Abstract
The incidence of developing circulating anti-human leukocyte antigen antibodies and the kinetics of T cell depletion and recovery among pediatric renal transplant recipients who receive alemtuzumab induction therapy are unknown. In a collaborative endeavor to minimize maintenance immunosuppression in pediatric renal transplant recipients, we enrolled 35 participants from four centers and treated them with alemtuzumab induction therapy and a steroid-free, calcineurin-inhibitor–withdrawal maintenance regimen. At 3 months after transplant, there was greater depletion of CD4+ than CD8+ T cells within the total, naive, memory, and effector memory subsets, although depletion of the central memory subset was similar for CD4+ and CD8+ cells. Although CD8+ T cells recovered faster than CD4+ subsets overall, they failed to return to pretransplant levels by 24 months after transplant. There was no evidence for greater recovery of either CD4+ or CD8+ memory cells than naïve cells. Alemtuzumab relatively spared CD4+CD25+FoxP3+ regulatory T cells, resulting in a rise in their numbers relative to total CD4+ cells and a ratio that remained at least at pretransplant levels throughout the study period. Seven participants (20%) developed anti-human leukocyte antigen antibodies without adversely affecting allograft function or histology on 2-year biopsies. Long-term follow-up is underway to assess the potential benefits of this regimen in children.
The effects of alemtuzumab on T cell subsets have been extensively studied in adults since its introduction in the 1990s. It has been associated with profound depletion of total T cells and differential recovery among T cell subsets, with early and near-complete recovery of CD8+ T cells, but late, partial recovery of CD4+ T cells.1–3 CD4+ memory T cells were relatively spared compared with other CD4+ subsets; some investigators reported preferential sparing of central memory (TCM) cells, whereas others observed preferential sparing of the effector memory (TEM) subset. Emergence of the TEM subset, whether identified peripherally or in the allograft, has been associated with acute rejection, raising concerns about the tolerogenic potential of alemtuzumab.1–4 Although the use of alemtuzumab was not associated with an increase in either FoxP3 expression or regulatory T cell counts in vitro, both transient and sustained expansion of regulatory T cells were observed when alemtuzumab was used in association with sirolimus in vivo.2,5,6 Notwithstanding this, however, the combination of alemtuzumab and sirolimus in protocols free of calcineurin inhibitor (CNI) was associated with rates of acute rejection exceeding 20%, with a humoral rejection rate as high as 62.5%.7,8 In the absence of long-term CNI treatment, alemtuzumab-treated adults may therefore have a propensity to develop alloantibodies and antibody-mediated rejection.9,10
In contrast, there is a paucity of data regarding the use of alemtuzumab in pediatric solid organ transplantation.11 From a clinical perspective, two small series investigating the outcomes of selected high-risk recipients of renal, liver, and intestinal transplants treated with alemtuzumab yielded conflicting results, whereas a recent series of 42 renal transplant recipients of living donor grafts reported few cases of acute rejection and excellent graft function up to 4 years after transplant.12–14 From a mechanistic perspective, only one study reported T cell counts in a single pediatric patient, demonstrating profound and prolonged depletion of CD3+, CD4+, CD8+, and CD20+ cells, with counts only reaching 50% of their baseline levels 12 months after transplant.12
In this study, we investigated the longitudinal immune profiles of pediatric renal transplant recipients treated with alemtuzumab induction therapy, followed by a CNI-withdrawal regimen. Specific aims were to characterize the depletion and recovery patterns of various T cell subsets and to screen for anti-human leukocyte antigen (anti-HLA) antibody development.
Results
Thirty-five participants were recruited. Of these, 23 participants completed screening for cAb at all six protocol-designated time points, whereas 12 patients completed screening up to between 6 and 12 months. One participant had a positive result for class I circulating antibody (cAb) pretransplant, with a panel reactive antibodies (PRAs) of 14% as determined by Luminex; seven participants (20%) developed cAb during the follow-up period.
Two participants experienced graft loss during the study period, the first due to focal and segmental glomerulosclerosis, the pretransplant existence of which was unrecognized, and the second due to medication nonadherence; neither of these patients developed cAb. Six participants suffered biopsy-proven acute rejection, one of which was antibody mediated. In three patients, the diagnosis was made on a nonprotocol biopsy performed for graft dysfunction, whereas in the remaining three, subclinical acute rejection was diagnosed on the 6-month protocol biopsy. There were no deaths.
Fourteen participants presented with at least one infectious episode. There were no cases of post-transplant lymphoproliferative disorder. Asymptomatic Epstein-Barr virus seroconversion was noted in three patients; valganciclovir prophylaxis was continued as planned. One subject developed BK viremia and another developed BK viruria, but neither progressed to BK nephropathy. Three participants had catheter-related bloodstream infections. Two participants developed pyelonephritis, whereas four developed cystitis. The remaining reported infections were pneumonia, Clostridium difficile colitis, sinusitis, a skin infection, and a toe infection, each of which occurred only once in different individuals. All infections responded to conventional therapy.
Depletion and Recovery of T Cell Subsets in Pediatric Kidney Transplant Recipients
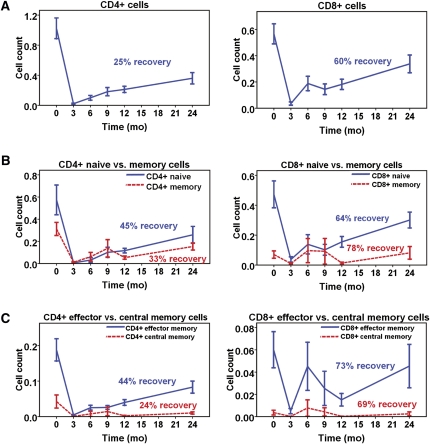
At 3 months after alemtuzumab induction, there was greater depletion of the total, 45RA+ naïve, 45RO+ memory, and 45RO+CCR7−62L− TEM cells within the CD4+ than CD8+ T cell subsets, whereas depletion of 45RO+CCR7+62L+ TCM cells was similar for both CD4+ and CD8+ T cells (Table 1, Figure 1). Within the CD4+ compartment, there was similar depletion of both memory and naïve cells (P=0.267), whereas within the CD4+ memory cell subset, there was slightly greater depletion of TCM compared with TEM cells (P=0.002). Within the CD8+ compartment, there was slight, albeit statistically significant, sparing of memory over naïve cells and, within the memory subset, sparing of TEM over TCM cells (both P=0.010).
Table 1.
Relative depletion of T cell subsets at 3 months after transplant
| T Cell Subset | CD4+ Cells | CD8+ Cells | P Value | ||||
|---|---|---|---|---|---|---|---|
| Baseline Cell Count (103 cells/µl) | 3-mo Cell Count (103 cells/µl) | (% Depletion)a | Baseline Cell Count (103 cells/µl) | 3-mo Cell Count (103 cells/µl) | (% Depletion)a | ||
| Total | 1.02±0.46 | 0.02±0.02 | 97.2±3.9 | 0.57±0.25 | 0.04±0.04 | 93.6±6.6 | 0.012 |
| Naïvea | 0.57±0.36 | 0.01±0.01 | 99.6±0.3 | 0.47±0.25 | 0.04±0.04 | 93.6±8.1 | 0.393 |
| Memory | 0.36±0.24 | 0.01±0.01 | 97.3±3.0 | 0.09±0.07 | 0.01±0.01 | 92.2±11.2 | 0.003 |
| Effector memory | 0.19±0.10 | 0.01±0.01 | 95.9±5.0 | 0.06±0.05 | 0.01±0.01 | 91.0±14.1 | 0.004 |
| Central memory | 0.04±0.06 | 0.01±0.01 | 98.5±1.6 | 0.01±0.01 | 0.01±0.01 | 98.3±1.7 | 0.079 |
| Regulatory | 0.03±0.01 | 0.01±0.01 | 90.3±7.6 | — | <0.001b | ||
Results are provided as mean ± SD. Percentage of depletion was calculated for each subject using the following formula: (absolute count at baseline − absolute count at 3 mo)/absolute count at baseline. P values were computed for the difference between CD4+ and CD8+ cells of the same subset. Analysis of the change in absolute cell counts over time was performed using generalized estimating equations.
aThe number of available samples was 11 for all subsets, except for 45RA+ naïve (n=5) and 25+FoxP3+ regulatory (n=8).
bCD4+25+FoxP3+ versus Total CD4+.
Figure 1.
Depletion and recovery of T cell subsets after induction with alemtuzumab. Cells were available from 11 participants, except for TCM cells in which cells were available for five participants. (A) After alemtuzumab induction, there was profound depletion of both CD4+ and CD8+ T cells, but greater recovery of CD8+ than CD4+ T cells at 24 months compared with baseline (P=0.014). (B) For CD4+ T cells, there was no evidence that the recovery of memory cells was favored over naïve cells (P=0.740), whereas there was a nonsignificant trend toward greater recovery of the CD8+ memory subset (P=0.163). (C) Within the CD4+ memory subset, the recovery of TEM cells was almost twice that of TCM cells (P=0.027); within the CD8+ memory subset, recovery of both TEM and TCM was similar (P=0.556). Cell counts are expressed as 103 cells/µl.
At 24 months after transplant, whereas the recovery rate of total CD4+ T cells was similar to that observed in adults, it was significantly slower than that of total CD8+ T cells (P=0.014; Figure 1). Within the CD4+ compartment, there was no significant difference in the magnitude of recovery of naïve versus memory cells (P=0.740); within the CD4+ memory cell subset, however, the extent of TEM cell recovery was almost twice that of TCM cells (P=0.027). There was a trend toward greater recovery of CD8+ memory rather than naïve cells (P=0.163), although the magnitude of recovery of TEM and TCM cells was similar (P=0.555). Taken together, these results demonstrate greater and more prolonged depletion of CD4+ compared with CD8+ T cells after the use of alemtuzumab in pediatric transplant recipients.
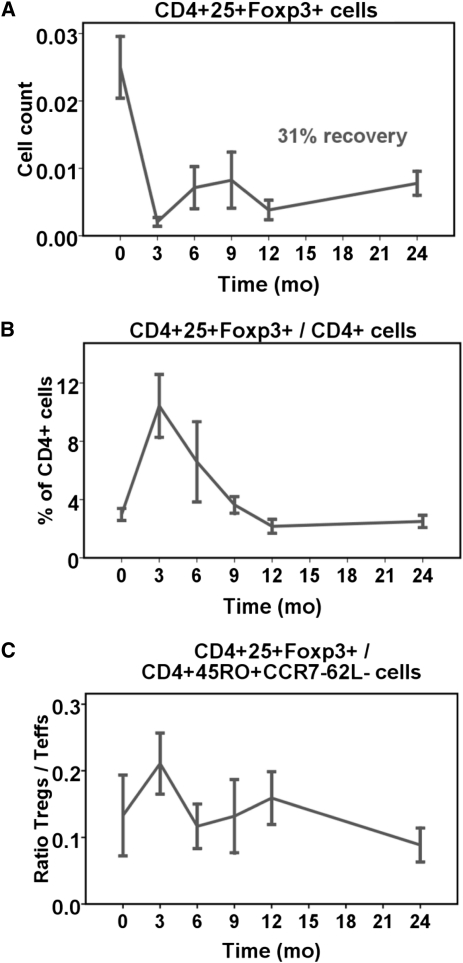
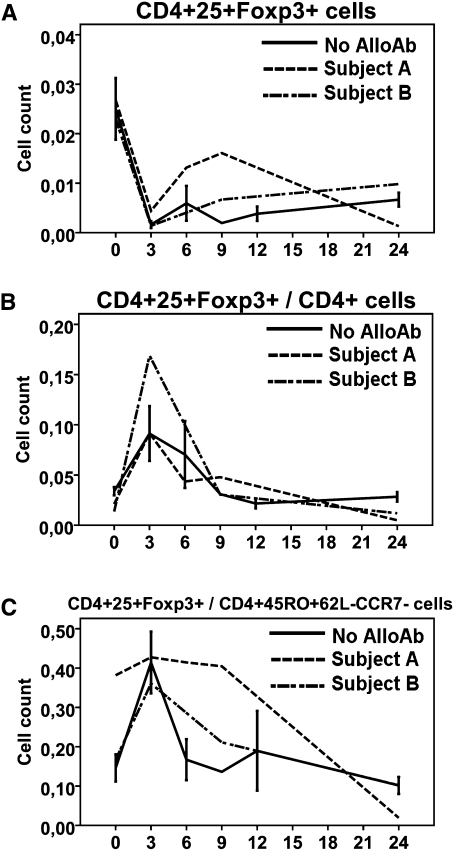
Relative Sparing of Regulatory T Cells after Depletion with Alemtuzumab
At 3 months after transplant, there was less depletion of CD4+25+FoxP3+ regulatory T cells (Tregs) relative to total CD4+ T cells (P<0.001; Table 1, Figure 2). This initial sparing, succeeded by brisk recovery of Treg counts between 3 and 12 months, caused the proportion of Tregs within the total CD4+ T cell compartment to peak at 3 months and subsequently slowly decline to the 12-month time point (Figure 2B). Thereafter, the pattern of recovery of Tregs was similar to that of total CD4+ T cells. Similarly, the ratio of Tregs/TEM increased significantly from baseline to 3 months after transplant (P=0.009), returned to baseline between 6 and 12 months, and slightly decreased thereafter (Figure 2C).
Figure 2.
Depletion and recovery of CD4+25+FoxP3+ Tregs in comparison to total CD4+ and CD4+45RO+CCR7-62L- TEM cells. Cells were available from eight participants. (A and B) The initial depletion of Tregs was relatively less than that of total CD4+ T cells, resulting in a sharp increase in the percentage of Tregs within total CD4+ T cells that persisted for the first 12 months post-transplant. (C) Ratio of Tregs/TEM. Cell counts are expressed as 103 cells/µl.
Incidence of Anti-HLA cAb Development
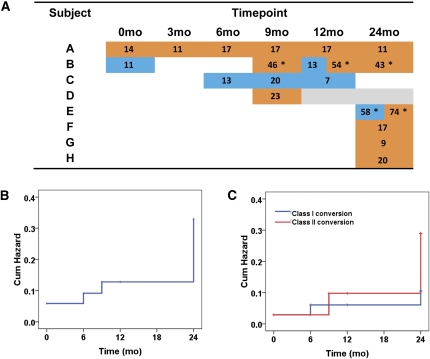
Figure 3A shows the longitudinal cAb status, along with the PRA level, for the seven participants who developed cAb, plus the patient who had a positive pretransplant result. Of these, one participant (patient C) developed class I cAb alone, showing only transient positivity between 6 and 12 months. Five participants developed class II cAb alone: the first (patient A) was positive for all time points during the course of the study at low PRA levels; one participant (patient D) converted at 9 months but was then lost to follow-up; and three participants (patients F, G, and H) converted at 24 months. Two participants had both detectable class I and class II cAb during the course of the study: the first (patient B) was transiently positive for class I cAbs at baseline and again at 12 months, but developed persistent class II cAbs at 9 months; the second (patient E) developed high levels of class I and II cAbs at 24 months. Two participants had donor-specific cAb, as denoted by an asterisk (Figure 3A). Overall, the cumulative hazard of cAb development was 13% until 24 months, at which it reached 33% (Figure 3B); the cumulative hazard for development of class I versus class II cAb was similar (Figure 3C).
Figure 3.
Development of circulating anti-HLA alloantibodies. (A) Anti-HLA alloantibodies were considered positive using a PRA >5% as threshold: the blue color represents anti-HLA class I positivity; the orange color anti-HLA class II positivity; the PRA values are shown in each positive cell; gray cells represent missing samples. (B) Cumulative hazard of development of any anti-HLA alloantibody type. (C) Cumulative hazards of anti-HLA class I versus class II alloantibody development. Asterisk indicates donor-specific alloantibodies.
A summary of the biopsy findings is presented along with cAb status in Table 2. Of the eight participants who had detectable cAbs during the study period, two received a diagnosis of cellular acute rejection. In both cases, the cAb developed after the diagnosis of acute rejection, which was made on their 6-month protocol biopsy, at which point renal function was stable. A third participant received a diagnosis of borderline cellular changes concurrent with the development of cAbs at 24 months. With the exception of the two patients who developed acute rejection, these participants had stable renal function throughout the study period (Supplemental Figure 1).
Table 2.
Longitudinal biopsy reports for participants who developed anti-HLA alloantibodies
| Patient | Time Point | |||||||
|---|---|---|---|---|---|---|---|---|
| Intraoperative | Before Conversion to Sirolimus | 6 mo | 9 mo | 12 mo | 24 mo | |||
| A | N | CTCb | CTCb | N | ||||
| B | N | N | CTC | N | ||||
| C | ND | ND | ND | CG | ||||
| D | N | ND | ACR | — | ||||
| E | N | ND | ACR | N | N | |||
| F | N | ND | ND | CTC, CA, CNI toxicity | ||||
| G | a | ND | ND | BLC, CA | ||||
| H | N | N | N | N | ||||
Dark gray shading represents anti-HLA class I positivity, medium gray shading represents anti-HLA class II positivity, and light gray shading represents missing information about alloantibody status. N, normal biopsy; CTC, chronic tubular changes; ND, nondiagnostic biopsy; CG, chronic glomerulitis; ACR, acute cellular rejection; CA, chronic arteriopathy; BLC, borderline changes.
aBiopsy was not done because the patient was taking heparin.
bChronic tubular changes secondary to ureteropelvic junction obstruction after initial bleeding.
Detailed 24-month protocol biopsies of the above eight participants were compared with the 17 biopsies available from participants negative for cAb (Table 3). Although limited by the small sample size, there was no evidence of an association between cAb development and chronic histologic lesions.
Table 3.
Comparisons of 24-month protocol biopsies for cAb-positive versus cAb-negative participants
| Histological Lesion | cAb Positive (n=7) | cAb Negative (n=18) | P Value |
|---|---|---|---|
| Chronic tubular changes | 1 (14) | 5 (28) | 0.637 |
| Chronic arterial changes | 2 (28) | 1 (6) | 0.180 |
| Chronic glomerular changes | 1 (14) | 1 (6) | 0.490 |
| CNI toxicity | 1 (14) | 0 (0) | 0.280 |
Longitudinal Immunoprofiles Associated with cAb
Cells were available from two participants with detectable cAb: the first (patient A) had a positive result for anti-HLA class II cAb at all time points, whereas the second (patient B) had transiently detectable class I cAb at baseline and 12 months only, but developed persistent, donor-specific class II cAb at 9 months.
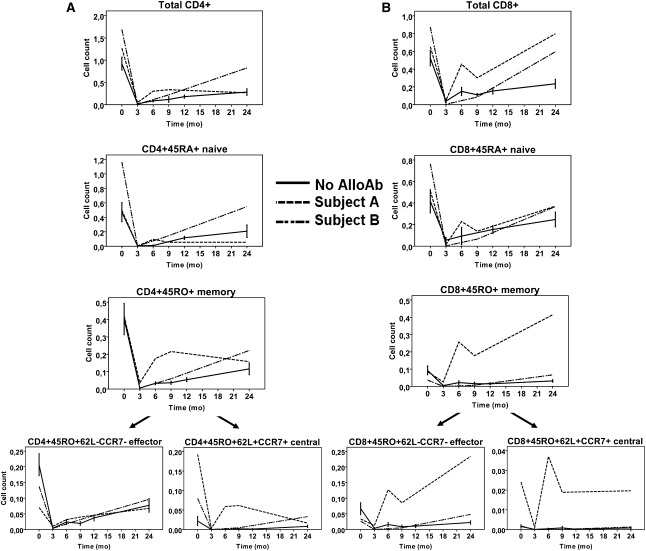
As shown in Figure 4, whereas CD4+ naïve cells in the first participant (patient A) had a similar depletion and repopulation profile as the controls, the memory cells were less depleted and recovered much faster; this accelerated recovery was mainly seen within the TCM subset. The Tregs demonstrated both a high magnitude and rate of recovery between 6 and 9 months, followed by a slow decline in cell count (Figure 5A), resulting in a high ratio of Tregs/TEM cells from 0–9 months with a pronounced decline thereafter (Figure 5C). Within the CD8+ subset, the memory cells appeared more resistant to depletion (Figure 4B) than naïve cells, and, furthermore, recovered to a level four times higher than their pretransplant value, an effect seen predominantly in the TEM compartment.
Figure 4.
Longitudinal T cell profiles for available participants who developed cAb during the course of the study. Means (± SEM) of available participants who did not develop cAb are shown as controls (n=9; except for TCM, n=3). (A) CD4+ subsets, (B) CD8+ subsets. Cell counts are expressed as 103 cells/µl. AlloAb, alloantibodies.
Figure 5.
Longitudinal CD4+25+FoxP3+ Tregs profiles for available participants who developed cAb during the course of the study. Means (± SEM) of available participants who did not develop cAb are shown as controls (n=6). (A) Absolute Tregs counts, (B) ratio of Tregs/Total CD4+ cells, (C) ratio of Tregs/TEM cells. Cell counts are expressed as 103 cells/µl. AlloAb, alloantibodies.
Compared with controls, the second participant (patient B) demonstrated a similar pattern of depletion of CD4+ subsets but a greater magnitude and rate of recovery of both naïve and memory cells (Figure 5A), whereas the Treg/TEM ratio showed a similar early peak (Figure 5C). For the CD8+ naïve and memory subsets, the depletion and recovery patterns were similar to those of controls (Figure 5B).
Discussion
This report, the first extensive immune profile of alemtuzumab-treated pediatric renal transplant recipients, reveals both differences and similarities in the pediatric and adult patterns of T cell depletion and reconstitution after alemtuzumab induction. In contrast to the adult response, pediatric naïve T cells displayed a similar recovery pattern to that of memory cells; overall, the magnitude of pediatric CD4+ and CD8+ T cell subset recovery was less than that of adults.1,2,9,15 Notwithstanding these differences, important parallels with the adult response included relatively less depletion of Tregs than total CD4+ cells and TEM cells, resulting in a transient increase in the ratio of Tregs/TEM cells,16 and within the memory subset, relatively less depletion of TEM than TCM cells.
The prolonged T cell depletion observed in our study is at variance with the general theory that, because of more active lymphopoiesis, depletion would be more transient in children.17 The recovery rates in adults were achieved with immunosuppression protocols similar to that used in this study. Trzonkowsi et al. used an induction regimen of alemtuzumab, followed by a steroid-free maintenance regimen consisting initially of mycophenolate mofetil (MMF) and tacrolimus; tacrolimus was switched to sirolimus at 6 months, whereas MMF was discontinued at 12 months.2 Knechtle et al. administered an induction regimen of alemtuzumab; tacrolimus and sirolimus were both started on day 1, tacrolimus subsequently stopped on day 60 after transplant.9
After T cell depletion, memory cells are reconstituted through homeostatic proliferation, whereas naïve T cell repopulation occurs primarily via thymic-dependent pathways.15,18 As mentioned, the capacity of the thymus to regenerate T cells seems to be inversely correlated with age, which explains why children usually show faster recovery of naïve T cells after chemotherapy-induced depletion.18 It was recently suggested that alemtuzumab, in addition to peripheral T cell depletion, induces prolonged depletion of the thymic output in adult renal transplant recipients.19 It is thus possible that the slow T cell reconstitution observed is due to a direct effect of alemtuzumab on the thymus. The pharmacokinetics of alemtuzumab may also differ between adults and children. For instance, during the pharmacological development of alemtuzumab, a humanized antibody, its t1/2 in adults was found to be substantially longer than the original rat mAb Campath-1G (14 to 21 days versus approximately 1 day), resulting in delayed lymphocyte recovery.20 To date, there are no published pharmacokinetic data regarding the use of alemtuzumab in pediatric solid organ transplantation.
The mechanism underlying the relative emergence of Tregs is poorly defined: It remains unclear if Tregs are comparatively resistant to depletion or if alemtuzumab is capable of inducing Treg proliferation in the period subsequent to T cell depletion. Importantly, in this study, this ratio peaked at 3 months after transplant, before the introduction of sirolimus. Although caution must be exercised before attributing tolerogenic properties to alemtuzumab in the pediatric setting, our data suggest that alemtuzumab does not have the initial detrimental effect on Treg frequency seen with other induction agents.21,22 The slow decrease in the Tregs/TEM ratio observed after 12 months is consonant with the hypothesis that the ratio of Tregs/memory T cells might decrease after homeostatic repopulation of the T cell compartment.23 All in all, it is tempting to speculate that the use of alemtuzumab in children might hamper the rapid homeostatic proliferation of memory T cells seen initially after depletion, potentially favoring long-term hyporesponsiveness to the graft.
The relative sparing of TEM cells seen in both adults and children is, however, of concern. Because TEM cells seem to be the predominant cell type involved in acute rejection, it follows that their emergence would be detrimental for allograft outcome, and, conversely, their depletion beneficial. In adults, Pearl et al. reported that at 1 week after transplant, the TEM subtype accounted for 88% of the remaining T cells after depletion.1 They and others have suggested that sparing of memory cells might be due to either preferential migration of memory cells to lymphoid and nonlymphoid organs or the upregulation of cell survival factors.23 Memory cells are characterized by the expression of antiapoptotic molecules and the responsiveness to homeostatic cytokines such as IL-7, both of which increase progressively with increasing antigenic exposure.24 It is conceivable that, given the relative lack of antigen exposure in children, their memory T cells lack the features essential to resist depletion upon contact with alemtuzumab. It is also possible that the more active pediatric thymus regenerates naïve T cells somewhat faster, providing competition for peripheral expansion of memory T cells for occupation of the empty T cell space.18 Regardless of the mechanism involved, although it seems to have a differential effect on the 24-month recovery of CD4+ TEM compared with TCM cells, it is reassuring that the overall effect of alemtuzumab is to increase the Treg/TEM ratio in vivo.
Further reassurance can be derived from the observation that although the cumulative hazard of cAb development was higher than described in large adult trials, in which the reported incidence ranges from 15%–20% at 1–5 years of follow-up,25–27 the development of cAb was neither associated with a decline in graft function, nor with detrimental histologic changes on the 2-year protocol biopsy. However, given the small sample size, the biopsy results must be interpreted with caution. Our findings suggest that the development of cAb, particularly when donor specific, may be associated with marked changes in T cell recovery. Moreover, they suggest that these changes are not widespread; rather, they are limited to specific T cell subsets.
In summary, our results indicate both differences and similarities in the adult and pediatric patterns of T cell depletion and repopulation after induction with alemtuzumab. Mainly, we found a relative sparing of Tregs over TEM cells, resulting in a transient increase in the Treg/TEM ratio that might be pro-tolerogenic. Long-term follow-up is underway to assess the potential benefits of this regimen in children.
Concise Methods
Study Population
PC-01 is a multicenter, single-arm, prospective phase II trial sponsored by the National Institute of Allergy and Infectious Disease. Thirty-five participants were enrolled from four centers (Children’s Hospital Boston; Children’s Hospital of Philadelphia; University of California, San Francisco; and Seattle Children’s Hospital). Patients who were unsensitized, first-time recipients with living donors, and between 1 and 20 years of age were eligible for inclusion. Deceased donor transplants, second transplants, presensitized patients, and those with a focal and segmental glomerulosclerosis were excluded. The duration of study follow-up was 2 years. There were 20 female and 15 male participants, with a mean (±SD) age of 12.6 (±5.4) years. Causes of renal failure included obstructive or dysplastic disorder (n=7 participants), glomerular or tubulo-interstitial disease (n=7 participants), genetic disorder (n=5 participants), or undefined disorder (n=16 participants).
Therapeutic Protocol and Sample Collection
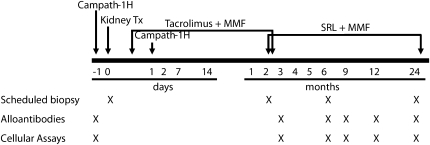
The therapeutic protocol, protocol biopsies, and blood sample collection schedule are presented in Figure 6. Tacrolimus was switched to sirolimus between 2 and 3 months after transplant, with an overlap period between 3 and 10 days. PBMCs were isolated from heparinized blood by density gradient centrifugation using Ficoll-Paque PLUS (GE Healthcare Biosciences AB, Uppsala, Sweden), washed twice with PBS, counted, and frozen at −140°C in liquid nitrogen. Cells were thawed by slow reconstitution with RPMI 1640 medium (Cambrex Bioscience, Walkersville, MD).
Figure 6.
Therapeutic and sample collection protocols. Each subject received 0.3 mg/kg (to a maximum of 20 mg) of alemtuzumab. Tacrolimus was initiated at a dose of 0.05–0.1 mg/kg twice daily, with a target trough level of 5–10 ng/ml, and was switched to sirolimus between 2 and 3 months after transplant (10 mg/m2 of loading dose followed by 3 mg/m2 every 12 hours, adjusted to attain trough levels of 12–15 ng/ml during the first 6 months and 8–12 ng/ml thereafter). MMF was administered at doses of 1200 mg/m2 per day (max 2 g) twice or thrice daily until day 14 after transplant, 900 mg/m2 per day until tacrolimus was switched to sirolimus, and 600 mg/m2 per day for the remainder of the study. SRL, sirolimus.
Flow Cytometry
Reconstituted cells were stained with the extracellular markers CD4, CD8, CD25, CD45RA, CD45RO, CD62L, and CCR7 (BD Biosciences, San Jose, CA), as well as intracellular marker FoxP3 (eBioscience Inc, San Diego, CA), and were analyzed as described previously.28
Alloantibody Screening
For the purpose of the mechanistic study, serum was assayed for anti-HLA cAb using the following One Λ kits on the Luminex 200 platform (Luminex Corp, Austin, TX): Labscreen, PRA Class I and Class II, and Single Antigen Beads (One Λ Inc, Canoga Park, CA). In the clinical setting, however, cross-matching was performed using the C-dependent cytotoxicity assay.
Statistical Analyses
Statistical analysis was performed using SAS 9.2 software (SAS Institute Inc, Cary, NC). Cell counts were analyzed longitudinally using generalized estimating equations. Alloantibody development was analyzed using the Kaplan–Meier technique. All P values were two tailed, and P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the following principal investigators and research coordinators of the four clinical sites for enrolling patients and obtaining research samples: Kevin E.C. Meyers and Jo Ann Palmer, CRNP (Nephrology Division, Department of Pediatrics, Children’s Hospital of Philadelphia); Ruth A. McDonald and Kara Farquharson (Division of Nephrology, Department of Pediatrics, Seattle Children’s Hospital); Anthony A. Portale and Marilyn McEnhill, PNP (Nephrology Division, Department of Pediatrics, University of California, San Francisco); and William E. Harmon and Leslie D. Spaneas (Nephrology Division, Department of Medicine, Children’s Hospital Boston). We also thank Dr. Nancy Bridges (National Institute of Allergy and Infectious Diseases) for her insightful comments and review of the manuscript, as well as Mrs. Yvonne Morrison for her tireless and continuous administrative guidance throughout the duration of this study.
This work was supported by the Cooperative Clinical Trials in Pediatric Transplantation (CCTPT) through the National Institutes of Health (NIH) Grant 5UO1-AI055801. S.A.D.S. is the recipient of a Kidney Research Scientist Core Education and National Training (KRESCENT) Program Post-Doctoral Fellowship Award and a McLaughlin’s Scholarship from Université Laval, Québec, Canada. N.N. received support from the Clinical Trials in Organ Transplantation (CTOT) cooperative research program through NIH Grant U01 AI 063623.
The data reported within this article were presented in a plenary session at the 2010 American Transplant Congress, May 4, 2010, in San Diego, California.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011040360/-/DCSupplemental.
References
- 1.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, Swanson SJ, Mannon RB, Roederer M, Kirk AD: Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant 5: 465–474, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Trzonkowski P, Zilvetti M, Chapman S, Wieckiewicz J, Sutherland A, Friend P, Wood KJ: Homeostatic repopulation by CD28-CD8+ T cells in alemtuzumab-depleted kidney transplant recipients treated with reduced immunosuppression. Am J Transplant 8: 338–347, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Ciancio G, Burke GW, 3rd: Alemtuzumab (Campath-1H) in kidney transplantation. Am J Transplant 8: 15–20, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Gallon L, Gagliardini E, Benigni A, Kaufman D, Waheed A, Noris M, Remuzzi G: Immunophenotypic analysis of cellular infiltrate of renal allograft biopsies in patients with acute rejection after induction with alemtuzumab (Campath-1H). Clin J Am Soc Nephrol 1: 539–545, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, Aiello S, Cassis L, Gotti E, Gaspari F, Cattaneo D, Perico N, Remuzzi G: Regulatory T cells and T cell depletion: Role of immunosuppressive drugs. J Am Soc Nephrol 18: 1007–1018, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N: A novel mechanism of action for anti-thymocyte globulin: Induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol 17: 2844–2853, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Knechtle SJ, Pirsch JD, Fechner JH, Jr, Becker BN, Friedl A, Colvin RB, Lebeck LK, Chin LT, Becker YT, Odorico JS, D’Alessandro AM, Kalayoglu M, Hamawy MM, Hu H, Bloom DD, Sollinger HW: Campath-1H induction plus rapamycin monotherapy for renal transplantation: Results of a pilot study. Am J Transplant 3: 722–730, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Flechner SM, Friend PJ, Brockmann J, Ismail HR, Zilvetti M, Goldfarb D, Modlin C, Mastroianni B, Savas K, Devaney A, Simmonds M, Cook DJ: Alemtuzumab induction and sirolimus plus mycophenolate mofetil maintenance for CNI and steroid-free kidney transplant immunosuppression. Am J Transplant 5: 3009–3014, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Knechtle SJ, Pascual J, Bloom DD, Torrealba JR, Jankowska-Gan E, Burlingham WJ, Kwun J, Colvin RB, Seyfert-Margolis V, Bourcier K, Sollinger HW: Early and limited use of tacrolimus to avoid rejection in an alemtuzumab and sirolimus regimen for kidney transplantation: Clinical results and immune monitoring. Am J Transplant 9: 1087–1098, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Pascual J, Pirsch JD, Odorico JS, Torrealba JR, Djamali A, Becker YT, Voss B, Leverson GE, Knechtle SJ, Sollinger HW, Samaniego-Picota MD: Alemtuzumab induction and antibody-mediated kidney rejection after simultaneous pancreas-kidney transplantation. Transplantation 87: 125–132, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Moudgil A, Puliyanda D: Induction therapy in pediatric renal transplant recipients: An overview. Paediatr Drugs 9: 323–341, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Bartosh SM, Knechtle SJ, Sollinger HW: Campath-1H use in pediatric renal transplantation. Am J Transplant 5: 1569–1573, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Tan HP, Donaldson J, Ellis D, Moritz ML, Basu A, Morgan C, Vats AN, Erkan E, Shapiro R: Pediatric living donor kidney transplantation under alemtuzumab pretreatment and tacrolimus monotherapy: 4-year experience. Transplantation 86: 1725–1731, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Tzakis AG, Kato T, Nishida S, Levi DM, Madariaga JR, Nery JR, Mittal N, Regev A, Cantwell P, Gyamfi A, Weppler D, Miller J, Tryphonopoulos P, Ruiz P: Preliminary experience with campath 1H (C1H) in intestinal and liver transplantation. Transplantation 75: 1227–1231, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Mueller TF: Phenotypic changes with immunosuppression in human recipients. Front Biosci 8: d1254–d1274, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Bloom DD, Chang Z, Fechner JH, Dar W, Polster SP, Pascual J, Turka LA, Knechtle SJ: CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am J Transplant 8: 793–802, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Knechtle SJ: Present experience with Campath-1H in organ transplantation and its potential use in pediatric recipients. Pediatr Transplant 8: 106–112, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Mackall CL, Gress RE: Thymic aging and T-cell regeneration. Immunol Rev 160: 91–102, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Scarsi M, Bossini N, Malacarne F, Valerio F, Sandrini S, Airò P: The number of circulating recent thymic emigrants is severely reduced 1 year after a single dose of alemtuzumab in renal transplant recipients. Transpl Int 23: 786–795, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Rebello P, Cwynarski K, Varughese M, Eades A, Apperley JF, Hale G: Pharmacokinetics of CAMPATH-1H in BMT patients. Cytotherapy 3: 261–267, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Pearl JP, Preston E, Kirk AD: Tolerance: Is it achievable in pediatric solid organ transplantation? Pediatr Clin North Am 50: 1261–1281, vii, 2003 [DOI] [PubMed]
- 22.De Serres SA, Sayegh MH, Najafian N: Immunosuppressive drugs and Tregs: A critical evaluation! Clin J Am Soc Nephrol 4: 1661–1669, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Neujahr DC, Chen C, Huang X, Markmann JF, Cobbold S, Waldmann H, Sayegh MH, Hancock WW, Turka LA: Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol 176: 4632–4639, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Lanzavecchia A, Sallusto F: Understanding the generation and function of memory T cell subsets. Curr Opin Immunol 17: 326–332, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Terasaki PI, Ozawa M: Predicting kidney graft failure by HLA antibodies: A prospective trial. Am J Transplant 4: 438–443, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Hourmant M, Cesbron-Gautier A, Terasaki PI, Mizutani K, Moreau A, Meurette A, Dantal J, Giral M, Blancho G, Cantarovich D, Karam G, Follea G, Soulillou JP, Bignon JD: Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol 16: 2804–2812, 2005 [DOI] [PubMed] [Google Scholar]
- 27.De Serres SA, Mfarrej BG, Guleria I, Najafian N, Morrison Y, Bridges N, Ikle D, Vincenti F, Harmon WH, Sayegh MH, Chandraker A: Silent development of de novo anti-HLA antibodies following transplantation is associated with histological changes in the allograft. Interim report on behalf of the CTOT-02/CCTPT-02 study [abstract SA-FC446]. J Am Soc Nephrol 21: 102A, 2010 [Google Scholar]
- 28.Najafian N, Salama AD, Fedoseyeva EV, Benichou G, Sayegh MH: Enzyme-linked immunosorbent spot assay analysis of peripheral blood lymphocyte reactivity to donor HLA-DR peptides: Potential novel assay for prediction of outcomes for renal transplant recipients. J Am Soc Nephrol 13: 252–259, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.