016/j.neurobiolaging.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Khoe W, Mitchell JF, Reynolds JH, Hillyard SA. Exogenous attentional selection of transparent superimposed surfaces modulates early event-related potentials. Vision research. 2005;45:3004–14. doi: 10.1016/j.visres.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Levin EA, Savostyanov AN. A failure to stop and attention fluctuations: an evoked oscillations study of the stop-signal paradigm. Clin Neurophysiol. 2008;119:556–67. doi: 10.1016/j.clinph.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Kok A. Effects of degradation of visual stimulation on components of the event-related potential (ERP) in go/nogo reaction tasks. Biol Psychol. 1986;23:21–38. doi: 10.1016/0301-0511(86)90087-6. [DOI] [PubMed] [Google Scholar]
- Kok A. Varieties of inhibition: manifestations in cognition, event-related potentials and aging. Acta Psychol (Amst) 1999;101:129–58. doi: 10.1016/s0001-6918(99)00003-7. [DOI] [PubMed] [Google Scholar]
- Kok A, Ramautar JR, De Ruiter MB, Band GP, Ridderinkhof KR. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology. 2004;41:9–20. doi: 10.1046/j.1469-8986.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Humphrey DG, Larish JF, Logan GD, Strayer DL. Aging and inhibition: beyond a unitary view of inhibitory processing in attention. Psychol Aging. 1994;9:491–512. [PubMed] [Google Scholar]
- Li CS, Yan P, Sinha R, Lee TW. Subcortical processes of motor response inhibition during a stop signal task. NeuroImage. 2008;41:1352–63. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Lindenberger U. Cross-level unification: A computational exploration of the link between deterioration of neurotransmitter systems dedifferentiation of cognitive abilities in old age. In: Nilsson LG, Markowitsch HJ, editors. Cognitive Neuroscience of Memory. Seattle: Hogrefe & Huber; 1999. [Google Scholar]
- Liotti M, Pliszka SR, Perez R, Kothmann D, Woldorff MG. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41:377–88. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–91. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Lopez M, Rodriguez V, Valdes-Sosa M. Two-object attentional interference depends on attentional set. Int J Psychophysiol. 2004;53:127–34. doi: 10.1016/j.ijpsycho.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lustig C, Hasher L, Zacks RT. Inhibitory Deficit Theory: Recent Developments in a “New View”. Washington, DC: American Psychological Association; 2007. [Google Scholar]
- Mani TM, Bedwell JS, Miller LS. Age-related decrements in performance on a brief continuous performance test. Arch Clin Neuropsychol. 2005;20:575–86. doi: 10.1016/j.acn.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Maurer JP, Bach M. Isolating motion responses in visual evoked potentials by preadapting flicker-sensitive mechanisms. Experimental brain research Experimentelle Hirnforschung. 2003;151:536–41. doi: 10.1007/s00221-003-1509-2. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L. Synchrony effects in inhibitory control over thought and action. J Exp Psychol Hum Percept Perform. 1998;24:363–79. doi: 10.1037//0096-1523.24.2.363. [DOI] [PubMed] [Google Scholar]
- Mercier M, Schwartz S, Michel CM, Blanke O. Motion direction tuning in human visual cortex. Eur J Neurosci. 2009;29:424–34. doi: 10.1111/j.1460-9568.2008.06583.x. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. Arch Neurol. 1963;9:90–100. [Google Scholar]
- Mitrushina M, Satz P. Changes in cognitive functioning associated with normal aging. Arch Clin Neuropsychol. 1991;6:49–60. [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- Ossmann JM, Mulligan NW. Inhibition and attention deficit hyperactivity disorder in adults. Am J Psychol. 2003;116:35–50. [PubMed] [Google Scholar]
- Overtoom CC, Kenemans JL, Verbaten MN, Kemner C, van der Molen MW, van Engeland H, et al. Inhibition in children with attention-deficit/hyperactivity disorder: a psychophysiological study of the stop task. Biol Psychiatry. 2002;51:668–76. doi: 10.1016/s0006-3223(01)01290-2. [DOI] [PubMed] [Google Scholar]
- Passingham RE. Non-reversal shifts after selective prefrontal ablations in monkeys (Macaca mulatta) Neuropsychologia. 1972;10:41–6. doi: 10.1016/0028-3932(72)90041-3. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM. ERPs to stimuli requiring response production and inhibition: effects of age, probability and visual noise. Electroencephalogr Clin Neurophysiol. 1988;71:55–63. doi: 10.1016/0168-5597(88)90019-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroencephalogr Clin Neurophysiol. 1985;60:423–34. doi: 10.1016/0013-4694(85)91017-x. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Liotti M, Woldorff MG. Inhibitory control in children with attention-deficit/hyperactivity disorder: event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biol Psychiatry. 2000;48:238–46. doi: 10.1016/s0006-3223(00)00890-8. [DOI] [PubMed] [Google Scholar]
- Potter LM, Grealy MA. Aging and inhibition of a prepotent motor response during an ongoing action. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2008;15:232–55. doi: 10.1080/13825580701336882. [DOI] [PubMed] [Google Scholar]
- Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–83. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Kok A, Ridderinkhof KR. Effects of stop-signal probability in the stop-signal paradigm: the N2/P3 complex further validated. Brain Cogn. 2004;56:234–52. doi: 10.1016/j.bandc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–82. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Williamson A, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microsc Res Tech. 2000;51:85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Span MM, van der Molen MW. Perseverative behavior and adaptive control in older adults: performance monitoring, rule induction, and set shifting. Brain Cogn. 2002;49:382–401. doi: 10.1006/brcg.2001.1506. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Rau H, Lutzenberger W, Birbaumer N. Mapping P300 waves onto inhibition: Go/No-Go discrimination. Electroencephalogr Clin Neurophysiol. 1994;92:44–55. doi: 10.1016/0168-5597(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage. 2003;20:351–8. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Rush BK, Barch DM, Braver TS. Accounting for cognitive aging: context processing, inhibition or processing speed? Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2006;13:588–610. doi: 10.1080/13825580600680703. [DOI] [PubMed] [Google Scholar]
- Rutman AM, Clapp WC, Chadick JZ, Gazzaley A. Early top-down control of visual processing predicts working memory performance. Journal of cognitive neuroscience. 2010;22:1224–34. doi: 10.1162/jocn.2009.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmajuk M, Liotti M, Busse L, Woldorff MG. Electrophysiological activity underlying inhibitory control processes in normal adults. Neuropsychologia. 2006;44:384–95. doi: 10.1016/j.neuropsychologia.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Hopf JM, Martinez A, Mai HM, Sattler C, Gasde A, et al. Spatio-temporal analysis of feature-based attention. Cereb Cortex. 2007;17:2468–77. doi: 10.1093/cercor/bhl154. [DOI] [PubMed] [Google Scholar]
- Shilling VM, Chetwynd A, Rabbitt PM. Individual inconsistency across measures of inhibition: an investigation of the construct validity of inhibition in older adults. Neuropsychologia. 2002;40:605–19. doi: 10.1016/s0028-3932(01)00157-9. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Movement-related potentials in the Go/NoGo task: the P3 reflects both cognitive and motor inhibition. Clin Neurophysiol. 2008;119:704–14. doi: 10.1016/j.clinph.2007.11.042. [DOI] [PubMed] [Google Scholar]
- Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643. [Google Scholar]
- Tachibana H, Aragane K, Sugita M. Age-related changes in event-related potentials in visual discrimination tasks. Electroencephalogr Clin Neurophysiol. 1996;100:299–309. doi: 10.1016/0168-5597(96)95108-4. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex. 2003;39:1107–28. doi: 10.1016/s0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex. 2004;14:966–73. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–14. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Stuss DT. Excessive sub-threshold motor preparation for non-target stimuli in normal aging. NeuroImage. 2010;50:1251–7. doi: 10.1016/j.neuroimage.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Stuss DT, McIntosh AR, Picton TW. Age-related differences in processing irrelevant information: evidence from event-related potentials. Neuropsychologia. 2009;47:577–86. doi: 10.1016/j.neuropsychologia.2008.10.018. [DOI] [PubMed] [Google Scholar]
- van Boxtel GJ, van der Molen MW, Jennings JR, Brunia CH. A psychophysiological analysis of inhibitory motor control in the stop-signal paradigm. Biol Psychol. 2001;58:229–62. doi: 10.1016/s0301-0511(01)00117-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-revised Manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- West R, Alain C. Age-related decline in inhibitory control contributes to the increased Stroop effect observed in older adults. Psychophysiology. 2000;37:179–89. [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–92. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wild-Wall N, Falkenstein M, Hohnsbein J. Flanker interference in young and older participants as reflected in event-related potentials. Brain Res. 2008;1211:72–84. doi: 10.1016/j.brainres.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Woldorff MG. Distortion of ERP averages due to overlap from temporally adjacent ERPs: analysis and correction. Psychophysiology. 1993;30:98–119. doi: 10.1111/j.1469-8986.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Hennigan K, Ostberg M, Clapp WC, Gazzaley A. Predictive knowledge of stimulus relevance does not influence top-down suppression of irrelevant information in older adults. Cortex. 2010a;46:564–74. doi: 10.1016/j.cortex.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Toy B, Gazzaley A. Delays in neural processing during working memory encoding in normal aging. Neuropsychologia. 2010b;48:13–25. doi: 10.1016/j.neuropsychologia.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. 64 electrode montage of the ERPs from the selective attention, delayed-recognition task averaged across all participants. The black line indicates the ERP to attended faces stimuli, the red line reflects ERPs to passively viewed faces, and the blue line reflects the ERPs to ignored face stimuli.
Supplementary Figure S2. 64 electrode montage of the ERPs from the stop-signal task averaged across all participants. The blue line indicates ERPs time-locked to STOP signal for successful inhibition (SI) stop trials, while the red line reflects ERPs time-locked to the STOP signal for failed inhibition (FI) stop trials.