Abstract
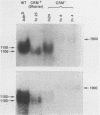
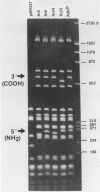
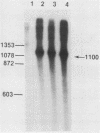
Two formaldehyde-induced, homozygous viable ADH-negative mutants, Adhfn4 and Adhfn6, possess no material that cross-reacts with antibody directed against ADH, no mature mRNA of wild-type size, and greatly reduced amounts of RNA that hybridizes with an Adh probe. We have cloned the genomic DNA sequences from these mutants in bacteriophage lambda Charon 4 and subcloned the Adh region into plasmid vector pBR327. Restriction analyses revealed one small deletion in each of these mutants and DNA sequencing showed that the splice junctions of the 65-base pair (bp) intervening sequence (IVS) were altered. Both cloned mutant Adh genes, as well as the wild-type gene, are capable of promoting correct specific transcription initiation in HeLa cell nuclear extracts in vitro. We conclude that Adhfn4 and Adhfn6 are defective in RNA processing. Our results provide evidence for the importance of the splice junction sequences in normal ADH RNA processing and stabilization in Drosophila. We also speculate that splicing of ADH RNA proceeds in a nonrandom manner: mutations in one of the intervening sequences appear to cause accumulation of a large ADH RNA containing at least one other IVS.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird M., Driscoll C., Schreiner H., Sciarratta G. V., Sansone G., Niazi G., Ramirez F., Bank A. A nucleotide change at a splice junction in the human beta-globin gene is associated with beta 0-thalassemia. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4218–4221. doi: 10.1073/pnas.78.7.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Place A. R., Powers D. A., Sofer W. Alcohol dehydrogenase gene of Drosophila melanogaster: relationship of intervening sequences to functional domains in the protein. Proc Natl Acad Sci U S A. 1981 May;78(5):2717–2721. doi: 10.1073/pnas.78.5.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Wang N., Reddy A., Weinberg E., Sofer W. Alcohol dehydrogenase in Drosophila: isolation and characterization of messenger RNA and cDNA clone. Nucleic Acids Res. 1980 Dec 11;8(23):5649–5667. doi: 10.1093/nar/8.23.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe R. Secondary structure and evolution of ribosomal RNA. Nature. 1981 Nov 19;294(5838):209–210. doi: 10.1038/294209a0. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumaki Y., Ghosh P. K., Benz E. J., Jr, Reddy V. B., Lebowitz P., Forget B. G., Weissman S. M. Abnormally spliced messenger RNA in erythroid cells from patients with beta+ thalassemia and monkey cells expressing a cloned beta+-thalassemic gene. Cell. 1982 Mar;28(3):585–593. doi: 10.1016/0092-8674(82)90213-6. [DOI] [PubMed] [Google Scholar]
- Gallwitz D. Construction of a yeast actin gene intron deletion mutant that is defective in splicing and leads to the accumulation of precursor RNA in transformed yeast cells. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3493–3497. doi: 10.1073/pnas.79.11.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Lai C. J., Dhar R., Khoury G. Splicing as a requirement for biogenesis of functional 16S mRNA of simian virus 40. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4317–4321. doi: 10.1073/pnas.76.9.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi N., Wu G. E., Murialdo H., Roberts L., Vetter D., Fife W. L., Whiteley M., Sadowski P. RNA splicing mutation in an aberrantly rearranged immunoglobulin lambda I gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7019–7023. doi: 10.1073/pnas.78.11.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Luse D. S., Roeder R. G. Accurate transcription initiation on a purified mouse beta-globin DNA fragment in a cell-free system. Cell. 1980 Jul;20(3):691–699. doi: 10.1016/0092-8674(80)90315-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., Vogeli G., Yamamoto T., de Crombrugghe B., Pastan I. Accurate in vitro transcriptional initiation of the chick alpha 2 (Type I) collagen gene. J Biol Chem. 1981 Nov 10;256(21):11251–11258. [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature. 1982 Feb 4;295(5848):380–384. doi: 10.1038/295380a0. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell J., Gerace L., Leister F., Sofer W. Chemical selection of mutants that affect alcohol dehydrogenase in Drosophila. II. Use of 1-pentyne-3-ol. Genetics. 1975 Jan;79(1):73–83. doi: 10.1093/genetics/79.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell J., Mandel H. C., Krauss M., Sofer W. Genetic and cytogenetic analysis of the Adh region in Drosophila melanogaster. Genetics. 1977 Jul;86(3):553–566. doi: 10.1093/genetics/86.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Goff S. C., Hechtman R. L. Mutation in an intervening sequence splice junction in man. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5041–5045. doi: 10.1073/pnas.78.8.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Shander M. H., Manley J. L., Gefter M. L., Maniatis T. Structure and in vitro transcription of human globin genes. Science. 1980 Sep 19;209(4463):1329–1336. doi: 10.1126/science.6158093. [DOI] [PubMed] [Google Scholar]
- Scherer G., Telford J., Baldari C., Pirrotta V. Isolation of cloned genes differentially expressed at early and late stages of Drosophila embryonic development. Dev Biol. 1981 Sep;86(2):438–447. doi: 10.1016/0012-1606(81)90202-5. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Sofer W. Alcohol dehydrogenase-negative mutants in Drosophila: defects at the structural locus? Genetics. 1976 May;83(1):125–136. doi: 10.1093/genetics/83.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Sofer W. H., Hatkoff M. A. Chemical selection of alcohol dehydrogenase negative mutants in drosophila. Genetics. 1972 Nov;72(3):545–549. doi: 10.1093/genetics/72.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnick D. An adenovirus mutant defective in splicing RNA from early region 1A. Nature. 1981 Jun 11;291(5815):508–510. doi: 10.1038/291508a0. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Jagadeeswaran P., Choudary P. V., Biro P. A., Elder J. T., deRiel J. K., Manley J. L., Gefter M. L., Forget B. G., Weissman S. M. Base substitution in an intervening sequence of a beta+-thalassemic human globin gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2455–2459. doi: 10.1073/pnas.78.4.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkington C. A., Nishioka Y., Leder P. In vitro transcription of normal, mutant, and truncated mouse alpha-globin genes. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7132–7136. doi: 10.1073/pnas.77.12.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Hirose S., Tsuda M., Suzuki Y. Promoter sequence of fibroin gene assigned by in vitro transcription system. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4838–4842. doi: 10.1073/pnas.78.8.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Kédinger C., Corden J., Brison O., Chambon P. Specific in vitro initiation of transcription on conalbumin and ovalbumin genes and comparison with adenovirus-2 early and late genes. Nature. 1980 Jun 5;285(5764):367–373. doi: 10.1038/285367a0. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Segall J., Harris B., Ng S. Y., Roeder R. G. Faithful transcription of eukaryotic genes by RNA polymerase III in systems reconstituted with purified DNA templates. J Biol Chem. 1979 Jul 10;254(13):6163–6173. [PubMed] [Google Scholar]
- Williams B. G., Blattner F. R. Construction and characterization of the hybrid bacteriophage lambda Charon vectors for DNA cloning. J Virol. 1979 Feb;29(2):555–575. doi: 10.1128/jvi.29.2.555-575.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]