Abstract
Context
Heart failure increases with advancing age, and approximately half of patients have preserved left ventricular ejection fraction. Although diastolic dysfunction plays a role in heart failure with preserved ejection fraction, little is known about age-dependent longitudinal changes in diastolic function in community populations.
Objective
To measure longitudinal change in diastolic function and heart failure incidence in a population-based cohort.
Design
2042 randomly selected participants underwent clinical evaluation, medical record abstraction, and echocardiography (1997–2000). Diastolic left ventricular function was graded as mild, moderate, or severe by validated Doppler techniques. After four years participants were invited to return for re-examination, and 1402 did so (2001–2004). The cohort was then followed for ascertainment of new onset heart failure (2004–2010).
Setting
Community population; Olmsted County, Minnesota
Participants
Population-based cohort of persons ≥45 years old
Main Outcome Measures
Incident heart failure
Results
Over 4 ± 0.3 years diastolic dysfunction prevalence increased from 23.8% (95% CI 21.2–26.4) to 39.2% (95% CI 36.3–42.2) (P <0.001). Diastolic function grade worsened in 23.4% (95% CI 20.9–26.0) of participants, was unchanged in 67.8% (95% CI 64.9–70.6), and improved in 8.8% (95% CI 7.1–10.5). Worsened diastolic dysfunction was associated with age ≥65 years (OR 2.85; 95% CI 1.77–4.72). During 6.3 ± 2.3 years of additional follow-up, heart failure occurred in 2.6% (95% CI 1.4–3.8), 7.8% (95% CI 5.8–13.0), and 12.2% (95% CI 8.5–18.4) of persons whose diastolic function normalized or remained normal, remained or progressed to mild dysfunction, or remained or progressed to moderate-severe dysfunction, respectively. (P <0.001) Diastolic dysfunction was associated with incident heart failure after adjustment for age, hypertension, diabetes, and coronary disease (HR 1.81; 95% CI 1.01–3.48).
Conclusion
In a population-based cohort followed for four years, diastolic dysfunction prevalence increased. Diastolic dysfunction was associated with development of heart failure during six years of subsequent follow-up.
INTRODUCTION
Heart failure is a progressive condition that increases in incidence with advancing age.1–10 There is an emerging emphasis on understanding the progression from heart failure risk factors to asymptomatic ventricular dysfunction, and eventually to symptomatic heart failure and death.6,7 Therefore, it is important to have population-based information on changes in cardiac function over time.
Heart failure may develop with reduced or preserved left ventricular ejection fraction, each form accounting for approximately half of cases.4, 7, 10–15 Echocardiographic classification of diastolic function in cross-sectional community studies has shown it to be highly prevalent and associated with heart failure. 11–14 However, little is known about time-dependent changes in diastolic function.
We randomly selected a cohort of 2042 persons ≥45 years old, the Olmsted County Heart Function Study (OCHFS).11 A cross-sectional evaluation of diastolic function in Exam 1 (1997–2000) has been reported.11 We report now a re-evaluation of this cohort, Exam 2 (2001–2004). After Exam 2 the cohort was followed passively and incident heart failure events ascertained (2004–2010). The objectives were to measure changes in diastolic function over time, to identify factors predictive of change in diastolic function, and to determine the relationship between diastolic dysfunction and the risk of subsequent heart failure.
METHODS
The Institutional Review Boards of Mayo Clinic and Olmsted Medical Center approved this study. Participants provided written informed consent for evaluation and medical record follow-up.
Subjects
In 2000 the population of Olmsted County, MN was 112, 255; 90% were white, 81% urban, and 11% ≥ 65 years old. Characteristics of this community and its use in population-based research (Rochester Epidemiology Project) have been described.16
In 1997 a random sample of County residents ≥45 years of age was identified by applying a sampling fraction of 7% within each gender- and age-specific (5 years) stratum. 4203 persons were invited and 2042 participated in Exam 1. (eFigure) A comparison of invited participants and nonparticipants was performed.17 Exam 1 (1997–2000) included physical examination, echocardiography, and medical record abstraction. Four years later all participants were invited to return and 1402 participated in Exam 2 (2001–2004) All Exam 1 data were recollected at Exam 2. Incident heart failure between Exams 1 and 2 was diagnosed by the Framingham criteria 2, 11 Diabetes was based on physician diagnosis and treatment. Myocardial infarction and hypertension were diagnosed according to criteria from the World Health Organization and the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, respectively.11
After Exam 2 long term surveillance for incident heart failure was accomplished by methodology previously validated in Olmsted County.9 From Exam 2 to November 2010 incident heart failure was identified using code 428 from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), which identifies 90% of Framingham criteria validated cases.9 Codes were assigned by trained coders according to physician diagnoses in outpatient and inpatient records (not by hospital billing records).
Healthy Subgroup
At Exam 1, a subset of participants was identified without heart failure, hypertension, coronary disease, diabetes, or cardiovascular medication use.
Echocardiograms
Ejection fraction was measured by quantitative 2-D methodology, as previously reported. 11 Systolic dysfunction was defined as ejection fraction <50%. Decreased ejection fraction was defined as a decrease of >7.5% (i.e., 1 SD decrease).
Diastolic function was assessed by pulse wave Doppler examination of mitral flow (before and during Valsalva maneuver), pulmonary venous flow, and Doppler imaging of the medial mitral annulus.11, 19, 25–28 Diastolic dysfunction was graded on a four-point ordinal scale: 1) normal; 2) mild diastolic dysfunction = abnormal relaxation without increased LV end-diastolic filling pressure (decreased E/A ratio <0.75); 3) moderate or “pseudonormal” diastolic dysfunction = abnormal relaxation with increased LV end-diastolic filling pressure (E/A 0.75 to 1.5, deceleration time >140 ms, plus 2 other Doppler indices of elevated end-diastolic filling pressure); 4) or severe diastolic dysfunction = advanced reduction in compliance, (i.e. markedly increased stiffness) with restrictive filling (E/A ratio of >1.5, deceleration time <140 ms, and Doppler indices of elevated LV end-diastolic filling pressure). For participants in atrial fibrillation, diastolic function was classified as indeterminate unless restrictive physiology (E/A >1.5, deceleration time <140 ms) was present. Valvular heart disease was assigned for moderate to severe echocardiographic valvular stenosis or regurgitation.
Echocardiogram Reading Agreement
Exam 1 and 2 echocardiograms were performed by the same three echocardiographers according to standardized protocols and reviewed by an echocardiologist (BK and MMR)11. Echocardiographers and echocardiologists were masked to clinical and Exam 1 echocardiogram findings. Inter-reader agreement was assessed for the echocardiologists, who independently reviewed sets of echocardiograms chosen to represent a range of ventricular function, and was comparable.
Nonreturning Subjects
To assess for Exam 2 participation bias, characteristics of Exam 1 participants who returned for Exam 2 were compared to those who did not.
Statistical Analysis
Means are expressed as ± SD. Comparisons between Exams 1 and 2 categorical variables were made with McNemar’s test and continuous variables were compared using the Wilcoxon signed-rank test. Ordinal logistic regression was used to adjust the association of clinical variables with the progression of diastolic dysfunction for age and gender. Cox proportional hazards regression models were used to identify factors associated with incident heart failure after Exam 2. Models were developed using stepwise techniques with consideration of clinically relevant variables having P<0.1 by univariate analysis: age, sex, hypertension, diabetes, coronary disease, incident myocardial infarction, ejection fraction, diastolic dysfunction, left atrial volume index, and E/e′. For persons with incomplete data additional categorical variables (test done, test not done) were included. Time-varying effects of covariates were assessed using two models: the first censored everyone at the median event time (3.5 years), and the second involved only those subjects at risk beyond 3.5 years. Long-term follow-up is based on the Kaplan-Meier product-limit method and compared between groups using the log-rank test. Analyses were performed with SAS versions 8.0 and 9.2 and JMP version 8.0 (SAS Institute, Cary, NC). Analyses were two sided, and significance judged at P<0.05.
RESULTS
Cohort Characteristics
Of the 4203 eligible Olmsted County residents invited to participate in Exam 1, 2042 (49%) participated. Analysis of potential participation bias in Exam 1 has been reported in 500 randomly selected participants and 500 nonparticipants: there was no significant difference in the prevalence of cardiovascular disease.17 Cross-sectional analyses of Exam 1 participants have been reported.7, 11, 18–24 Eighty-two Exam 1 participants died before Exam 2 and 72% (1402/1960) of the surviving Exam 1 participants returned for Exam 2. These 1402 participants are the focus of the present analysis. (eFigure)
The mean age of the 1,402 study participants at Exam 1 was 61 ± 9.5 years with 34.1% aged ≥65 years. Exam 2 was performed 4.0 ± 0.3 years after Exam 1, by which time 46.9% of participants were aged ≥65 years. At Exam 2 there was an increase in the prevalence of co-morbid conditions: hypertension increased from 25.8% (361/1402) to 42.4% (594/1402) (P < 0.001), diabetes increased from 6.3% (88/1402) to 10.3% (145/1402) (P <0.001) and heart failure increased from 1.1% (16/1402) to 2.2% (31/1402) (P = 0.027). Despite the increased number of participants fulfilling diagnostic criteria for hypertension, mean systolic blood pressure decreased from 130.7 ± 19.8 mm Hg at Exam 1 to 126.0 ± 19.1 at Exam 2 (P <0.001). Concomitantly, angiotensin converting enzyme inhibitor or angiotensin receptor blocker use increased from 8.7% (123/1402) to 17.9%, (251/1402) and beta blocker use from 13.6% (190/1402) to 21.6%. (303/1402). (both P <0.001)
Changes in Diastolic Function
Diastolic function grade could be assigned in 75.5% (1058/1402) of participants in both Exams 1 and 2. At Exam 2 diastolic dysfunction was present but not gradable in 112 persons and diastolic function could not be measured in 139 persons due to arrhythmia or incomplete echocardiographic data. (Table 1)
Table 1.
Clinical and echocardiographic characteristics of participants (n = 1402). Data presented as number (%) or mean (SD).
| Exam 1 | Exam 2 | P | |
|---|---|---|---|
| 1997–2000 | 2001–2004 | ||
| Clinical | |||
| Sex, male | 692 (49.4%) | ||
| Age, years | 61.0 (9.5) | 65.2 (9.5) | <0.001 |
| 45–64 | 924 (65.9%) | 744 (53.1%) | |
| ≥ 65 | 478 (34.1%) | 658 (46.9%) | |
| BMI, kg/m2 | 28.3 (5.1) | 28.5 (5.2) | <0.001 |
| <25 | 355 (25.3%) | 367 (26.2%) | |
| 25–30 | 609 (43.4%) | 594 (42.4%) | |
| >30 | 438 (31.2%) | 441 (31.5%) | |
| Systolic BP, mmHg | 130.7 (19.8) | 126.0 (19.1) | <0.001 |
| Diastolic BP, mmHg | 73.4 (9.9) | 69.5 (10.4) | <0.001 |
| Hypertensiona | 361 (25.8%) | 594 (42.4%) | <0.001 |
| Diabetes mellitusa | 88 (6.3%) | 145 (10.3%) | <0.001 |
| Coronary artery diseasea | 148 (10.6%) | 234 (16.7 %) | <0.001 |
| Myocardial infarctiona | 48 (3.4%) | 50 (3.6%) | 0.16 |
| Heart failure (Framingham criteria) a | 16 (1.1%) | 31 (2.2%) | 0.001 |
| ACEI/ARB use | 123 (8.7%) | 251 (17.9%) | <0.001 |
| Beta blocker use | 190 (13.6%) | 303 (21.6%) | <0.001 |
| Calcium channel blocker use | 81 (5.8%) | 105 (7.5%) | 0.07 |
| Lipid lowering agent use | 245 (17.5%) | 431 (30.7%) | <0.001 |
| Other cardiovascular drug use | 463 (33.0%) | 614 (43.8%) | <0.001 |
| Echocardiography | |||
| LV ejection fraction (947)b | 63.9 (6.6) | 65.9 (7.5) | <0.001 |
| ≥ 50% | 925 (97.7%) | 924 (97.6%) | 0.99 |
| ≥ 40% – <50% | 17 (1.8%) | 18 (1.9%) | |
| < 40% | 5 (0.5%) | 5 (0.5%) | |
| LV EDD/height, mm/m (1148;1078)c | 26.1 (2.9) | 26.1 (2.8) | 0.12 |
| LA Volume Index (1316; 1352) c | 24.3 (7.5) | 24.7 (8.5) | 0.05 |
| E, m/sec (1388; 1389) c | 0.67 (0.15) | 0.73 (0.18) | <0.001 |
| e′, m/sec (1182; 1387) c | 0.09 (0.04) | 0.08 (0.05) | <0.001 |
| Ratio of E/e′ (1178; 1377) c | 8.5 (2.8) | 10.7 (4.5) | <0.001 |
| < 10 | 818 (58.4%) | 589 (42.0%) | <0.001 |
| ≥ 10 | 360 (25.7%) | 788 (56.2%) | |
| Diastolic function (1310; 1263) | |||
| normal | 981 (70.0%) | 688 (49.1%) | <0.001 |
| mild dysfunction | 213 (15.2%) | 271 (19.3%) | |
| moderate dysfunction | 87 (6.2%) | 190 (13.6%) | |
| severe dysfunction | 3 (0.2%) | 2 (0.1%) | |
| dysfunction, indeterminate grade | 26 (1.9%) | 112 (8.0%) | |
| indeterminate function– insufficient data | 77 (5.5%) | 120 (8.6%) | |
| indeterminate function– arrhythmia | 15 (1.1%) | 19 (1.4%) | |
Ascertained by chart abstraction.
number of persons whose 2D ejection fraction could be measured
the number of persons in Exams 1 and 2 in whom the measurement could be made
BP = blood pressure; ACE1=angiotensin converting enzyme inhibitor. ARB=angiotensin receptor blocker. BSA=body surface area, BMI=body mass index, LVEDD=LV end diastolic dimension
e′=medial mitral annulus tissue velocity.
LV = left ventricular.
LVEDD = left ventricular end diastolic dimension.
From Exam 1 to Exam 2 the prevalence of diastolic dysfunction of any degree increased from 23.8% (95% CI 21.2–26.4) to 39.2% (95% CI 36.3–42.2) (P <0.001). Moderate-severe diastolic dysfunction increased from 6.4% (95% CI 4.9–7.9) to 16.0% (95% CI 13.7–18.2) (P <0.001). (Table 1)
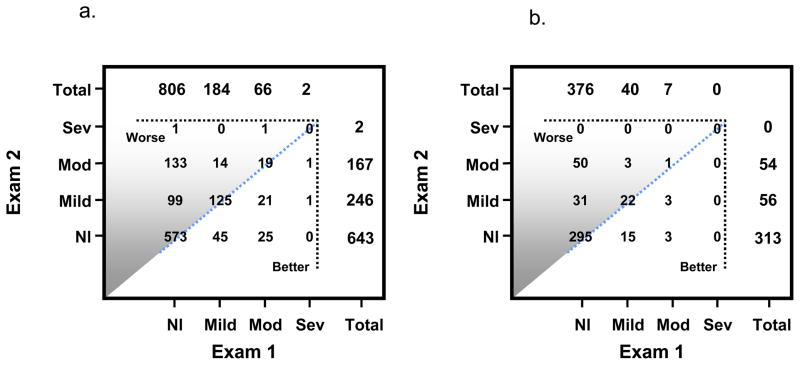
Within-individual changes are depicted in Figure 1a. Over four years, 23.4% (95% CI 20.9–26.0) of participants experienced worsening of diastolic function, 67.8% (95% CI 64.8–70.6) were unchanged, and 8.8% (95% CI 7.1–10.5) improved diastolic function. Elevated ventricular filling pressure, E/e′ ≥ 10, increased from 30.6% (95% CI 27.9–33.6) to 57.2% (95% CI 139.3–54.6) (P <0.001).
Figure 1.
Figure 1a – Within-individual changes in diastolic function classification from Exam 1 to Exam 2 in all participants diastolic function could be classified at both Exams in 1058 of 1402 participants. Participants above the diagonal line manifested a worse diastolic function grade at Exam 2, those on the diagonal line no change in diastolic function grade, and those below the line an improvement in diastolic function grade
1b – Within-individual changes in diastolic function classification from Exam 1 to Exam 2 in 423 (of 531) participants without hypertension, diabetes, coronary disease, heart failure, or cardiovascular medications whose diastolic function grade could be classified at both Exams. Participants above the diagonal line manifested a worse diastolic function grade at Exam 2, those on the diagonal line no change in diastolic function grade, and those below the line an improvement in diastolic function grade.
Factors Predictive of Worsening Diastolic Function
Age was predictive of the development of diastolic dysfunction, especially age ≥ 65 years (OR 2.85; 95% CI 1.77–4.72), as was E/e′ at Exam 1 (OR 1.14; 95% CI 1.06–1.23) (Table 2)
Table 2.
Factors predictive of the development of diastolic dysfunction from Exam 1 to Exam 2 (n=892 in whom diastolic function was normal at Exam 1 and could be classified as normal or diastolic dysfunction of any degree at Exam 2. Data presented as number (%) or mean (25th, 75th percentile).
| Development of diastolic dysfunction n = 319a |
Diastolic function remains normal n = 573 |
OR (95% CI) | OR adjusted for age & gender | |
|---|---|---|---|---|
| Age, years | 65.4 (57, 73) | 60.6 (55, 65) | 2.06 (1.72–2.45)b | |
| 45–64 | 166 (52.0%) | 430 (75.0%) | ||
| ≥ 65 | 153 (48%) | 143 (25%) | 2.85 (1.77–4.72)c | |
| Gender, female | 179 (56%) | 285 (50%) | 1.06 (0.93–1.21) | 1.05 (0.92–1.19) |
| Body mass index, kg/m2 | 28.1 (24.8, 30.5) | 28.1 (24.6, 30.7) | 0.99 (0.99–1.01) | 1.0 (0.99–1.01) |
| Hypertension (Exam 1) | 74 (23.2%) | 92 (16.1%) | 1.58 (1.12–2.22) | 1.23 (0.85–1.75) |
| Diabetes mellitus (Exam 1) | 17 (5.3%) | 22 (3.8%) | 0.71 (0.37–1.41) | 0.83 (0.42–1.6) |
| Ejection fraction (Exam 1) | 64.5 (60.7, 68.9) | 63.7 (60.4, 67.3) | 0.99 (0.94–1.05)c | 0.97 (0.92–1.03)c |
| E/e′ (Exam 1) | 8.4 (6.7, 10.0) | 7.5 (6.0, 8.75) | 1.19 (1.10–1.27) | 1.14 (1.06–1.23) |
| Hypertension (Exam 2) | 140 (43.9%) | 165 (28.8%) | 1.93 (1.45–2.57) | 1.54 (1.14–12.07) |
| Incident myocardial infarction | 3 (0.9%) | 3 (0.05%) | 1.17 (0.47–2.38) | 1.04 (0.41–2.17) |
Comprised of 99 persons with mild and 134 with moderate-severe diastolic dysfunction, and 86 with diastolic dysfunction that could not be confidently assisgned a grade
per 10 years of age;
compared to those 45–64 years of age;
per 5% change in LVEF
For abbreviations see Table 1. OR = odds ratio.
Diastolic function in Healthy Participants
531 participants were without hypertension, diabetes, coronary artery disease, heart failure, or cardiovascular drug use. Diastolic dysfunction of any degree increased from 11.3% (60/531; 95% CI 8.6–14.0) at Exam 1 to 29.8% (158/531; 95% CI 25.9–33.7) at Exam 2 (P <0.001). (eTable) In 79.7% (423/531) diastolic function grade could be classified at both Exams. Among healthy participants 19.9% (84/423; 95% CI 16.0–23.7) evidenced worsening diastolic function, 75.2% (318/423; 95% CI 70.8–79.1) remained the same, and 5.0% (21/423; 95% CI 2.9–7.0) improved. (Figure 1b) Consistent with these findings, E/e′ ≥ 10 prevalence increased from 17.9% (95/531; 95% CI 14.6–21.2) to 45.0% (239/531; 95% CI 40.8–49.3) (P < 0.001). (eTable)
Change in Systolic Function
2-D ejection fraction could be measured in 67.5% (947/1402) of participants at both Exams. Within-individual decrease in ejection fraction, defined as a decrease of >7.5% (i.e., >1 SD), occurred in 8.0% (76/947) of participants. However, ejection fraction <50% was unchanged: 2.3% (24/1402) at Exam 1 and 2.4% (23/1402) at Exam 2. (Table 1)
Incident Heart Failure Between Exam 1 and Exam 2
Among participants without heart failure at Exam 1 Framingham criteria heart failure developed by Exam 2 in 0.9% (12/1386). Among these cases, 83% (5/6 with gradable diastolic function) had diastolic dysfunction at Exam 1, compared to 24% (310/1289) who did not develop heart failure between Exams. (P<0.001) Consistent with this observation, left atrial volume index was greater in heart failure cases, 36 ± 12.9 cc/m2 versus 24 ± 6.8 cc/m2 in those without heart failure (OR 1.73; 95% CI 1.12–2.70).
None of the 12 incident heart failure participants had EF <50% at Exam 1, and only one had a ejection fraction <50% at Exam 2. Medical record abstraction revealed that four of the 12 participants experienced a transient decrease in ejection fraction to <50% when clinically symptomatic, only to recover normal ejection fraction by Exam 2. Causes of these transient systolic heart failure events were uncontrolled hypertension (n = 2), rapid atrial fibrillation (n = 1), and apical ballooning syndrome (n = 1). The one incident heart failure participant whose ejection fraction was <50% at Exam 2 had suffered a myocardial infarction.
Heart Failure Surveillance After Exam 2
Surveillance after Exam 2 indentified both new inpatient and outpatient heart failure diagnoses. During 6.3 ± 2.3 years, 81 participants developed heart failure. Age ≥ 65 years was the most potent predictor of heart failure (HR 8.38; 95% CI 4.4–16.0). (Table 3) Multivariable analysis demonstrated the independent predictive power of diastolic dysfunction (HR 1.81;95% CI 1.01–3.48), hypertension (HR 2.21; 95% CI 1.32–3.84), diabetes (HR 1.77; 95% CI 1.0–3.01) and coronary disease (HR 2.07; 95% CI 1.27–3.32).
Table 3.
Baseline factors predictive of the development of incident heart failure after Exam 2. Data presented as number (%) or mean (25th, 75th percentile). Heart failure cases ascertained by diagnostic code ICD-9-CM 428.
| Variable | HF** (n=81) | No HF (n=1277) | HR (95% CI) | HR (adjusted for age and gender) | HR multivariable model* |
|---|---|---|---|---|---|
| Clinical Variables | |||||
| Age (years) | 75.0 (69.5, 81.4) | 64.2 (56.6, 70.7) | 3.1 (2.4–3.9)b | 2.53 (1.94–3.31) | |
| 45–64 | 11 (14%) | 726 (57%) | |||
| ≥65 | 70 (86%) | 551 (43%) | 8.38 (4.4–16.0)a | ||
| Gender (female) | 43 (53%) | 648 (51%) | 1.10 (0.70–1.72) | ||
| Hypertension (Exam 2) | 59 (73%) | 505 (40%) | 4.10 (2.48–6.77) | 2.66 (1.58–4.48) | 2.21 (1.32–3.84) |
| Diabetes (Exam 2) | 17 (23%) | 114 (10%) | 2.64 (1.48–4.70) | 2.43 (1.31–4.51) | 1.77 (1.0–3.01) |
| Coronary disease (Exam 2) | 31 (38%) | 172 (13.5%) | 3.72 (2.35–5.78) | 2.33 (1.43–3.75) | 2.07 (1.27–3.32) |
| Incident MI between Exams | 3 (4%) | 13 (1%) | 3.89 (1.08–13.9) | 1.91 (0.48–7.66) | |
| Echo Variables (Exam 2) | |||||
| EF, Mean | 65.3 (60.4, 73.0) | 66.1 (62.5, 70.9) | 0.88 (0.75–1.06)c | 0.83 (0.71–0.97)c | |
| EF<50% | 4 (9%) | 16 (2%) | 5.03 (1.61–15.7) | 7.08 (2.01–24.9) | |
| Diastolic Dysfunction, any degree | 49 (75%) | 478 (43%) | 3.93 (2.29–7.14) | 2.05 (1.17–3.80) | 1.81 (1.01–3.48) |
| Left atrial Volume Index | 31.4 (23.6, 37.7) | 25.4 (19.2, 27.3) | 2.43 (2.02–2.87)d | 2.02 (1.61–2.49)d | |
| E/e′ | 12.6 (8.8, 16.7) | 10.4 (8.0, 12.0) | 1.20 (1.06–1.31)e | 1.15 (0.96–1.28)e | |
compared to age 45–64;
per 10 years of age;
per 5% change in ejection fraction;
per 10 cc/m2;
per 5 unit change
HR = hazard ratio;
multivariable model adjusted for age, hypertension, diabetes, coronary disease.
Individuals with ICD-9-CM 428 heart failure at Exam 2 are excluded.
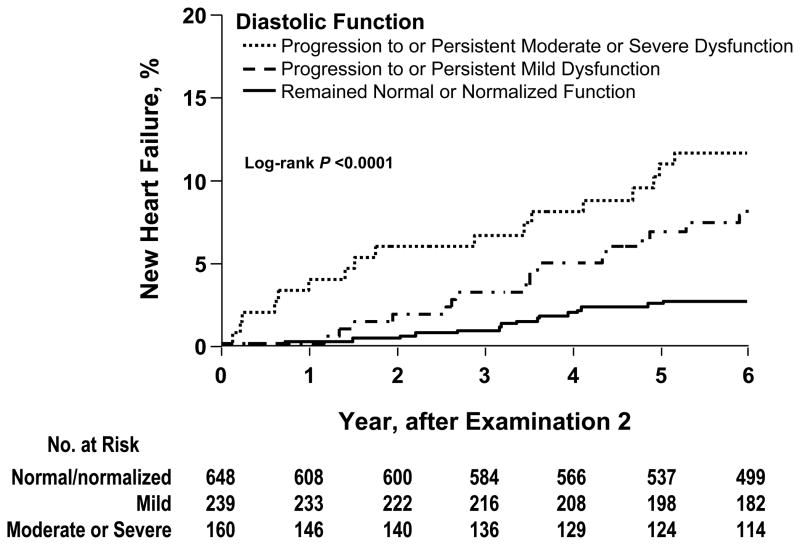
Persistent or worsening diastolic dysfunction was associated with heart failure. (Figure 2) Participants whose diastolic function remained normal or normalized between Exams had a 2.6% (95% CI 1.4–3.8) cumulative heart failure incidence. Participants with persistent, or progression to, mild diastolic dysfunction had a 7.8% (95% CI 5.8–13.0) cumulative heart failure incidence. Participants with persistent, or progression to, moderate-severe diastolic dysfunction experienced a cumulative heart failure incidence of 12.2% (95% CI 8.5–18.4). (P<0.001)
Figure 2.
The cumulative incidence of heart failure after Exam 2. The groups represent three grades of severity and change in diastolic dysfunction from Exam 1 and Exam 2. Persons with heart failure at Exam 2 and those in whom diastolic function could not be classified at both Exams 1 and 2 are excluded, leaving 1047 persons at risk after Exam 2.
Time-varying effects of covariants were examined using Cox models before and after the median event time of 3.5 years. In the initial 3.5 years after Exam 2, age, hypertension, diabetes, coronary artery disease, diastolic dysfunction, left atrial volume index and E/e′ were associated with an increased heart failure risk. In persons still at risk past 3.5 years the effects of diabetes and E/e′ disappeared.
Potential for participation bias at Exam 2
Among Exam 1 participants, 82/2042 died before Exam 2 and 71.5% (1402/1960) of survivors returned for Exam 2. Survivors who did not return were older (66.3 ± 12 versus 61.1 ± 10 years, P <0.001) and had a higher prevalence of co-morbidities at Exam 1: hypertension 33.4% versus 24.5% (P <0.001); diabetes mellitus 9.8% versus 6.3% (P = 0.004); prior myocardial infarction 7.8% versus 2.1% (P <0.001); heart failure 4.5% versus 1.1%, (P <0.001). Exam 1 diastolic dysfunction was more prevalent among non-returning than among returning participants (41.1% versus 25.1%, P <0.001), as was left ventricular EF ≤ 50% (8.9% versus 2.4%, P <0.001). Mortality follow-up demonstrated better 1-, 3- and 5-year survival for returning (98%, 96%, and 90%) compared to non-returning participants (96%, 91%, and 86%). Non-returning participants experienced greater mortality risk (HR 4.0; 95% CI 2.9–5.4; P <0.0001).
DISCUSSION
Our initial report from the OCHFS cohort provided cross-sectional estimates of left ventricular dysfunction prevalence in the community, and characterized the relationship between ventricular dysfunction and clinical status.11 This report adds a longitudinal “change within individual” dimension to left ventricular function measurements and clinical status. There was a marked progression of diastolic dysfunction: 23% of participants showing worse diastolic function, 68% were unchanged and 9% improved. A similar pattern of worsening diastolic function was also observed in a subset of healthy participants. Incident heart failure during 6.3 ± 2.3 years of follow-up was associated with age, hypertension, diabetes, coronary disease, and diastolic dysfunction. Persistent or worsening diastolic dysfunction between Exams 1 and 2 was an independent risk factor for subsequent heart failure.
Temporal Change in Left Ventricular Diastolic Function
Community population studies report that approximately half of heart failure patients have preserved ejection fraction.11, 15 Heart failure, with or without reduced ejection fraction, is marked by recurrent hospitalizations and a five year mortality in the range of 30–35%.9, 10 Echocardiographic measurement of diastolic function in population-based cohorts show that approximately 7% of persons over age 45 years have moderate to severe diastolic dysfunction, most of whom report few, if any, symptoms.11, 13 The current longitudinal data confirm and extend the cross-sectional association reported between age and diastolic dysfunction: over a four-year interval middle-aged and elderly persons were three times more likely to manifest poorer diastolic function than better diastolic function.11–14. The fact that diastolic dysfunction worsened even in healthy persons supports the concept that aging itself may be accompanied by progressive deterioration in diastolic function. This age-related progression of diastolic dysfunction in the population contributes to the pathophysiologic substrate from which overt heart failure emerges.
The biological pathways leading to heart failure with preserved ejection fraction are manifold, and understanding its pathophysiology remains a work in progress. Contributing factors include changes in myocardial relaxation and elastic recoil, changes in ventricular load and diastolic stiffness, external constraint and abnormal systolic function.26–36 Age-related loss of peripheral vascular elasticity, and its effect on left ventricular load and stiffness, may play an important role in this process.18, 37–42 Measurements of the interaction between left ventricular function and vascular load suggest that ventriculo-vascular coupling may play in the development of the diastolic dysfunction component of heart failure with preserved ejection fraction.30, 31, 33–35, 37 Indeed, previous cross-sectional analyses from this OCHFS cohort have shown significant correlations between age and vascular, ventricular end-systolic, and ventricular end-diastolic stiffness.18
Incident Heart Failure
Surveillance studies of the entire Olmsted County population from 1987–2001 (n= 100,000–125,000 persons) have documented a constant incidence of heart failure with reduced ejection fraction, but an increase in heart failure with preserved ejection fraction15 The current analysis identifies diastolic dysfunction as an independent predictor of these heart failure events.
However, to put diastolic dysfunction in context it should be noted that only about one in four persons with moderate-severe diastolic dysfunction at Exam 2 developed incident heart failure during long-term follow-up. This suggests that superimposed clinical events play an important role in the transition from asymptomatic diastolic dysfunction to overt heart failure with preserved ejection fraction. Specifically, our findings are consistent with the hypothesis that a combination of cardiovascular aging and superimposed cardiovascular disease accelerates the deterioration in diastolic function, setting the stage for symptomatic heart failure with preserved ejection fraction in the elderly.18, 35–37 The detailed assessment of the twelve heart failure events between Exams 1 and 2 documents some of these superimposed cardiovascular disease processes. An important clinical implication is that prevention of risk factors for superimposed events, especially hypertension, is fundamental to reducing heart failure with preserved ejection fraction.
Returning and non-returning Participants
Comparison of participants who returned for Exam 2 to those who did not return indicates that non-returning subjects had more baseline hypertension, diabetes, myocardial infarction, heart failure, diastolic dysfunction, and increased subsequent mortality. Therefore, the worsening diastolic dysfunction we report in returning subjects may underestimate that in the whole cohort.
Strengths and weaknesses
Strengths of this study include its population-based randomly selected cohort, the ability to make protocolized serial observations for research purposes, the opportunity to examine interval clinical events in the cohort, and the ability to collect data on nonreturning participants. Study participants underwent a uniform evaluation at Exams 1 and 2 using the same measurement methodologies. The ability to ascertain both inpatient and outpatient heart failure diagnoses during long term follow-up using a validated ICD-9-CM code methodology is a strength.
Survival bias and participation bias may contribute to underestimation of the overall impact of diastolic dysfunction on heart failure in the cohort. Finally, our cohort was over 95% white, so inferences to other ethnic or racial populations may not be valid.
CONCLUSION
Longitudinal evaluation of participants in the population-based OCHFS cohort reveals that left ventricular diastolic dysfunction is highly prevalent, tends to worsen over time, and is associated with advancing age. Worsening diastolic function can be detected even in apparently healthy persons. The persistence or progression of diastolic dysfunction is an independent risk factor for heart failure in the elderly.
Acknowledgments
Funding/Support: The research was supported by NIH HL-R01-55502 (Dr. Rodeheffer), NIH AR-36582 (Dr. Jacobsen), NIH HL-63281 (Redfield). The project was support by NIH/NCRR CTSA Grant UL RR024150, the Rochester Epidemiology Project R01-AG034676 from the National Institute on Aging, and by the Mayo Foundation.
Role of the Sponsors: The study sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review or approval of the manuscript.
Footnotes
Author Contributions: Drs. Kane and Rodeheffer had full access to all study data and to be responsible for data accuracy and integrity.
Study concept and design: Rodeheffer, Redfield, Burnett.
Analysis and interpretation of the data: Kane, Rodeheffer, Mahoney, Redfield, Jacobson, Roger.
Drafting of the manuscript: Kane, Rodeheffer, Jacobsen.
Statistical analysis: Mahoney, Jacobsen, Kane.
Obtaining funding: Rodeheffer.
Study supervision: Rodeheffer.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Intersst.
Additional Contributions: We thank Tammy Burns and Kasey Muetzel for invaluable assistance in manuscript preparation. They did not receive compensation apart from employment at Mayo Clinic.
Online-Only Material: The eTables and eFigures are available at http://www.jama.com
This publication is dedicated to the memory of Kenneth L. Baughman, M.D., physician and mentor.
References
- 1.Rodeheffer RJ, Jacobsen SJ, Gersh BJ, et al. The incidence and prevalence of congestive heart failure in Rochester, Minnesota. Mayo Clin Proc. 1993;68:1143–1150. doi: 10.1016/s0025-6196(12)60063-9. [DOI] [PubMed] [Google Scholar]
- 2.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 4.Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: trends in incidence and survival in a 10-year period. Arch Intern Med. 1999;159:29–34. doi: 10.1001/archinte.159.1.29. [DOI] [PubMed] [Google Scholar]
- 5.Mosterd A, Hoes AW, de Bruyne MC, et al. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur Heart J. 1999;20:447–455. [PubMed] [Google Scholar]
- 6.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 7.Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115:1563–1570. doi: 10.1161/CIRCULATIONAHA.106.666818. [DOI] [PubMed] [Google Scholar]
- 8.Redfield MM. Heart failure - an epidemic of uncertain proportions. N Engl J Med. 2002;347:1442–1444. doi: 10.1056/NEJMe020115. [DOI] [PubMed] [Google Scholar]
- 9.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 11.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 12.Davies M, Hobbs F, Davis R, et al. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet. 2001;358:439–444. doi: 10.1016/s0140-6736(01)05620-3. [DOI] [PubMed] [Google Scholar]
- 13.Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92:1259–1264. doi: 10.1136/hrt.2005.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 15.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 16.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen SJ, Mahoney DW, Redfield MM, Bailey KR, Burnett JC, Jr, Rodeheffer RJ. Participation bias in a population-based echocardiography study. Ann Epidemiol. 2004;14:579–584. doi: 10.1016/j.annepidem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 19.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 20.Okura Y, Ohno Y, Ramadan MM, et al. Characterization of outpatients with isolated diastolic dysfunction and evaluation of the burden in a Japanese community: Sado Heart Failure Study. Circ J. 2007;71:1013–1021. doi: 10.1253/circj.71.1013. [DOI] [PubMed] [Google Scholar]
- 21.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: a community-based study. Circulation. 2004;109:3176–3181. doi: 10.1161/01.CIR.0000130845.38133.8F. [DOI] [PubMed] [Google Scholar]
- 22.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: Impact of age and gender. JACC. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 23.McKie PM, Rodeheffer RJ, Cataliotti A, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuetic peptide. Hypertension. 2006;47:874–880. doi: 10.1161/01.HYP.0000216794.24161.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mungala VK, Jacobsen SJ, Mahoney MS, Rodeheffer RJ, Bailey KR, Redfield MM. Association of newer diastolic function parameters with age in healthy subjects: a population-based study. J Am Soc Echocardiogr. 2003;16 (10):1049–1056. doi: 10.1016/S0894-7317(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 25.The sixth report of the Joint National Committee on prevention, detection evaluation, and treatment of high blood pressure. Arch Intern Med. 1998;158:573. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 26.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta Stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 28.Hurrell DG, Nishimura RA, Ilstrup DM, Appleton CP. Utility of preload alteration in assessment of left ventricular filling pressure by Doppler echocardiography: a simultaneous catheterization and Doppler echocardiographic study. J Am Coll Cardiol. 1997;30:459–467. doi: 10.1016/s0735-1097(97)00184-8. [DOI] [PubMed] [Google Scholar]
- 29.Redfield MM. Understanding “diastolic” heart failure. N Engl J Med. 2004;350:1930–1931. doi: 10.1056/NEJMp048064. [DOI] [PubMed] [Google Scholar]
- 30.Borlaug BA, Lam CSP, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease. J Am Coll Cardiol. 2009;54:410–8. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: Mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol. 2008;105:1342–1351. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure - abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 33.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Failure Clin. 2008;4:23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kass DA, Bronzwaer JGF, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004;94:1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 35.Kass DA. Ventricular arterial stiffening: Integrating the pathophysiology. Hypertension. 2005;46:185–193. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- 36.Zile MR, Gaasch WH, Carroll JD, et al. Heart failure with normal ejection fraction. Circulation. 2001;104:779–782. doi: 10.1161/hc3201.094226. [DOI] [PubMed] [Google Scholar]
- 37.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises. Part II: The aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 38.Abhayaratna WP, Barnes ME, O’Rourke MF, et al. Relation of arterial stiffness to left ventricular diastolic function and cardiovascular risk prediction in patients >65 years of age. Am J Cardiol. 2006;98:1387–1392. doi: 10.1016/j.amjcard.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 39.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises. Part I: Aging arteries: A “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 40.Gerstenblith G, Frederiksen J, Yin FCP, Fortuin NJ, Lakatta EG, Weisfeldt ML. Echocardiographic assessment of a normal adult aging population. Circulation. 1977;56:273–278. doi: 10.1161/01.cir.56.2.273. [DOI] [PubMed] [Google Scholar]
- 41.Grewal J, McCully RB, Kane GC, Lam C, Pellikka PA. Left ventricular function and exercise capacity. JAMA. 2009;301 (3):286–294. doi: 10.1001/jama.2008.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng S, Fernandes VRS, Bluemke DA, McClelland RL, Kronmal RA, Lima JAC. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes. Circ Cardiovasc Imaging. 2009;2:191–198. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]