Abstract
Aims
It is uncertain if the higher blood pressure (BP) observed in the offspring of hypertensive pregnancies is an isolated abnormality or one that is accompanied by impaired vascular function and alterations in lipid and inflammation markers that would be indicative of a more general cardiometabolic disturbance of the type observed in the mother during pre-eclampsia.
Methods and results
In a large UK cohort of maternal-offspring pairs (n = 3537–4654), assessed at age 9–12 years, we examined the associations of maternal gestational hypertension and pre-eclampsia with offspring BP, endothelial function assessed by brachial artery flow-mediated dilatation; arterial stiffness assessed by carotid to radial pulse wave velocity; brachial artery distensibility and BP (vascular outcomes); as well as markers of inflammation, lipids and apolipoproteins A1 and B. Offspring of women with pre-eclampsia or gestational hypertension had higher systolic blood pressure by 2.04 mmHg (95% CI: 1.33, 2.76) and 1.82 mmHg (95% CI: 0.03, 3.62), respectively, and higher diastolic blood pressure by 1.10 mmHg (95% CI: 0.47, 1.73) and 1.26 mmHg (95% CI: −0.32, 2.85), respectively, in analyses adjusted for maternal and offspring body mass index (BMI), offspring dietary sodium intake and other potential confounders. However, we found no associations of either hypertensive disorder of pregnancy with the other vascular outcomes or with inflammatory markers, lipids, and apolipoproteins.
Conclusion
Pre-eclampsia and gestational hypertension are associated with higher offspring BP in childhood in the absence of other vascular alterations or metabolic derangements. The findings support the existence of shared mother-offspring risk factors that are specific for higher BP, rather than the additional cardiometabolic abnormalities of hypertensive disorder of pregnancy having long-term consequences for offspring.
Keywords: Hypertensive disorder of pregnancy, Endothelial function, Arterial stiffness, Blood pressure, Lipids, ALSPAC
Introduction
Pre-eclampsia or gestational hypertension (hypertensive disorders of pregnancy; HDP) is associated with adverse perinatal outcomes for the mother and neonate, and also with higher maternal and offspring blood pressure in later life,1–7 though the mechanisms underlying the association with later offspring blood pressure remain unclear.8
Pre-eclampsia is a disease characterized by systemic inflammation, endothelial dysfunction, and insulin resistance in the pregnant mother.9–12 It has been suggested that these systemic influences result in as yet undetermined vasculotoxic factors being released into the maternal circulation that have adverse influences on foetal development. This, it has been suggested, results in increased risk of vascular dysfunction and chronic inflammation in later life of the offspring.13 If this hypothesis were true one would anticipate associations of pre-eclampsia with offspring endothelial dysfunction, other markers of vascular dysfunction and markers of chronic inflammation in later life, but few studies have examined these associations. In contrast if associations of both pre-eclampsia and gestational hypertension with offspring outcomes are specific to blood pressure, this would suggest that the underlying mechanism is more likely to be due to inherited genetic variants that are related to higher blood pressure or shared familial environmental factors that are specifically (or much more strongly) related to blood pressure, rather than intrauterine mechanisms. Dietary sodium intake is a specific risk factor for higher blood pressure and, thus, it may underlie associations between higher maternal blood pressure in pregnancy and higher offspring blood pressure.
In one small study, preterm infants born of mothers with HDP (either pre-eclampsia or gestational hypertension; n = 19) had lower flow-mediated dilatation (FMD), a marker of endothelial dysfunction, as well as higher blood pressure and thicker carotid intima in their mid-20s, than a group who had been born preterm but whose mothers were normotensive throughout pregnancy (n = 52).14 The authors of that study interpreted their findings as indicating that either genetic predisposition to endothelial dysfunction or the exposure in utero to inflammatory markers were important in explaining the link between HDP and future offspring risk of an unhealthy vascular phenotype. In a more recent study of term infants, those whose mothers had pre-eclampsia during pregnancy (n = 24) had lower FMD and higher pulmonary arterial pressure in their teens, but similar blood pressure and brachial arterial diameter to age-matched controls (n = 27).13 The authors suggested that their results supported an intrauterine mechanism driven by the release of vascular toxins (including inflammatory markers) into the maternal and developing foetal circulation. They further explored this in a within sibling study and found that among 10 sibling pairs FMD was lower and pulmonary arterial pressure higher in the sibling exposed to maternal pre-eclampsia compared with the one born later during a normal pregnancy, suggesting that the mechanism was at least in part via intrauterine mechanisms rather than fully explained by shared familial lifestyles or genetic factors related to vascular dysfunction.13
The aim of this study was to examine the association of HDP with offspring vascular outcomes [endothelial function assessed by brachial artery FMD, arterial stiffness assessed by carotid to radial pulse wave velocity (PWV), brachial distensibility coefficient (DC), brachial artery diameter, and systolic blood pressure (SBP) and diastolic blood pressure (DBP)]; markers of inflammation [C-reactive protein and Interluekin-6 (IL-6)]; lipids [triglyceride levels, high density lipoprotein cholesterol (HDLc), non-HDLc]; and apolipoproteins A1 and B, assessed in offspring at age 9–12 years in a large UK cohort of children who have been followed since their mother's pregnancy. This builds on an earlier publication in which associations with blood pressure only were examined.1
Methods
Study population
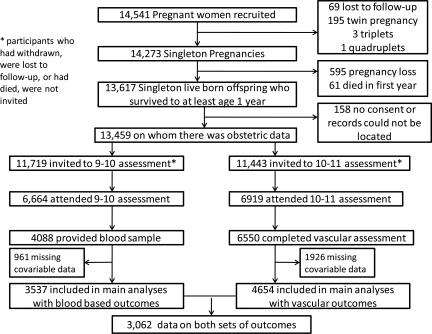
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a prospective population-based birth cohort study that recruited 14 541 pregnant women resident in Avon, UK with expected dates of delivery 1 April 1991 to 31 December 1992 (http://www.alspac.bris.ac.uk).15 Figure 1 shows the flow of participants through the study. In this study, we only included mother-offspring pairs where there was a singleton pregnancy resulting in a live birth with the child surviving to at least 1 year of age. The eligible cohort for the current analyses was the 4675 mother-offspring pairs who had complete data on maternal HDP and offspring blood assays of inflammatory markers, lipids, and apolipoproteins (obtained at the 9–10-year follow-up clinic) and the 6550 pairs who had complete data on HDP and had vascular outcome data (obtained at the 10–11-year follow-up clinic).
Figure 1.
Participant flow
Maternal pregnancy data
Six trained research midwives abstracted data from obstetric medical records. There were no between-midwife variation in mean values of abstracted data and repeated data entry checks demonstrated error rates consistently <1%. Obstetric data abstractions included every measurement of SBP, DBP, and proteinuria entered into the medical records and the corresponding gestational age and date at the time of these measurements. These measurements were obtained in routine clinical practice by trained midwives and obstetricians. The median number (inter-quartile range) of BP measurements in pregnancy was 14 (11, 16) and that of urine measurements was 11 (10, 14). We applied the International Society for the Study of Hypertension in Pregnancy16 criteria to all of the clinic data in order to determine women with pre-eclampsia and those with gestational hypertension. Using these criteria pre-eclampsia was defined as a SBP >139 mmHg or a DBP >89 mmHg, measured on at least two occasions after 20 weeks of gestation, with proteinuria, diagnosed if the protein reading on dipstick testing (Albustix; Ames Company, Elkhart, IN, USA) was at least 1+ (30 mg/dL), occurring at the same time as the elevated BP.16 Gestational hypertension was defined as the same pattern of elevated blood pressure but without proteinuria.16 Thus, all women were categorized into one of three mutually exclusive categories of no HDP, gestational hypertension, or pre-eclampsia. A total of 12 women with either pre-eclampsia or gestational hypertension and 9 with no evidence of HDP, reported a previous diagnosis of hypertension unrelated to pregnancy; none of these women were on antihypertensive treatment. A further 13 women had blood pressure ≥139/89 on at least two occasions prior to 20 weeks gestation (again none was on antihypertensive medication). When these 25 women were excluded from the analyses the results were unchanged from those presented here that include them.
Offspring outcome measurements
Current age of the child was recorded in months as they arrived at the assessment clinics. Inflammatory markers, lipids, and apolipoproteins were assessed at the 9–10-year assessment. Non-fasting blood samples were taken using standard procedures, with samples immediately spun and frozen at −80°C. The measurements were assayed after a median of 7.5 years in storage with no previous freeze–thaw cycles during this period. Plasma lipids (total cholesterol, triglycerides, and HDLc) were assayed by modification of the standard Lipid Research Clinics Protocol using enzymatic reagents for lipid determination. Apolipoprotein A1 and B were measured by immunoturbidimetric assays (Hitachi/Roche). Interluekin-6 was measured by ELISA (R&D systems, Abingdon, UK) and C-reactive protein was measured by automated particle-enhanced immunoturbidimetric assay (Roche UK, Welwyn Garden City, UK). Non-HDLc was calculated as total cholesterol minus HDLc.
Vascular phenotypes were assessed at the 10–11-year follow-up assessment and detailed descriptions of the measurements have been previously reported.17 For PWV, pressure pulse waveforms were recorded transcutaneously using a high-fidelity micromanometer (SPC-301, Millar Instruments, Houston, TX, USA) from the radial and carotid pulse, synchronous with the ECG signal. Integral software processed the data to calculate the mean time difference between R-wave and pressure wave on a beat-to-beat basis over 10 s, and the PWV was then calculated using the mean time difference and arterial path length between the two recording points (SphygmoCorversion 7.1, Scanmed, UK). Ultrasound images of the right brachial artery were used to measure FMD and DC. Images were recorded onto SVHS video using an ALOKA 5500 high-resolution ultrasound system with a 5–10 MHz linear array probe (Keymed, UK) and the measurements were undertaken later from the videos at the Vascular Physiology Unit, Institute of Child Health, London. The right brachial artery was imaged 5–10 cm above the antecubital fossa. Flow-mediated dilatation was induced by a 5-min inflation of a pneumatic cuff, placed around the forearm immediately below the medial epicondyle, to 200 mmHg followed by rapid deflation using an automatic air regulator. The diameter of the brachial artery was measured using edge detection software (Brachial Tools, MIA, IA, USA) from ECG-triggered ultrasound images captured at 3 s intervals throughout the 11-min recording protocol. Flow-mediated dilatation was expressed as an absolute value in our analyses. Flow-mediated dilatation expressed as the maximum percentage change in vessel diameter from baseline correlated very highly with this absolute value (Pearson's correlation coefficient = 0.95) and findings were essentially the same if % change was used rather than the absolute value. The distension of the artery was determined by measuring the luminal diameter excursion from diastole to systole. Distensibility coefficient was calculated from the distension and the pulse pressure and was expressed as mean per cent change in cross-sectional area per unit change in blood pressure.
Other characteristics
Maternal age, parity, mode of delivery (caesarean section/vaginal delivery), gestational age at delivery, and the child's sex and birthweight were obtained from the obstetric records. At the time of recruitment, mothers were asked to report their pre-pregnancy weight and height, which were used to calculate maternal pre-pregnancy body mass index (BMI). Maternal self-report of pre-pregnancy weight and her measured weight at the first antenatal clinic were highly correlated (Pearson's correlation coefficient = 0.95; P < 0.0001). Based on questionnaire responses, the highest parental occupation was used to allocate the children to family social class groups [classes I (professional/managerial) to V (unskilled manual workers), using the 1991 British Office of Population and Census Statistics (OPCS) classification]. Mothers were repeatedly asked about their smoking throughout pregnancy and these data were used to generate a categorical variable: never smoked; smoked before pregnancy or in the first trimester and then stopped; smoked throughout pregnancy.
At both clinic assessments, the child's age in months was recorded and their weight and height were measured in light clothing and without shoes. Weight was measured to the nearest 0.1 kg using Tanita scales. Height was measured to the nearest 0.1 cm using a Harpenden stadiometer. Waist circumference was measured to the nearest 1 mm at the mid-point between the lower ribs and the pelvic bone with a flexible tape. Dual energy X-ray absorptiometry (DXA) scans were used to measure total fat mass.
At the same time as the vascular measurements (mean age: 10.7), offspring diet was assessed using three 1-day unweighed dietary diaries in which children and carers recorded everything the child ate and drank in household measures for 2 weekdays and 1 weekend day. Data from these diaries were used to determine offspring dietary sodium (salt) intake. Completed diaries were brought to the clinic and nutrition fieldworkers checked through the diaries to increase completeness and remove uncertainties. Diet diaries were transformed into food codes with associated weights in grams for each item of food and drink recorded, by the same fieldworker using the Diet in, Diet out (DIDO) programme.18 Food codes and nutrient content, including sodium content, were derived from McCance and Widdowsons food tables and supplements.18
Statistical analysis
Triglyceride, C-reactive protein, IL-6 levels, DXA-determined fat mass, and DC were positively skewed and were natural log transformed to produce approximately normal distributions and normal residuals in the regression models. In the descriptive analyses, for these variables, geometric means are presented. In the regression analyses, associations of HDP with outcomes that were log transformed are presented as ratios of geometric means. Pearson's correlation coefficients were used to explore associations between all outcomes using the 3062 participants with complete data on both sets of outcomes (Figure 1).
To obtain reliable 95% confidence intervals in the context of multiple comparisons, we used a non-parametric bootstrap procedure in conjunction with OLS multivariable linear regression, based on 10 000 replications. Beta estimates and standard errors were calculated from the mean and standard deviation of the bootstrap distribution, respectively. The bootstrap means and standard errors, compared with a z-distribution, were used to calculate the 95% percentile confidence intervals. Multivariable linear regression was used to examine the associations of pre-eclampsia vs. no HDP and gestational hypertension vs. no HDP with each outcome. In the basic model, we adjusted only for the child's gender and age at the time of outcome measurement. We then adjusted for maternal characteristics that could confound the association (model 2)—maternal age, parity, pre-pregnancy BMI, smoking during pregnancy, and education, head of household social class. In model 3, we adjusted for offspring adiposity (BMI in the main analyses, but showing that substituting BMI for either fat mass or waist circumference, did not result in different effects to adjusting only for BMI). While offspring BMI occurs after the exposure, we consider it part of a confounding pathway via its relationship to maternal BMI.1 For vascular outcomes only, we then adjusted for offspring dietary sodium intake (model 4). As with offspring BMI, while this was measured after the exposure of HDP, we considered it to be potentially part of a confounding pathway reflecting familial dietary sodium intake, high levels of which in the mothers could result in increased risk of HDP and increased likelihood of their offspring having a high intake, which would result in higher offspring blood pressure. In the final model (model 4 for blood-based outcomes and 5 for vascular outcomes) we adjusted for possible mediation by markers of intrauterine mechanisms (gestational age, mode of delivery and birthweight). Lastly, as blood pressure is associated with most of the other vascular and biomarker outcomes that we have examined here, if we found any associations of HDP with other vascular outcomes these may be generated entirely or in part by the association of HDP with offspring blood pressure. We examined this by adjusting for SBP or DBP (first entering SBP and then removing it and entering DBP) in any regression models where an association was found between HDP and one of the other outcomes.
Because a previous study had suggested that HDP was associated with offspring endothelial dysfunction only in offspring who were born preterm,14 we examined whether there was any evidence that associations differed between those born preterm (gestational age <37 complete weeks) and those born term (≥37 complete weeks gestation). A likelihood ratio test was used to examine the interaction between preterm/term and HDP in their associations with outcomes.
Dealing with missing data
There were small amounts of missing data on some covariables included in the multivariable models (Figure 1). Of the 4088 participants who attended the 9–10-year follow-up clinic and provided a blood sample, 3537 (86%) had complete data on all outcomes, exposure, and covariables included in any model. Of the 6550 participants who attended the 10–11-year follow-up clinic and completed the vascular assessment, 4654 (71%) had complete data on all outcomes, exposure, and covariables included in any model. We undertook sensitivity analyses aimed at exploring whether missing data might have biased our results. In two of these, we only imputed missing covariable data and so conducted analyses on the 4088 mother-offspring pairs who had complete data on HDP and blood-based assays for the analyses with these outcomes and on the 6550 pairs with complete data on HDP and vascular outcomes for these outcomes. In a further two, we extended the imputation data sets by imputing up to the 6664 mother-offspring pairs where the offspring had attended the 9–10-year clinic (irrespective of whether they gave a blood sample) and the 6919 pairs who attended the 10–11 clinic (irrespective of whether the undertook any of the vascular outcome measurements). Finally, we imputed up to 8665 mother-offspring pairs where the offspring had attended either of the 9–10- or 10–11-year clinic. In all of these analyses, we used multivariable multiple imputation in Stata as described by Royston.19 We carried out 20 cycles of regression switching and generated 20 imputation data sets. The multiple multivariate imputation approach creates a number of copies of the data (in this case, we generated 20 copies) in which missing values are imputed by chained equations.19 The main analysis results are obtained by averaging across the results from each of these 20 data sets using Rubin's rules which ensure that the standard errors for any regression coefficients take account of uncertainty in the imputations as well as uncertainty in the estimation.19 The results from these sensitivity analyses did not differ substantively from the results presented here based only on those with complete data (main analysis cohorts in Figure 1); these results are available from the authors on request.
Results
Among the 13 459 eligible women with complete obstetric data (Figure 1), 11 043 (82%) had no HDP, 2097 (16%) had gestational hypertension, and 319 (2%) had pre-eclampsia. Proportions were similar in the 6550 who attended the vascular clinic [5368 (82%) no HDP; 1039 (16%) with gestational hypertension; 143 (2%) with pre-eclampsia] and the 4088 who had blood assay results [3327 (81%) no HDP; 689 (17%) with gestational hypertension; 88 (2%) with pre-eclampsia]; they were also similar in the groups with complete data on all covariables (see Tables 2 and 3 for numbers in each category). Table 1 shows the correlations between all outcomes in the 3062 participants with data from both the 9.9-year clinic at which blood-based assays were obtained and the 10.7-year clinic at which vascular measurements were obtained, as well as data on HDP and all covariables. Even where statistically significant at conventional 5% levels, the majority of correlations were weak. Expected strong positive correlations were observed between HDLc and Apolipoprotein A1, non-HDLc and Apolipoprotein B, and C-reactive protein and IL-6 and strong inverse correlations between HDLc and triglycerides.
Table 2.
Distributions of participant characteristics by hypertensive disorder of pregnancy status
| Characteristic | Mean (SD) [N] by HDP category |
P-valuea | ||
|---|---|---|---|---|
| No HDP | Gestational hypertension | Pre-eclampsia | ||
| Maternal age (years) | 29.0 (4.5) [5035] | 28.9 (4.8) [1000] | 29.5 (5.4) [137] | 0.36 |
| Maternal pre-pregnancy BMI (kg/m2) | 22.5 (3.2) [4567] | 24.6 (4.6) [890] | 26.1 (6.1) [123] | <0.001 |
| Gestational age (weeks) | 39.5 (1.7) [5035] | 39.6 (1.7) [1000] | 37.6 (3.2) [137] | <0.001 |
| Birthweight (g) | 3443 (507) [4975] | 3435 (566) [992] | 3042 (929) [133] | <0.001 |
| Offspring age (months)b | 118 (4) [4654] | 118 (4) [994] | 118 (3) [132] | 0.98 |
| Offspring BMI (kg/m2)b | 17.6 (2.8) [4599] | 18.1 (3.2) [935] | 17.9 (3.3) [132] | <0.001 |
| Offspring waist (mm)b | 62.6 (7.5) [4640] | 64.1 (8.6) [943] | 63.5 (9.2) [132] | <0.001 |
| Offspring fat mass (kg)b,c | 7.0 (6.8, 7.1) [3403] | 7.8 (7.5, 8.1) [715] | 7.3 (6.4, 8.2) [86] | <0.001 |
| Offspring HDLc (mmol/L)b | 1.40 (0.31) [3369] | 1.38 (0.29) [689] | 1.37 (0.30) [88] | 0.21 |
| Offspring non-HDLc (mmol/L)b | 2.87 (0.65) [3369] | 2.88 (0.64) [689] | 2.87 (0.59) [88] | 0.76 |
| Offspring triglyceride (mmol/L)b,c | 1.03 (1.01, 1.04) [3369] | 1.03 (1.00, 1.06) [689] | 0.99 (0.91, 1.09) [88] | 0.78 |
| Offspring apolipoprotein A1 (mg/dL)b | 136 (20) [3369] | 135 (19) [689] | 136 (17) [88] | 0.42 |
| Offspring apolipoprotein B (mg/dL)b | 59 (13) [3369] | 60 (13) [689] | 60 (12) [88] | 0.69 |
| Offspring C-reactive protein (mg/L)b,c | 0.27 (0.26, 0.28) [3369] | 0.29 (0.26, 0.31) [689] | 0.29 (0.23, 0.37) [88] | 0.30 |
| Offspring IL6 (pg/mL)b,c | 0.84 (0.82, 0.87) [3363] | 0.86 (0.81, 0.91) [688] | 0.94 (0.79, 1.11) [86] | 0.51 |
| Offspring age (months)d | 128 (3) [5367] | 128 (3) [1039] | 128 (3) [143] | 0.45 |
| Offspring SBP (mmHg)d | 104 (9) [5367] | 106 (9) [1039] | 107 (11) [143] | <0.001 |
| Offspring DBP (mmHg)d | 60 (8) [5367] | 61 (8) [1039] | 62 (8) [143] | <0.001 |
| Offspring FMD (mm)d | 0.21 (0.09) [4826] | 0.21 (0.09) [921] | 0.21 (0.08) [129] | 0.47 |
| Offspring PWV (m/s)d | 7.56 (1.23) [5434] | 7.54 (1.22) [1061] | 7.74 (1.26) [147] | 0.20 |
| Offspring DC (% per mmHg)d,c | 11.4 (11.3, 11.6) [4983] | 11.2 (10.9, 11.5) [961] | 10.9 (10.1, 11.9) [133] | 0.33 |
| Brachial artery diameter (mm)d | 2.67 (0.30) [4983] | 2.70 (0.31) [961] | 2.65 (0.33) [133] | 0.06 |
| Binary characteristics [n/N (%) by HDP category] | ||||
| First pregnancy for mother | 2129/4857 (44) | 587/970 (60) | 91/133 (68) | <0.001 |
| Mother smoked during pregnancy | 940/4914 (19) | 136/982 (14) | 16/135 (12) | <0.001 |
| Mother has university degree | 790/4845 (16) | 159/960 (16) | 15/132 (11) | 0.27 |
| Head of household in manual social class | 655/4619 (14) | 128/918 (14) | 21/125 (17) | 0.70 |
| Delivery by caesarean section | 449/4953 (9) | 165/997 (17) | 49/137 (36) | <0.001 |
| Infant male | 2443/5035 (49) | 520/1000 (52) | 76/137 (55) | 0.04 |
HDP, hypertensive disorder of pregnancy; BMI, body mass index; HDLc, high density lipoprotein cholesterol; IL-6, Interlukin-6; SBP, systolic blood pressure; DBP, diastolic blood pressure; FMD, flow-mediated dilatation; PWV, pulse wave velocity; DC, distensibility coefficient.
aF-test for continuous characteristics and χ2 for binary characteristics; both with 2 df testing the null hypothesis that distributions of characteristics are the same across the three HDP categories.
bAssessed at 9-year assessment.
cGeometric means and 95% confidence intervals of these means are presented.
dAssessed at 10-year assessment.
Table 3.
Multivariable associations of hypertensive disorder of pregnancy with offspring lipids, apolipoproteins, and inflammatory markers at mean age 9.9 years (n = 3537 with complete data on all variables used in any model)
| Outcome | Model | Mean difference (95% CI) |
||
|---|---|---|---|---|
| No HDP, n = 2869 | Gestational HT, n = 598 | Pre-eclampsia, n = 70 | ||
| HDLc (mmol/L) | M1 | 0 | −0.02 (−0.05, 0.01) | −0.04 (−0.11, 0.03) |
| M2 | 0 | 0.00 (−0.03, 0.03) | −0.02 (−0.09, 0.06) | |
| M3 | 0 | −0.01 (−0.03, 0.02) | −0.03 (−0.11, 0.04) | |
| M4 | 0 | 0.00 (−0.02, 0.03) | −0.04 (−0.11, 0.03) | |
| Non-HDLc (mmol/L) | M1 | 0 | 0.03 (−0.03, 0.08) | 0.03 (−0.12, 0.18) |
| M2 | 0 | 0.01 (−0.06, 0.07) | 0.00 (−0.15, 0.15) | |
| M3 | 0 | 0.01 (−0.05, 0.07) | −0.01 (−0.16, 0.14) | |
| M4 | 0 | 0.01 (−0.05, 0.06) | 0.01 (−0.14, 0.16) | |
| Triglycerides (ratio geometric means)a | M1 | 1 | 1.00 (0.96, 1.04) | 0.97 (0.86, 1.08) |
| M2 | 1 | 0.98 (0.94, 1.02) | 0.95 (0.84, 1.06) | |
| M3 | 1 | 0.98 (0.94, 1.02) | 0.96 (0.85, 1.07) | |
| M4 | 1 | 0.97 (0.93, 1.01) | 0.97 (0.86, 1.07) | |
| Apolipoprotein A1 (mg/dL) | M1 | 0 | −1.30 (−3.06, 0.45) | −0.44 (−5.18, 4.29) |
| M2 | 0 | −0.46 (−2.28, 1.37) | 0.72 (−4.08, 5.51) | |
| M3 | 0 | −0.61 (−2.43, 1.22) | 0.23 (−5.12, 4.66) | |
| M4 | 0 | −0.09 (−1.89, 1.72) | −0.61 (−5.43, 4.21) | |
| Apolipoprotein B (mg/dL) | M1 | 0 | 0.63 (−0.50, 1.76) | 1.09 (−1.96, 4.14) |
| M2 | 0 | 0.33 (−0.85, 1.50) | 0.57 (−2.52, 3.66) | |
| M3 | 0 | 0.28 (−0.90, 1.45) | 0.49 (−2.66, 3.64) | |
| M4 | 0 | 0.39 (−0.77, 1.55) | 0.85 (−2.23, 3.94) | |
| C-reactive protein (ratio geometric means)a | M1 | 1 | 1.13 (1.01, 1.25) | 1.19 (0.89, 1.47) |
| M2 | 1 | 1.03 (0.91, 1.14) | 1.05 (0.76, 1.35) | |
| M3 | 1 | 1.02 (0.91, 1.13) | 1.04 (0.76, 1.33) | |
| M4 | 1 | 0.99 (0.88, 1.09) | 1.10 (0.81, 1.38) | |
| IL-6 (ratio geometric means)a | M1 | 1 | 1.08 (1.00, 1.16) | 1.16 (0.95, 1.36) |
| M2 | 1 | 1.02 (0.94, 1.11) | 1.09 (0.88, 1.30) | |
| M3 | 1 | 1.02 (0.94, 1.11) | 1.04 (0.75, 1.27) | |
| M4 | 1 | 0.99 (0.89, 1.08) | 1.09 (0.88, 1.30) | |
M1: Adjusted for offspring sex and age at time of outcome measurement.
M2: Maternal confounder adjusted model: as M1 plus adjustment for maternal age, nulliparity, smoking during pregnancy, pre-pregnancy BMI, education and head of household social class.
M3: Confounder adjusted model taking account of offspring adiposity: as M2 plus adjustment for offspring BMI, height and height-squared (NB: substituting BMI for offspring waist circumference or for offspring fat mass did not substantively alter any of the results).
M4: Mediator for intrauterine characteristics model: as M3 plus adjustment for birthweight, gestational age and mode of delivery.
HDLc, high density lipoprotein cholesterol; IL-6, Interlukin-6.
aResults for these outcomes are ratios of geometric means. As indicated in column 1 the null value for these results is 1 (it is 0 for other results which are mean differences).
Table 1.
Correlation coefficients between biomarker and vascular outcomes assessed age 9–11 years (n = 3062)
| HDLc | N-HDLc | Trigs | ApoA1 | ApoB | C-reactive protein | IL-6 | SBP | DBP | FMD | PWV | DC | BD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HDLc | 1 | ||||||||||||
| N-HDLc | −0.20* | 1 | |||||||||||
| Trigs | −0.44* | 0.41* | 1 | ||||||||||
| ApoA1 | 0.82* | 0.00**** | −0.12* | 1 | |||||||||
| ApoB | −0.18* | 0.89* | 0.22* | −0.03**** | 1 | ||||||||
| C-reactive protein | −0.22* | 0.05** | 0.03*** | −0.20* | 0.10* | 1 | |||||||
| IL-6 | −0.18* | −0.01**** | 0.03*** | −0.19* | 0.01**** | 0.48* | 1 | ||||||
| SBP | −0.05** | 0.05** | 0.02**** | −0.04*** | 0.05** | 0.07* | 0.06** | 1 | |||||
| DBP | −0.05** | 0.03**** | −0.01**** | −0.06** | 0.06** | 0.09* | 0.07* | 0.58* | 1 | ||||
| FMD | −0.06** | 0.03**** | 0.07* | −0.03**** | 0.02**** | 0.06** | 0.04*** | 0.01**** | 0.03**** | 1 | |||
| PWV | 0.05** | −0.05** | −0.06** | 0.03**** | −0.04*** | −0.08* | −0.03*** | 0.09* | 0.21* | −0.01**** | 1 | ||
| DC | −0.05** | −0.03*** | −0.02*** | −0.05** | −0.01**** | 0.02 | 0.01**** | −0.11* | 0.06* | −0.13* | −0.16* | 1 | |
| BD | −0.06** | −0.02**** | 0.04**** | −0.03**** | −0.03*** | 0.02**** | 0.00**** | 0.04** | −0.08* | −0.01**** | −0.13* | −0.06* | 1 |
HDLc, high density lipoprotein cholesterol; N-HDLc, non-high density lipoprotein cholesterol; ApoA1, Apolipoprotein A1; ApoB, Apolipoprotein B; IL-6, Interlukin-6; SBP, systolic blood pressure; DBP, diastolic blood pressure; FMD, flow-mediated dilatation; PWV, pulse wave velocity; DC, distensibility coefficient; BD, Brachial diameter.
*P < 0.001.
**P ≥ 0.001 <0.01.
***P ≥ 0.01 <0.05.
****P ≥ 0.05.
Table 2 shows the distributions of potential confounding and mediating characteristics, as well as outcomes by whether the mother had no HDP, gestational hypertension, or pre-eclampsia. Women with either gestational hypertension or pre-eclampsia compared with those without HDP had higher pre-pregnancy BMI, were more likely to be in their first pregnancy, less likely to smoke and more likely to have a caesarean section; their offspring were more likely to be male, had earlier gestational age, lower birthweight and had greater mean BMI, waist circumference and fat mass at mean age 9.9 years. Other potential covariables did not vary by HDP category. With respect to outcomes, both offspring SBP and DBP assessed at mean age 10.7 years were greater in those whose mothers had experienced either gestational hypertension or pre-eclampsia (consistent with our previous paper where these were measured at age 9.9 years1). Mean brachial diameter was slightly higher in offspring of mothers who had previously experienced gestational hypertension, but slightly lower in those who had previously experienced pre-eclampsia. None of the distributions of the other vascular phenotypes, lipids, apolipoproteins, or inflammatory markers differed by HDP status in these unadjusted analyses.
Table 3 shows the multivariable associations of HDP with lipids, apolipoproteins, and inflammatory markers, assessed at mean age 9.9 years, in the 3537 participants with complete data exposure, blood-based outcomes and any covariables included in any model. With the exception of inflammatory markers, there were no associations of HDP with any of the blood-based outcomes in any models. Both C-reactive protein and IL-6 were elevated in offspring whose mothers had experienced either gestational hypertension or pre-eclampsia in the first model adjusting only for the offspring sex and age at outcome assessment, but following adjustment for maternal confounding factors (model 2) these associations attenuated to the null and remained null in the mediator adjusted models (models 3 and 4).
Of the 3537 participants included in multivariable analyses of associations with blood-based outcomes, 169 (5%) were born preterm. Associations in this subgroup born preterm were the same as those in offspring born at term, with point estimates all being very similar in the two groups and the P-values for interaction all being >0.3.
Table 4 shows the multivariable associations of HDP with vascular outcomes assessed at mean age 10.7 in the 4654 participants with complete data exposures, vascular outcomes, and on all covariables included in any models. Consistent with our previous publication with blood pressure measured at age 9.9 years, offspring of mothers who had experienced either gestational hypertension or pre-eclampsia had higher mean SBP and DBP in the first model. There was some attenuation with adjustment for maternal confounders, largely due to adjustment for maternal BMI and some further attenuation with adjustment for offspring BMI (model 1). However, positive associations remained even with these adjustments. After adjustment for confounders, including maternal and offspring BMI, the positive associations were stronger for SBP (∼2 mmHg greater in offspring whose mothers had either gestational hypertension or pre-eclampsia) than DBP (∼1 mmHg greater for each exposure). The associations were similar for gestational hypertension as they were for pre-eclampsia, though for the association of pre-eclampsia with DBP the confidence intervals were wide and included the null value. Adjustment for dietary sodium intake resulted in some attenuation of the association of pre-eclampsia with SBP and DBP, though positive associations remained, but did not alter associations of gestational hypertension with these outcomes. Adjustment for possible mediation by birthweight, gestational age, and mode of delivery resulted in attenuation towards the null of the association of pre-eclampsia with both SBP and DBP (model 5). With one exception, neither pre-eclampsia nor gestational hypertension was associated with endothelial dysfunction or any of the other vascular outcomes in any of the models. In the simple age- and gender-adjusted model offspring of women who had experienced gestational hypertension had slightly wider brachial artery diameters (model 1), but adjustment for confounding factors attenuated this to the null.
Table 4.
Multivariable associations of hypertensive disorder of pregnancy with offspring vascular phenotypes at mean age 10.7 years (n = 4654 with complete data on all variables used in any model)
| Outcome | Model | Mean difference (95% CI) |
||
|---|---|---|---|---|
| No HDP, n = 3781 | Gestational HT, n = 771 | Pre-eclampsia, n = 102 | ||
| SBP (mmHg) | M1 | 0 | 2.54 (1.83, 3.25) | 2.59 (0.80, 4.39) |
| M2 | 0 | 2.11 (1.39, 2.85) | 2.27 (0.49, 4.04) | |
| M3 | 0 | 2.04 (1.33, 2.76) | 2.09 (0.31, 3.89) | |
| M4 | 0 | 2.04 (1.33, 2.76) | 1.82 (0.03, 3.62) | |
| M5 | 0 | 2.09 (1.36, 2.81) | 1.30 (−0.54, 2.81) | |
| DBP (mmHg) | M1 | 0 | 1.38 (0.76, 1.99) | 1.72 (0.16, 3.28) |
| M2 | 0 | 1.13 (0.50, 1.77) | 1.26 (−0.32, 4.03) | |
| M3 | 0 | 1.10 (0.47, 1.73) | 1.12 (−0.49, 2.72) | |
| M4 | 0 | 1.10 (0.47, 1.73) | 1.40 (−0.17, 2.98) | |
| M5 | 0 | 1.10 (0.47, 1.73) | 1.26 (−0.32, 2.85) | |
| FMD (mm) | M1 | 0 | 0.00 (−0.01, 0.01) | 0.00 (−0.02, 0.02) |
| M2 | 0 | −0.01 (−0.01, 0.01) | −0.01 (−0.03, 0.01) | |
| M3 | 0 | −0.01 (−0.01, 0.00) | −0.01 (−0.03, 0.01) | |
| M4 | 0 | −0.01 (−0.01, 0.01) | −0.01 (−0.03, 0.01) | |
| M5 | 0 | −0.01 (−0.01, 0.01) | −0.01 (−0.02, 0.01) | |
| PWV (m/s) | M1 | 0 | −0.05 (−0.15, 0.04) | 0.08 (−0.16, 0.32) |
| M2 | 0 | −0.03 (−0.13, 0.07) | 0.11 (−0.13, 0.36) | |
| M3 | 0 | −0.02 (−0.12, 0.07) | 0.07 (−0.17, 0.32) | |
| M4 | 0 | −0.03 (−0.13, 0.07) | 0.10 (−0.15, 0.35) | |
| M5 | 0 | −0.02 (−0.12, 0.07) | 0.06 (−0.17, 0.31) | |
| DC (ratio of geometric means)a | M1 | 1 | 0.98 (0.94, 1.02) | 0.94 (0.84, 1.04) |
| M2 | 1 | 0.98 (0.94, 1.02) | 0.94 (0.84, 1.03) | |
| M3 | 1 | 0.97 (0.94, 1.01) | 0.93 (0.83, 1.03) | |
| M4 | 1 | 0.97 (0.94, 1.01) | 0.93 (0.83, 1.03) | |
| M5 | 1 | 0.97 (0.94, 1.01) | 0.93 (0.83, 1.03) | |
| Brachial artery diameter (mm) | M1 | 0 | 0.024 (0.001, 0.048) | −0.043 (−0.102, 0.016) |
| M2 | 0 | 0.010 (−0.015, 0.034) | −0.069 (−0.129, −0.008) | |
| M3 | 0 | 0.014 (−0.010, 0.039) | −0.059 (−0.119, 0.002) | |
| M4 | 0 | −0.002 (−0.002, −0.20) | −0.036 (−0.092, 0.019) | |
| M5 | 0 | 0.000 (−0.022, 0.022) | −0.037 (−0.092, 0.018) | |
M1: Adjusted for offspring sex and age at time of outcome measurement.
M2: Maternal confounder adjusted model: as M1 plus adjustment for maternal age, nulliparity, smoking during pregnancy, pre-pregnancy BMI, education and head of household social class.
M3: Confounder adjusted model taking account of offspring adiposity: as M2 plus adjustment for offspring BMI, height and height-squared (NB: substituting BMI for offspring waist circumference or for offspring fat mass did not substantively alter any of the results).
M4: As M3 plus adjustment for offspring dietary sodium.
M5: Mediator for intrauterine characteristics model: as M3 plus adjustment for birthweight, gestational age and mode of delivery.
SBP, systolic blood pressure; DBP, diastolic blood pressure; FMD, flow-mediated dilatation absolute; PWV, pulse wave velocity; DC, distensibility coefficient.
aResults for these outcomes are ratios of geometric means. As indicated in column 1 the null value for these results is 1 (it is 0 for other results which are mean differences).
Of the 4654 participants, 211 (5%) were born preterm of whom 25 had pre-eclampsia. Associations of either pre-eclampsia or gestational hypertension with all vascular outcomes in this subgroup who were born preterm were the same as those in offspring born at term, with point estimates all being very similar in the two groups and the P-values for interactions (between HDP and preterm/term) all being >0.5.
As HDP was not associated with outcomes other than offspring SBP and DBP, the associations with offspring blood pressure could not explain associations with other outcomes. Associations with other vascular and biomarker outcomes were essentially unchanged from those presented with further adjustment for SBP and DBP.
When all analyses were repeated in the 3062 with complete data on both sets of outcomes they were essentially unchanged from those presented here. In all statistical models, the residuals were approximately normally distributed and in all models overall model P-values were <0.0001. There was no evidence of multicollinearity in any models with all variance inflation factors being between 1.06 and 1.58.
Discussion
In this large general population birth cohort, we found no association of either gestational hypertension or pre-eclampsia with offspring lipids, apolipoproteins, inflammatory markers, FMD, PWV, DC, or brachial artery diameter, despite associations of HDP with offspring blood pressure assessed at mean age 9.9 years,1 and with blood pressure assessed ∼1 year later at mean age 10.7 years. The associations with blood pressure were somewhat attenuated by adjustment for maternal and offspring BMI, and further adjustment for dietary sodium intake, but positive associations remained. As we found previously,1 adjustment for birthweight, gestational age, and mode of delivery resulted in attenuation of the association of pre-eclampsia with offspring blood pressure but did not alter the associations of gestational hypertension with blood pressure.
Our findings contrast with two previous studies that found HDP14 and pre-eclampsia13 to be associated with lower FMD in offspring in later life. Both these studies were smaller and were based on selected populations. In the first, all offspring were born preterm and 19 mother-offspring pairs in which the mother had experienced HDP were compared with 52 controls where the mother had normal blood pressure in pregnancy. Of note, we found no associations of either pre-eclampsia or gestational hypertension with FMD or other vascular outcomes other than blood pressure in the subgroup in our cohort where the infant was born preterm (n = 215, of whom 25 had pre-eclampsia) and no evidence that any of the associations that we examined differed by whether the offspring were born preterm or at term. In the second study (n = 24 with pre-eclampsia and 27 controls with normal blood pressure in pregnancy), mothers and offspring had spent all of their lives living at high altitude and the authors argued that the stress on the vascular system at high altitude allowed associations to emerge in this population. We are unable to examine whether associations differ between individuals living at high altitude and those who do not, but our results suggest that, in a general population not living at high altitude, maternal HDP is not associated with cardiovascular risk factors in offspring beyond the established association with blood pressure. In both of these previous studies, outcomes were measured when offspring were somewhat older than in our study, in adolescence in the first14 and early 20 s in the second.13 It is possible that associations with a wider range of vascular outcomes do not emerge until after puberty and with further follow-up of the ALSPAC cohort we will have the potential to examine this. Indeed high blood pressure is a risk factor for later endothelial dysfunction. It is therefore possible that the primary effect of maternal HDP on offspring vascular risk is elevated blood pressure, but that this then leads to later adverse vascular outcomes, including endothelial dysfunction in later life.
We also found no long-term associations of HDP with offspring markers of chronic inflammation, lipids, or apolipoproteins, the latter being consistent with a previous, but much smaller, study that found no association of HDP with offspring lipids, but a positive association with blood pressure.5 Our findings are also consistent with the one study that has examined the association of HDP with offspring cardiovascular disease events, which found it to be associated with offspring stroke risk (more strongly influenced by blood pressure than lipids), but not coronary heart disease risk.20
The specific association of both pre-eclampsia and gestational hypertension with offspring blood pressure, but not with other cardiovascular risk factors, would support a mechanism that links higher blood pressure in mothers and their offspring. Such mechanisms would include the existence of common genetic variants that influence both HDP and higher offspring blood pressure, and also shared familial environmental factors that are specifically (or particularly strongly) associated with blood pressure. Greater adiposity is strongly associated with higher blood pressure outside of pregnancy and also with higher blood pressure during pregnancy21 and with HDP.1 Our findings suggest that familial correlations of adiposity may in part explain the association of HDP with offspring higher blood pressure. However, positive associations remained after adjustment for maternal and offspring BMI and greater adiposity is also strongly associated with markers of inflammation and adverse lipid levels and yet we found no associations of HDP with these. Dietary salt intake is a specific risk factor for higher blood pressure and therefore shared familial dietary patterns in relation to salt intake could result in a specific association of maternal blood pressure in pregnancy with offspring later blood pressure. We found that there was some attenuation of the association of pre-eclampsia with offspring blood pressure after adjustment for offspring dietary sodium intake, but this adjustment had no effect on the association of gestational hypertension with offspring blood pressure. Even after adjustment for maternal and offspring BMI and offspring dietary sodium intake, as well as other potential confounders a positive association of both gestational hypertension and pre-eclampsia with offspring blood pressure, particularly with SBP, remained. Further evidence for shared genetic or familial environmental characteristics explaining the associations for HDP with offspring blood pressure, come from findings that the association of maternal blood pressure with offspring blood pressure is of very similar magnitude to that of paternal blood pressure with offspring blood pressure.22 Essential hypertension in both parents has also been shown to associate with offspring forearm endothelial dysfunction, with similar magnitudes of association for each parent.23 Thus, even in studies finding associations with endothelial function this may be due to shared familial characteristics rather than specific intrauterine effects. We do not have measurements of blood pressure in the fathers in this study to be able to compare father-offspring blood pressure associations with the mother-offspring associations reported here.
Others have suggested that HDP results in vasculotoxic factors being released into the maternal circulation which cross the placenta and have adverse influences on foetal development, resulting in increased risk of vascular dysfunction and chronic inflammation in later life of the offspring.13 Our results from the largest study to date in a general population do not support this assertion, although as acknowledged above it is possible that associations with outcomes other than blood pressure may emerge as these offspring age.
Study strengths and limitations
The main strength of our study is that it is considerably larger than previous studies and examines a wider range of offspring outcomes that have been suggested as being plausibly influenced by exposure to maternal HDP in utero. Consistent with all general population birth cohorts, our study is affected by cohort attrition and missing data (Figure 1). The distribution of HDP was similar in all women with obstetric data, those whose child had attended either of the clinics and also those who had complete data on any variable included in any analyses. In a number of sensitivity analyses, using multivariate multiple imputation, results were similar to those presented here including only those with complete data. These findings suggest that our results have not been markedly biased by loss to follow-up or missing data. Offspring blood tests were completed on non-fasting blood samples but the majority of measures are not appreciably altered by this approach.24,25 Assessment of dietary sodium intake was by diet diaries rather than excretion in 24 h urine and measurement error in this dietary assessment may mean that we have been unable to fully account for its contribution to the observed association. All of our vascular measurements were done on the brachial artery and therefore do not necessarily reflect central arterial stiffness.26 However, these assessments are consistent with those used in other studies of these associations and despite blood pressure amplification from central to more peripheral arteries, peripheral measurements do correlate strongly with central ones and it is unlikely that important strong associations with central stiffness have been missed here.
In conclusion, our findings suggest that there is a specific association of both types of HDP with greater offspring blood pressure but that HDP is not associated with FMD, PWV, DC, lipids, apolipoproteins, or inflammatory markers.
Ethical approval
Ethical approval for all aspects of data collection was obtained from the ALSPAC Law and Ethics Committee (IRB 00003312) and the Local Research Ethics Committee. All participants provided signed written consent.
Contribution of Authors
D.A.L. and A.F. developed the study idea; D.A.L. developed the analysis plan and undertook all analyses; A.F. and C.M.W. cleaned obstetric data and generated the HDP variables; all authors were involved in obtaining research funds for some of the data used in this publication and all contributed critically to the interpretation of results. D.A.L. wrote the first draft of the paper and all authors contributed to the final version.
Funding
This work was funded by a grant from the NIH (R01 DK077659) and the Wellcome Trust (WT087997MA); the latter funds C.M.W.; The UK Medical Research Council (MRC), the Wellcome Trust and the University of Bristol provide core-funding support for ALSPAC; The blood assays were funded by a British Heart Foundation (BHF) grant (PG07/002); Vascular measures were funded by a BHF programme grant (RG/20011002); The MRC (G0600705) and the University of Bristol provide core-funding for MRC CAiTE; A.F. is funded by a MRC research fellowship; ADH is funded by a BHF Senior Research Fellowship (FS05/125); The views expressed in this manuscript are those of the authors and not necessarily any funding body. No funding body had access to data and none influenced the analysis plan or interpretation of its results. Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust.
Conflict of interest: none declared.
Acknowledgements
We are grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. D.A.L., A.F., and C.M.-W. had full access to all of the data in the study and D.A.L. takes responsibility for the integrity of the analyses.
References
- 1.Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey Smith G, Sattar N, Nelson S, Lawlor DA. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. 2010;122:1192–1199. doi: 10.1161/CIRCULATIONAHA.110.936674. doi:10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palti H, Rothschild E. Blood pressure and growth at 6 years of age among offsprings of mothers with hypertension of pregnancy. Early Hum Dev. 1989;19:263–269. doi: 10.1016/0378-3782(89)90061-3. doi:10.1016/0378-3782(89)90061-3. [DOI] [PubMed] [Google Scholar]
- 3.Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Pre-eclampsia and offspring's blood pressure, cognitive ability and physical development at 17-years-of-age. Br J Obstet Gynaecol. 1991;98:1009–1014. doi: 10.1111/j.1471-0528.1991.tb15339.x. doi:10.1111/j.1471-0528.1991.tb15339.x. [DOI] [PubMed] [Google Scholar]
- 4.Taittonen L, Nuutinen M, Turtinen J, Uhari M. Prenatal and postnatal factors in predicting later blood pressure among children: cardiovascular risk in young Finns. Pediatr Res. 1996;40:627–632. doi: 10.1203/00006450-199610000-00019. doi:10.1203/00006450-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Tenhola S, Rahiala E, Martikainen A, Halonen P, Voutilainen R. Blood pressure, serum lipids, fasting insulin, and adrenal hormones in 12-year-old children born with maternal preeclampsia. J Clin Endocrinol Metab. 2003;88:1217–1222. doi: 10.1210/jc.2002-020903. doi:10.1210/jc.2002-020903. [DOI] [PubMed] [Google Scholar]
- 6.Tenhola S, Rahiala E, Halonen P, Vanninen E, Voutilainen R. Maternal preeclampsia predicts elevated blood pressure in 12-year-old children: evaluation by ambulatory blood pressure monitoring. Pediatr Res. 2006;59:320–324. doi: 10.1203/01.pdr.0000196734.54473.e3. doi:10.1203/01.pdr.0000196734.54473.e3. [DOI] [PubMed] [Google Scholar]
- 7.Oglaend B, Forman MR, Romundstad PR, Nilsen ST, Vatten LJ. Blood pressure in early adolescence in the offspring of preeclamptic and normotensive pregnancies. J Hypertens. 2009;27:2051–2054. doi: 10.1097/HJH.0b013e328330052a. doi:10.1097/HJH.0b013e328330052a. [DOI] [PubMed] [Google Scholar]
- 8.Lawlor DA, Davey Smith G. Early life determinants of adult blood pressure. Curr Opin Nephrol Hypertens. 2005;14:259–264. doi: 10.1097/01.mnh.0000165893.13620.2b. doi:10.1097/01.mnh.0000165893.13620.2b. [DOI] [PubMed] [Google Scholar]
- 9.Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195:40–49. doi: 10.1016/j.ajog.2005.07.049. doi:10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 10.Sattar N, Bendomir A, Berry C, Shepherd J, Greer IA, Packard CJ. Lipoprotein subfraction concentrations in preeclampsia: pathogenic parallels to atherosclerosis. Obstet Gynecol. 1997;89:403–408. doi: 10.1016/S0029-7844(96)00514-5. doi:10.1016/S0029-7844(96)00514-5. [DOI] [PubMed] [Google Scholar]
- 11.Sattar N, Gaw A, Packard CJ, Greer IA. Potential pathogenic roles of aberrant lipoprotein and fatty acid metabolism in pre-eclampsia. Br J Obstet Gynaecol. 1996;103:614–620. doi: 10.1111/j.1471-0528.1996.tb09827.x. doi:10.1111/j.1471-0528.1996.tb09827.x. [DOI] [PubMed] [Google Scholar]
- 12.Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003;42:39–42. doi: 10.1161/01.HYP.0000074428.11168.EE. doi:10.1161/01.HYP.0000074428.11168.EE. [DOI] [PubMed] [Google Scholar]
- 13.Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori-Cucchia C, Nicod P, Villena M, Allemann Y, Scherrer U, Sartori C. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122:488–494. doi: 10.1161/CIRCULATIONAHA.110.941203. doi:10.1161/CIRCULATIONAHA.110.941203. [DOI] [PubMed] [Google Scholar]
- 14.Lazdam M, de la Horra A, Pitcher A, Mannie Z, Diesch J, Trevitt C, Kylintireas I, Contractor H, Singhal A, Lucas A, Neubauer S, Kharbanda R, Alp N, Kelly B, Leeson P. Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension. 2010;56:159–165. doi: 10.1161/HYPERTENSIONAHA.110.150235. doi:10.1161/HYPERTENSIONAHA.110.150235. [DOI] [PubMed] [Google Scholar]
- 15.Golding J, Pembrey M, Jones R The ALSPAC Study Team. ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatric Perinatal Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. doi:10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 16.Davey DA, MacGillivray I. The classification and definition of the hypertensive disorders of pregnancy. Am J Obstet Gynecol. 1988;158:892–898. doi: 10.1016/0002-9378(88)90090-7. [DOI] [PubMed] [Google Scholar]
- 17.Donald AE, Charakida M, Falaschetti E, Lawlor DA, Halcox JP, Golding J, Hingorani AD, Davey Smith G, Deanfield JE. Determinants of vascular phenotype in a large childhood population: the Avon Longitudinal Study of Parents and Children (ALSPAC) Eur Heart J. 2010 doi: 10.1093/eurheartj/ehq062. doi:10.1093/eurheartj/ehq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brion M-J A, Ness AR, Rogers I, Emmett P, Cribb V, Davey Smith G, Lawlor DA. Maternal macronutrient and energy intake in pregnancy and offspring intake at 10 years: exploring parental comparisons and prenatal effects. Am J Clin Nutr. 2010;91:748–756. doi: 10.3945/ajcn.2009.28623. doi:10.3945/ajcn.2009.28623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Royston P. Multiple imputation of missing values. Stata J. 2004;4:227–241. [Google Scholar]
- 20.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40:1176–1180. doi: 10.1161/STROKEAHA.108.538025. doi:10.1161/STROKEAHA.108.538025. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Established pre-eclampsia risk factors are related to patterns of blood pressure change in normal term pregnancy: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC) J Hyperten. 2011 doi: 10.1097/HJH.0b013e328349eec6. in press. [DOI] [PubMed] [Google Scholar]
- 22.Walker BR, McConnachie A, Noon JP, Webb DJ, Watt GC. Contribution of parental blood pressures to association between low birth weight and adult high blood pressure: cross sectional study. BMJ. 1998;316:834–837. doi: 10.1136/bmj.316.7134.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taddei S, Virdis A, Mattei P, Arzilli F, Salvetti A. Defective L-Arginine-nitric oxide pathway in offspring of essential hypertensive parents. Circulation. 1996;94:1298–1303. doi: 10.1161/01.cir.94.6.1298. [DOI] [PubMed] [Google Scholar]
- 24.Rifai N, Merrill JR, Holly RG. Postprandial effect of a high fat meal on plasma lipid, lipoprotein cholesterol and apolipoprotein measurements. Ann Clin Biochem. 1990;27(Pt 5):489–493. doi: 10.1177/000456329002700512. [DOI] [PubMed] [Google Scholar]
- 25.Poppitt SD, Keogh GF, Lithander FE, Wang Y, Mulvey TB, Chan YK, McArdle BH, Cooper GJ. Postprandial response of adiponectin, interleukin-6, tumor necrosis factor-alpha, and C-reactive protein to a high-fat dietary load. Nutrition. 2008;24:322–329. doi: 10.1016/j.nut.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Bondier H. on behalf of the European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. doi:10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]